Abstract
Objective: To estimate the prevalence and study the clinical presentation of mild cognitive impairment (MCI), assess its outcome in terms of cognition and quality of life, identify factors for reversion to baseline, and compare these factors in the modifiable and nonmodifiable risk factor groups.
Methods: Individuals aged >50 years with memory/cognitive complaint(s) were screened using the Mini-Cog over 1 year (August 2018–August 2019). Those meeting the DSM-5 criteria for MCI were enrolled, and risk factors (modifiable and nonmodifiable) were noted. Assessments were done using the Hindi version of the Montreal Cognitive Assessment (H-MoCA), the Clinical Dementia Rating (CDR)-Hindi version, and the World Health Organization Quality of Life-Brief Hindi version. Treatment outcome was assessed at 6 months and compared between the risk factor groups. Factors for reversion of MCI were assessed.
Results: A total of 124 patients (22.1% of 561 with cognitive complaints) had MCI, and 100 patients (50 patients from the modifiable group and 50 patients from the nonmodifiable group) completed the study. Depression (52%) and hypertension (48%) were common risk factors. End point cognition scores were similar in both groups, with quality of life better in the modifiable group (P = .023). Age was negatively correlated with cognition in total patients and the nonmodifiable group (r =0.283–0.420; P = .002–.004). In total patients, cognition moderately correlated with education and somewhat with quality of life; 31% and 57% reverted to normal on the MoCA and CDR scales, respectively, while 1 progressed to dementia. Reverters had higher baseline H-MoCA scores (odds ratio [OR] = 6.996; P < .001) and were treated with cholinesterase inhibitors + vitamin E (OR = 28.999; P = .007).
Conclusion: Short-term outcome for both the modifiable and nonmodifiable risk factor groups was favorable. Higher education positively correlated with cognition, which itself predicted a better quality of life. Reverters of MCI had better baseline cognition and were treated with cholinesterase inhibitors + vitamin E.
Prim Care Companion CNS Disord 2024;26(4):24m03708
Author affiliations are listed at the end of this article.
Mild cognitive impairment (MCI) is the transitional state between the cognition of normal aging and mild dementia.1 Global prevalence in the general population varies from 6% to 12%2; however, rates vary widely in Asian studies. A study from Malaysia quoted MCI in persons aged approximately 65–70 years as 68%,3 and a hospital-based Indian study4 found a prevalence of 31.5%. Varying study population or diagnostic guidelines for MCI may be a cause, warranting standardization and age-appropriate estimates. The DSM-5 describes MCI as mild neurocognitive disorder, divided into amnestic and nonamnestic, single or multiple domain types.5 Outcome of MCI is varied; some progress to dementia (20%–40%), some remain stable, some improve over time, and some go back and forth and eventually develop dementia.6 Outcome is influenced by modifiable and nonmodifiable risk factors such as neuropsychiatric manifestations, medical conditions, and socioeconomic factors.7 Having no specific diagnostic modalities/treatment, early detection is of paramount importance to prevent its progression to dementia.8 Considering scarce Indian research on MCI and comparative data on the risk factors, the current study aimed to estimate the prevalence and study clinical presentation of MCI, assess its outcome in terms of cognition and quality of life, identify factors for reversion to baseline, and compare these factors in potentially modifiable and nonmodifiable risk factor groups.
METHODS
This was a longitudinal comparative study conducted at a tertiary-care government hospital in Punjab, India. Enrollment was done over 1 year (August 2018–August 2019) after approval by the Institutional Ethics Committee (No. BFUHS/2K18p-TH/14436).
Inclusion and Exclusion Criteria
Individuals aged >50 years with memory or cognitive complaints, presenting for the first time to the memory clinic of the psychiatry department of the hospital, who gave written informed consent for participation were included. Subjects with intellectual disability, those with a history of recent head injury or severe medical/ psychiatric morbidities, those taking anticholinergic drugs, and those who took cognitive enhancers/ cholinesterase inhibitors in the last year were excluded.
Procedure
Individuals aged >50 years with memory/cognitive complaints were screened with the Mini-Cog scale.9 Subjects screening positive for MCI were interviewed, and diagnosis was made using DSM-5 criteria.5 Written informed consent was taken from patients and primary caregivers. Diagnostic confirmation and severity assessment were done using 2 scales: the Hindi version of the Montreal Cognitive Assessment (H-MoCA)10 and the Clinical Dementia Rating (CDR) Hindi version.11 Sociodemographic profile and illness-related data were collected using a semistructured proforma. Assessment of quality of life was done using the World Health Organization-Quality of Life-Brief (WHOQOL-BREF) Hindi scale.12 To justify the inclusion/exclusion criteria, patients were examined (general physical and mental status examination) and investigated (complete blood counts; liver, renal, and thyroid function tests; serum electrolytes; HIV/hepatitis B surface antigen/hepatitis C virus testing; fasting blood sugar and lipids; vitamin B12; electrocardiogram; and specific investigations like electroencephalogram or magnetic resonance imaging of the brain, wherever required). Based on the above information, risk factors were noted. Potential modifiable risk factors included medical comorbidities, such as diabetes mellitus type II, hypertension, hypothyroidism, hyperlipidemia, vitamin B12/folate deficiency, and obesity, and psychiatric comorbidities, such as alcohol/ nicotine use disorders, depressive disorder, anxiety disorder, and bipolar affective disorder. Diagnosis of medical disorders was made using the previously mentioned investigations and medicine/neurology consult(s). Psychiatric risk factors and disorders were assessed based on history/case records and mental status examination and corroborated with scales: Patient Health Questionnaire–Somatic, Anxiety, and Depressive Symptom Scale13 for depression, anxiety, and somatoform disorders and the Young Mania Rating Scale14 for bipolar disorder. Education is an important modifiable risk factor; however, considering that the level of education was similar in the whole population, sensible cutoffs for comparison of low versus high education could not be made, with significant overlap with the risk factors enumerated previously. Effect of education was hence separately studied in the overall population and not in light of modifiable versus nonmodifiable risk factors. The potentially nonmodifiable risk factors included a family history of neurocognitive disorder like Alzheimer disease, a history of head injury, and those with none of any of the previously mentioned risk factors. At enrollment, patients with modifiable risk factors were either not in the active phase of illness or were treated until its passing. Patients were started on treatment for MCI and followed up on a weekly, biweekly, or monthly basis to ensure treatment adherence and/or dose adjustments. A phone call was made to the primary caregiver every 2 weeks ensuring compliance. Along with pharmacologic treatment, all patients were advised for lifestyle modification including healthy diet, physical activity, social engagement, and any home-based cognitive activities. Outcome was assessed in terms of change in cognitive scores and quality of life at 6 months. Statistical comparison based on sociodemographic, illness, and outcome variables was done between the risk factor groups. Factors for reversion from MCI to normal cognition were assessed.
Instruments
Mini-Cog. An excellent screening measure for cognitive impairment, the Mini-Cog,9 takes ∼3 minutes to administer. It has a 3-item recall component (3 points) and a clock drawing test (2 points), with a total score of 5. Scores are not affected by low literacy or physical illness. A cut point of <4 is recommended for further evaluation and <3 for dementia. The developers indicated that a low sensitivity for MCI can be increased using a higher cut point.15 Hence, patients with a Mini-Cog score of 3–4 were taken as a positive screen for MCI.
H-MoCA. Designed for MCI detection, the H-MoCA is a 30-point test, administered in <10 minutes.10 It assesses cognitive domains of memory (5 points); visuospatial abilities (4 points); executive functions (4 points); attention, concentration, and working memory (6 points); language (5 points); and orientation (6 points). Patients scoring 18–25 are considered to have MCI.
CDR Hindi version. A 5-point scale used for MCI and dementia, the CDR Hindi version is rated by both patient and caregiver.11 It contains 6 domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Scoring is not affected by physical illness. Scores range from 0: no impairment to 0.5: MCI, with 1, 2, and 3, respectively, as mild, moderate, and severe dementia. With many individuals being illiterate, a score of 0.5–1 is considered MCI.
WHOQOL-BREF. The Hindi version of the WHOQOL BREF was used.12 It assesses one’s subjective perception of quality of life. The 26-item self-administered scale measures 4 domains: physical health, psychological health, social relationships, and environment. Each item is scored from 1 to 5, and domain scores are transformed to a 100-point scale.
The H-MoCA assessed cognitive domains in detail, while the CDR also assessed practical domains. The H-MoCA has tremendous utility in MCI, while the CDR can track progress from MCI to dementia better. Hence, both scales were used.
Statistical Analysis
Data were analyzed using IBM SPSS v23. Descriptive data were presented as frequency, mean ± SD, and median. Categorical variables were compared using the Pearson χ2 test, with Bonferroni correction for multiple comparisons. The Fisher exact test was used when the expected cell count (≥20% cells) was less than 5. Comparison of continuous variables was made using the Mann-Whitney U test. The Wilcoxon signed-rank test was used for related samples. Correlation analysis was done using the Kendall τ-b test. Binomial logistic regression was used to evaluate factors of reversion. P values <.05 were considered significant.
RESULTS
Of the 619 newly registered patients in the memory clinic, 561 patients were aged >50 years with memory/ cognitive complaint(s). A total of 177 patients screened positive for MCI on the Mini-Cog. Of these, 124 patients (22.1%) were diagnosed with MCI, with 68 and 56 patients having potentially modifiable and nonmodifiable risk factors, respectively. Within the modifiable and nonmodifiable risk factor groups, 6 and 2 patients did not fulfill inclusion/exclusion criteria, 1 in each group did not provide consent, 3 and 1 patient died or suffered from severe medical ailment(s) during the follow-up, and 8 and 2 patients were lost to follow-up (2 in each group due to nonimprovement in symptoms and the rest for unknown reasons). The final analysis included 100 patients, with N = 50 in each group.
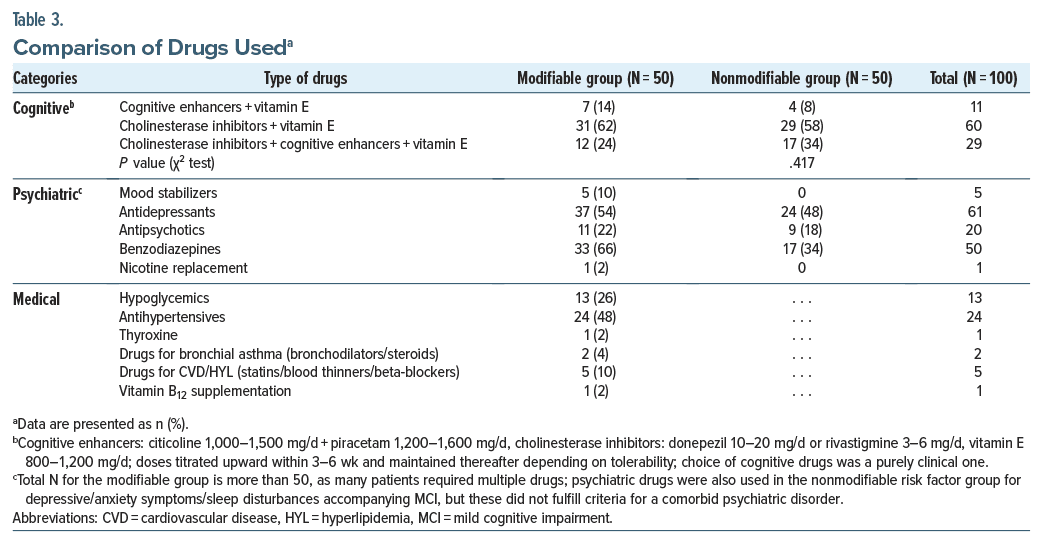
Patients’ median age and duration of illness (DOI) were 63 and 0.8 years, respectively. Most were male, educated until the primary school level (5 years), farmers, married, Sikhs, and from upper middle class joint families. Sociodemographic comparisons between the risk factor groups (Table 1) were mostly insignificant, except that more patients in the modifiable group were urban (n = 33, 66%) than in the nonmodifiable group who were rural (n = 32, 64%) (P = .003). Risk factors and drugs used are shown in Tables 2 and 3. The most common modifiable risk factor was depression (n = 26, 52%) among psychiatric factors and hypertension (n = 24, 48%) followed by diabetes mellitus (n = 13, 26%) among medical factors. More than half (n = 26, 52%) of patients in the modifiable group had combined psychiatric and medical risk factors. No group differences were found based on medication.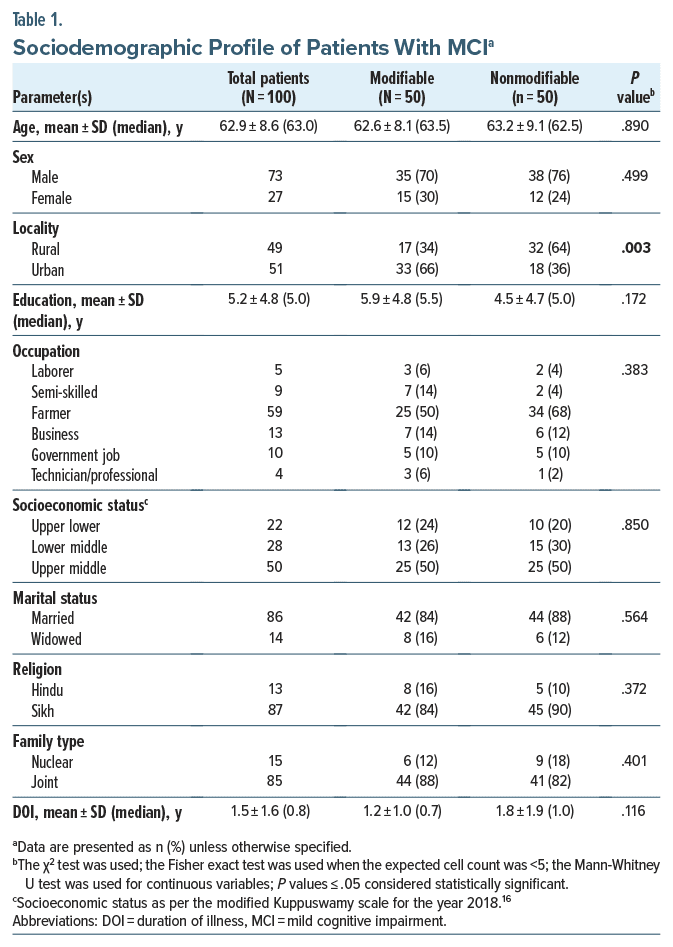
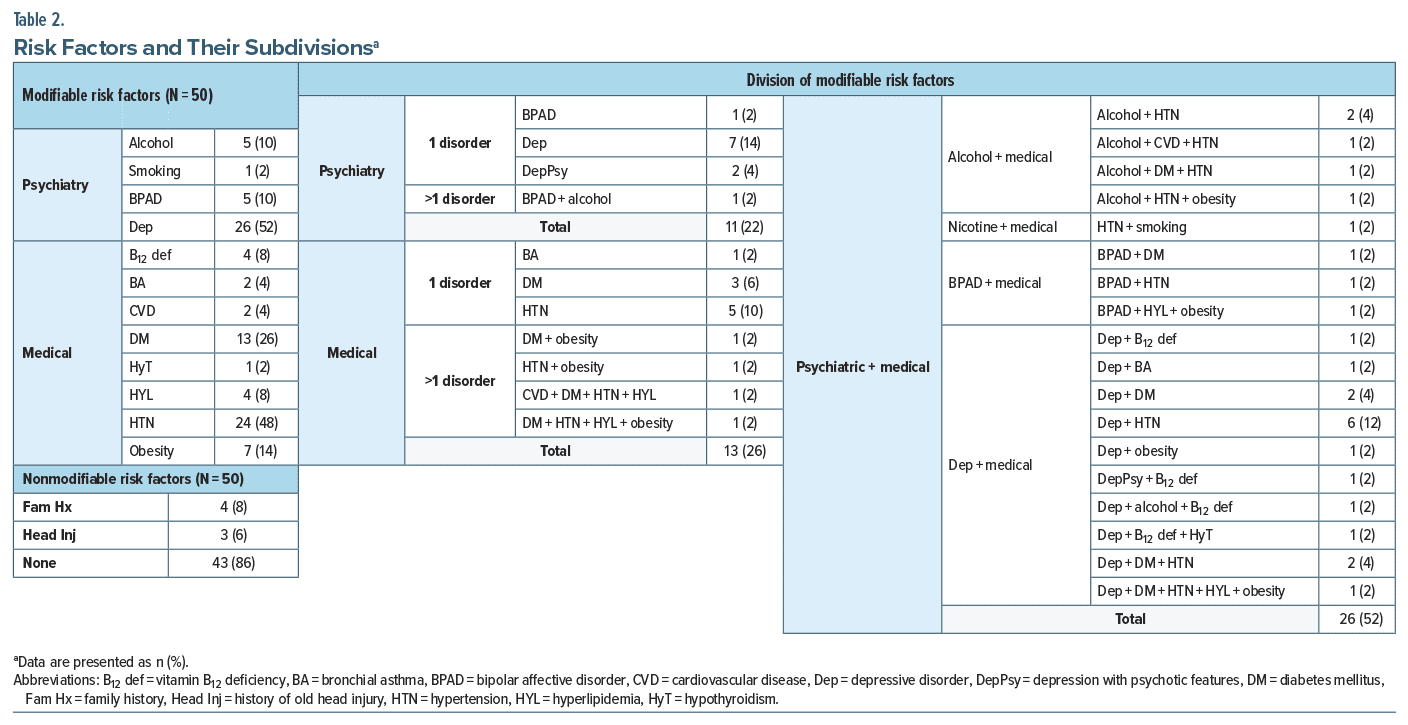
 Mean (median) baseline and end point (6 months) H-MoCA, CDR, and WHOQOL scores in total patients (N = 100) were 20.3 ± 1.9 (20.0), 0.7 ± 0.2 (0.5), 46.0 ± 4.9 (45.5) and 24.8 ± 2.6 (25.0), 0.2 ± 0.3 (0.0), 61.0 ± 6.2 (61.2), respectively. At end point, the Wilcoxon signed-rank test showed a significant increase in H-MoCA and WHOQOL scores and a significant decrease in CDR scores (P < .0001 for all pairs). Table 4 compares illness variables between the risk factor groups based on MoCA, CDR, and WHOQOL scores. Following treatment, at 6 months, both groups individually showed an increase in cognitive scores and quality of life (P < .001 for all). But comparing intergroup at baseline and end point, cognitive scores were similar. At 6 months, however, the quality of life in the modifiable risk factor group was significantly better than its counterpart (P = .023). Comparison of scale categories at baseline and 6 months showed no difference, with many patients improving from MCI to normal cognition. One patient in the nonmodifiable group progressed to mild dementia.
Mean (median) baseline and end point (6 months) H-MoCA, CDR, and WHOQOL scores in total patients (N = 100) were 20.3 ± 1.9 (20.0), 0.7 ± 0.2 (0.5), 46.0 ± 4.9 (45.5) and 24.8 ± 2.6 (25.0), 0.2 ± 0.3 (0.0), 61.0 ± 6.2 (61.2), respectively. At end point, the Wilcoxon signed-rank test showed a significant increase in H-MoCA and WHOQOL scores and a significant decrease in CDR scores (P < .0001 for all pairs). Table 4 compares illness variables between the risk factor groups based on MoCA, CDR, and WHOQOL scores. Following treatment, at 6 months, both groups individually showed an increase in cognitive scores and quality of life (P < .001 for all). But comparing intergroup at baseline and end point, cognitive scores were similar. At 6 months, however, the quality of life in the modifiable risk factor group was significantly better than its counterpart (P = .023). Comparison of scale categories at baseline and 6 months showed no difference, with many patients improving from MCI to normal cognition. One patient in the nonmodifiable group progressed to mild dementia.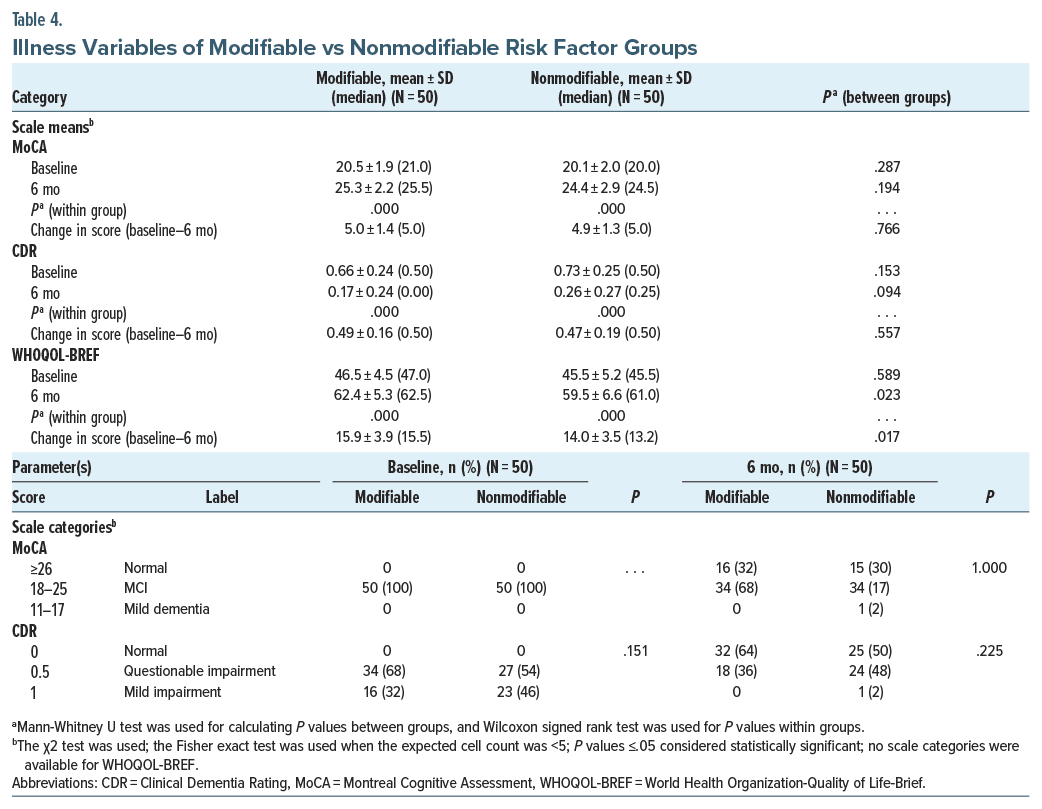 Zero-order correlations showed that in the total population (N = 100) and in the nonmodifiable group (N = 50), age had a consistent negative correlation with H-MoCA scores at baseline (r = −0.283, P = .004; r = −0.420, P = .002, respectively) and H-MoCA (r = −0.295, P = .003; r = −0.404, P = .004, respectively) and CDR (r = −0.284, P = .004; r = −0.306, P = .025, respectively) scores at 6 months, but not in the modifiable risk factor group (r = −0.228–0.260, P = .068–.310, N = 50). Male sex correlated with H-MoCA scores at baseline (r = −0.206, P = .040, N = 100). Other variables including DOI and cognitive drugs did not correlate with outcome measures. Controlling for age and sex, partial nonparametric correlations (Table 5) depicted education having a positive correlation with cognitive scores and quality of life at baseline and end point for all, but not with quality of life at end point for the modifiable group. Controlling for age, sex, and education, quality of life was inconsistently correlated with cognitive measures at baseline. At 6 months, however, it was more consistent.
Zero-order correlations showed that in the total population (N = 100) and in the nonmodifiable group (N = 50), age had a consistent negative correlation with H-MoCA scores at baseline (r = −0.283, P = .004; r = −0.420, P = .002, respectively) and H-MoCA (r = −0.295, P = .003; r = −0.404, P = .004, respectively) and CDR (r = −0.284, P = .004; r = −0.306, P = .025, respectively) scores at 6 months, but not in the modifiable risk factor group (r = −0.228–0.260, P = .068–.310, N = 50). Male sex correlated with H-MoCA scores at baseline (r = −0.206, P = .040, N = 100). Other variables including DOI and cognitive drugs did not correlate with outcome measures. Controlling for age and sex, partial nonparametric correlations (Table 5) depicted education having a positive correlation with cognitive scores and quality of life at baseline and end point for all, but not with quality of life at end point for the modifiable group. Controlling for age, sex, and education, quality of life was inconsistently correlated with cognitive measures at baseline. At 6 months, however, it was more consistent.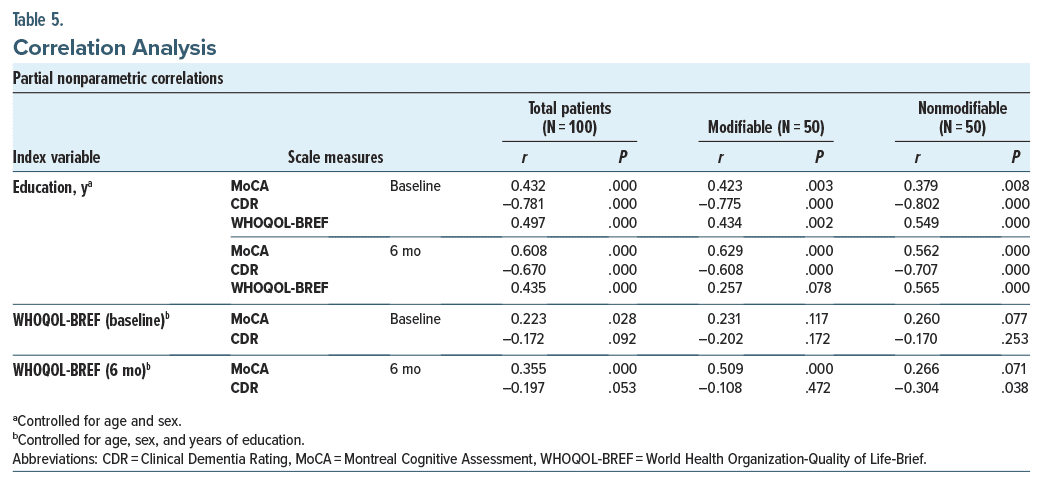 MCI patients reverting to normal were 31 (31%) and 57 (57%) on the H-MoCA and CDR, respectively (N = 100). Binomial logistic regression was performed to ascertain the effects of the risk factor group, age, sex, education, occupation, socioeconomic status, DOI, cognitive drugs used, cognitive scores (MoCA/CDR), and WHOQOL scores at baseline on the likelihood of reverting to normal cognition at end point. All other factors remaining the same, H-MoCA and CDR scores at baseline were interchanged in 2 separate models utilizing “reversion on H-MoCA” and “reversion on CDR” as dependent variables, respectively. Both models were statistically significant (χ2 = 86.625 and 93.714, respectively, df = 16 and P < .0001 for both), and both explained 81.6% (Nagelkerke R2) of variance. Patients having a higher baseline H-MoCA score and treated with cholinesterase inhibitors + vitamin E were more likely to revert on the H-MoCA and CDR, respectively (odds ratio [OR] = 6.996, P = .0004 and OR = 28.999, P = .007, respectively). Models were tried to evaluate the effect of individual risk factor as covariate for reversion on the H-MoCA or CDR; however, these were not good fits.
MCI patients reverting to normal were 31 (31%) and 57 (57%) on the H-MoCA and CDR, respectively (N = 100). Binomial logistic regression was performed to ascertain the effects of the risk factor group, age, sex, education, occupation, socioeconomic status, DOI, cognitive drugs used, cognitive scores (MoCA/CDR), and WHOQOL scores at baseline on the likelihood of reverting to normal cognition at end point. All other factors remaining the same, H-MoCA and CDR scores at baseline were interchanged in 2 separate models utilizing “reversion on H-MoCA” and “reversion on CDR” as dependent variables, respectively. Both models were statistically significant (χ2 = 86.625 and 93.714, respectively, df = 16 and P < .0001 for both), and both explained 81.6% (Nagelkerke R2) of variance. Patients having a higher baseline H-MoCA score and treated with cholinesterase inhibitors + vitamin E were more likely to revert on the H-MoCA and CDR, respectively (odds ratio [OR] = 6.996, P = .0004 and OR = 28.999, P = .007, respectively). Models were tried to evaluate the effect of individual risk factor as covariate for reversion on the H-MoCA or CDR; however, these were not good fits.
DISCUSSION
Clinical Presentation and Outcome of MCI
The prevalence of MCI of 22.1% in the present study, which was lower than a previous hospital-based study from north India (31.5%),4 may be due to different age inclusions (>50 years in the current study and ≥65 years in the previous study). Our results, however, fall within the prevalence range quoted by an Indian report (4.3%–26.06%).17 Variability in study design and MCI criteria were reasons for the high range. Regarding sociodemographic and illness-related data, a European study by Hanninen et al18 found a higher mean age (69.9 ± 4.3 years) and mean years of education (7.4 ± 2.7) and no sex difference. In the Indian context, a study by Mohan et al19 found that the majority were 60–69 years of age and female, had <8 years of education, and were married, unemployed, pensioners, homemakers, and above the poverty line. Difference from studies conducted abroad may be due to geography. Indian data were similar to the current study, except for sex. Population variance due to study location may be a cause rather than actual sex disparity.
In terms of outcome, there was a significant improvement in cognition and quality of life scores. Although improvement in quality of life was evidently better (a change of ∼15 points), a “clinically relevant” improvement in cognition is better understood in terms of reversion and nonreversion, which is discussed later. Among sociodemographic factors, poor cognition at end point was correlated with higher age and lower education. Previous research by Samy et al20 and Wilhalme et al21 also found age to be correlated with lower cognitive scores, but with no sex differences. Histories of depression, stroke, and diabetes were also reported as predictors,20,21 which was not the case in the current study, possibly due to design/population differences. The design of the current study eliminated biases raised by active psychiatric/medical illnesses. History of stroke, however, could not be assessed, as such patients either had a recent history of stroke and hence were excluded or had dementia rather than MCI or presented to other departments of the hospital.
Modifiable and Nonmodifiable Risk Factors
Depression was the most common psychiatric risk factor, and hypertension and diabetes were the most common medical risk factors. More than half of patients had combined psychiatric and medical risk factors, with depression being common again. Nonmodifiable factors included a small total percentage (14%) with a positive family history of cognitive impairment or a history of old head injury. The rest either had none of the above factors or more likely had genetic or specific biochemical markers that were out of scope of the current study. Previous studies22–24 also report depression, smoking, and medical factors such as hypertension, diabetes, physical inactivity, hearing loss, heart disease, stroke, and poor sleep as common modifiable factors. Similar to the current study, Rolandi et al25 reported nonmodifiable demographic factors of age and sex, apolipoprotein E ε4, and history of traumatic brain injury. Another study by Locke et al26 found that chances of cognitive impairment increased with a family history of cognitive impairment (risk ratio = 1.011). The current study did not find family history to be relevant, probably because of its small percentage, hence lower power for significance. An increase in sample size could help.
No sociodemographic differences were found between the risk factor groups, except that more patients with potentially modifiable risk factors were urban and vice versa. The hospital is located in an urban setting; hence, easy accessibility and relatively frequent visits for treatment by urban patients may cause this difference. Other patient-related factors may be sedentary lifestyle and stress in urban settings, making them more prone to MCI than rural patients.27 Higher age in the current study had a negative impact on cognition in the nonmodifiable risk factor group but not in the modifiable group. It may be that the modifiable illnesses themselves played a major role in impacting cognition. Although direct impact was ruled out as part of the study design, indirect impact by way of cell messenger systems or inflammatory pathways may be a possibility, which was also suggested by previous research.28,29 Education positively impacted cognition and quality of life in both groups. Previous research also shows education as a protective and increasing age as a negative factor for MCI.26,30
Quality of Life
At end point, quality of life correlated with education and cognition scores. For the latter, correlation was found on distinct scales in the 2 risk factor groups, probably due to scale differences, but overall, better cognition indicated better quality of life. The same was proposed by Hussenoeder et al,31 with an emphasis on the importance of individual domains rather than on total quality of life scores.
Among the risk factor groups, quality of life was better in the modifiable group. Although statistically significant, clinical significance is doubtful, considering a difference of about 2 points on the WHOQOL scale. If taken statistically, compared to modifiable factors, the nonmodifiable factors seem to be more resilient to drugs/other interventions focused on increasing functionality and hence quality of life due to lack of specific drug targets and/or biomarkers. Recent drug developments, eg, donanemab and lecanemab, are underway; however, their use requires research in larger populations even for dementia, much less its use in MCI. In terms of quality of life, supporting the present study’s results, recent research by Mank et al32 found that it declined in individuals with MCI who were amyloid positive, a nonmodifiable factor, compared to those who were not.
Reverters and Nonreverters
Many patients reverted to normal cognition at end point (31% and 57%, depending on the scale used), at least in the short term of 6 months. One progressed to mild dementia, while the rest remained stable. Previous studies report variable results. Angevaare et al33 quoted around 48% improving and not meeting criteria for MCI on follow-ups. A community MCI sample from Australia followed for 2 years showed only 29.6% reverting to normal cognition,30 while the rest did not revert, similar to the current study. In contrast, some studies, 1 from the United States by Koepsell and Monsell34 and the other from the National Institute of Mental Health and Neurosciences, India (Mukku et al35), found that only 16% and none reverted, respectively. This high range of outcomes may be due to differences in study population/ design (hospital based in the current study), tool differences, variable duration of follow-ups, and difference in intervention (some were naturalistic and some intervention). Of note, the current study found more reverters on the CDR rather than on the MoCA. Scale difference is a factor. It may be that since the CDR also assesses practical domains like activities of daily living (ADLs), a not-so-large improvement in cognition may have caused satisfactory improvement in ADLs for the patient and hence better scores on the CDR rather than on the H-MoCA. Also, the CDR is rated by both the patient and caregiver. Improvement seen from the caregiver’s perspective potentially accounted for the same. This caregiver/patient-rated discrepancy in cognition ratings exists in previous research as well, albeit in an opposite direction (ie, primary caregivers tend to rate patients poorly than patients themselves for cognition).36 In summary, the caregiver’s information and personal bias need to be scrutinized during interviews. Caregiver-reported improvement in the current study may be due to joint families dividing burden and better social engagement; however, this is speculative.
In the current study, better cognition (higher MoCA scores) at baseline and treatment with cholinesterase inhibitors (donepezil 10–20 mg/d or rivastigmine 3–6 mg/d) + vitamin E (800–1,200 mg/d) supplementation predicted reversion at end point. This points us toward the importance of timely intervention. MCI patients should be treated when symptoms are few. Reversion was not linked to DOI, which may seem to undermine the role of early detection. However, this should not be inferred, as most patients presenting to this center had a DOI of 9.6 months, who may have yet been excused by the underlying pathology. Another point of discussion is that putting patients on a combination of cholinesterase inhibitors + cognitive enhancers + vitamin E could not predict reversion, even though this strategy combines more drugs. Since drugs were chosen based on the clinical expertise of senior consultant(s), it may be that nonimprovement during the initial follow-ups led to combination drug treatment in these patients, who then did not revert at 6 months. Drugs may not have a linear relation with improvement in all patients, and other factors like genetic/ neuroimaging/biochemical disease or response markers are at play. Also, more time on treatment may be required for these patients to see if they revert later or progress to dementia. Previous literature employing regression or multivariate analyses mention similar findings. In the study by Sachdev et al,30 less reversion was found in those with moderate-severe cognitive domain impairment and in those in whom multiple cognition domains were involved. The study by Koepsell and Monsell34 similarly found that patients with lower baseline CDR scores (better overall cognition) had more chances of reversion (P < .001). Regarding pharmacologic agents, previous literature holds mixed results on the efficacy of cognitive enhancers in halting the progression of MCI to dementia. According to a previous systematic review by Cooper et al,37 clinical studies found cognitive enhancers to have a modest role in MCI. But this was not consistently found, as shown in an epidemiologic study by Luck et al38 and a meta-analysis by Tricco et al.39 Another systematic review and meta analysis on MCI by Matsunaga et al40 found no significant clinical improvement in cognitive scores using pharmacologic intervention. However, compared to a placebo, there was a positive chance of preventing progression to dementia.40 Yet again, as previously mentioned, the role of longer follow-ups, hidden genetic factors,41 and lack of drugs with specific targets is a cause for stark differences from the current study.
Limitations of the study included small sample, which was biased toward including more farmers and male population. Generalizability was limited by a hospital based design. The placebo effect of intervention could not be studied. Long-term follow-up would better establish maintenance of treatment response or progression of MCI to dementia. The effect of concomitant psychiatric/medical drugs, including over the-counter supplements, if taken was not studied. Psychiatric comorbidities other than the ones studied were not assessed (eg, posttraumatic stress disorder). Due to nonfeasibility, effect of specific genetic, neuroimaging, and biochemical markers was not studied.
CONCLUSION
Short-term outcome for both the modifiable and nonmodifiable risk factor groups was favorable, with depression and hypertension as frequent risk factors. Education positively affected cognition, which itself predicted better quality of life. Reverters of MCI had better baseline cognition and were treated with cholinesterase inhibitors + vitamin E.
Article Information
Published Online: July 18, 2024. https://doi.org/10.4088/PCC.24m03708
© 2024 Physicians Postgraduate Press, Inc.
Submitted: January 13, 2024; accepted March 25, 2024.
To Cite: Moria K, Sharma A, Bansal PD, et al. Mild cognitive impairment: clinical presentation and short-term comparative outcome of patients with potentially modifiable and nonmodifiable risk factors. Prim Care Companion CNS Disord. 2024;26(4):24m03708.
Author Affiliations: Department of Psychiatry, District Hospital, Tarn Taran, India (Moria); Department of Psychiatry, Guru Gobind Singh Medical College and Hospital, Faridkot, India (Sharma, P.D. Bansal, Bahetra, Saini); Department of Psychiatry, Government Medical College and Rajindra Hospital, Patiala, India (P. Bansal, J. Singh, H. Kaur); Department of Psychiatry, Christian Medical College and Hospital, Ludhiana, India (V. Kaur); District Hospital, Tarn Taran, India (G. Singh); Department of Neurology (Medicine), Government Medical College and Rajindra Hospital, Patiala, India (J. Singh, H. Kaur).
Corresponding Author: Arvind Sharma, MD, Department of Psychiatry, Guru Gobind Singh Medical College and Hospital, Faridkot, India, House no. 4, Friends colony, 22 no. Phatak, Behind Lakshmi Palace, Patiala 147001, Punjab, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Previous Presentation: Presented at the International Medical Sciences Academy Conference (IMSACON) held at PGIMS; December 13, 2020; Rohtak, Haryana, India.
Acknowledgments: The authors thank Jaskaran Singh, MD (Department of Surgery, GGSMCH, Faridkot, India), Deepak Meena, MD (Department of Surgery, GGSMCH, Faridkot, India), and Sonam Singh, MD (Department of Surgery, GGSMCH, Faridkot, India) for their help in data collection, compilation, and heuristics. They also thank Baltej Singh, MSc Biostatistics (Department of Community Medicine, GGSMCH, Faridkot, India) for help with statistical analysis. None of the acknowledged individuals have relevant financial relationships to declare.
ORCID: Bhavneesh Saini: https://orcid.org/0000-0002-0565-261X; Priyanka Bansal: https://orcid.org/0000-0002-4659-185X; Jaskaran Singh: https://orcid.org/0009-0006-2465-8730; Harshdeep Kaur: https://orcid.org/0009-0005-1020-153X
Clinical Points
- Outcome of mild cognitive impairment (MCI) is influenced by modifiable and nonmodifiable risk factors.
- Short-term outcome for both the modifiable and nonmodifiable risk factor groups was favorable, emphasizing early intervention.
- The current study supports the positive role of education in preventing cognitive decline.
- Reverters of MCI had better baseline cognition and were treated with cholinesterase inhibitors + vitamin E.
References (41)

- Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–772. PubMed CrossRef
- Sachdev PS, Lipnicki DM, Kochan NA, et al. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10(11):e0142388. PubMed CrossRef
- Khairiah K, Mooi CS, Hamid TA. Prevalence and factors associated with mild cognitive impairment on screening in older Malaysians. Dusunen Adam. 2016;29(4):298–306.
- Kaur J, Sidhu BS, Sibia RS, et al. Prevalence of mild cognitive impairment among hospital patients aged 65 and above. Delhi Psychiatry J. 2014;17(1):60–64.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. APA; 2013.
- Lopez OL. Mild cognitive impairment. Continuum (Minneap Minn). 2013;19(2 Dementia):411–424. PubMed CrossRef
- Feldman H, Scheltens P, Scarpini E, et al. Behavioral symptoms in mild cognitive impairment. Neurology. 2004;62(7):1199–1201. PubMed CrossRef
- Olazaran J, Torrero P, Cruz I, et al. Mild cognitive impairment and dementia in primary care: the value of medical history. Fam Pract. 2011;28(4):385–392. PubMed CrossRef
- Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. PubMed CrossRef
- Gupta M, Gupta V, Nagar Buckshee R, et al. Validity and reliability of Hindi translated version of Montreal Cognitive Assessment in older adults. Asian J Psychiatr. 2019;45:125–128. PubMed CrossRef
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. PubMed CrossRef
- Saxena S, Chandiramani K, Bhargava R. WHOQOL-Hindi: a questionnaire for assessing quality of life in health care settings in India. World Health Organization Quality of Life. Natl Med J India. 1998;11(4):160–165. PubMed
- Kroenke K, Spitzer RL, Williams JBW, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. PubMed CrossRef
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. PubMed CrossRef
- Borson S, Scanlan JM, Watanabe J, et al. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874. PubMed CrossRef
- Saleem SM. Modified Kuppuswamy scale updated for year 2018. Paripex Indian J Res. 2018;7(3):217–218.
- Kumar CT, Shaji KS, Varghese M, editors. Dementia in India 2020. In: Alzheimer’s and Related Disorders Society of India (ARDSI); 2019.Cochin Chapter.
- Hanninen T, Hallikainen M, Tuomainen S, et al. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol Scand. 2002;106(3):148–154. PubMed CrossRef
- Mohan D, Iype T, Varghese S, et al. A cross-sectional study to assess prevalence and factors associated with mild cognitive impairment among older adults in an urban area of Kerala, South India. BMJ Open. 2019;9(3):e025473. PubMed CrossRef
- Samy AL, Kamaruzzaman SB, Krishnaswamy S, et al. Predictors of quality of life among older people with mild cognitive impairment attending urban primary care clinics. Clin Gerontol. 2020;43(4):441–454. PubMed CrossRef
- Wilhalme H, Goukasian N, De Leon F, et al. A comparison of theoretical and statistically derived indices for predicting cognitive decline. Alzheimers Dement (Amst). 2017;6:171–181. PubMed CrossRef
- Assaf G, El Khoury J, Jawhar S, et al. Mild cognitive impairment and modifiable risk factors among Lebanese older adults in primary care. Asian J Psychiatr. 2021;65:102828. PubMed CrossRef
- Fu J, Liu Q, Du Y, et al. Age- and sex-specific prevalence and modifiable risk factors of mild cognitive impairment among older adults in China: a population based observational study. Front Aging Neurosci. 2020;12:578742. PubMed CrossRef
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1):97–137. PubMed CrossRef
- Rolandi E, Zaccaria D, Vaccaro R, et al. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: a population-based study. Alzheimers Res Ther. 2020;12(1):94. PubMed CrossRef
- Locke DE, Ivnik RJ, Cha RH, et al. Age, family history, and memory and future risk for cognitive impairment. J Clin Exp Neuropsychol. 2009;31(1):111–116. PubMed CrossRef
- Lin CH, Lee YY, Liu CC, et al. Urbanization and prevalence of depression in diabetes. Public Health. 2012;126(2):104–111. PubMed CrossRef
- Kuo CY, Lin CH, Lane HY. Molecular basis of late-life depression. Int J Mol Sci. 2021;22(14):7421. PubMed
- Zheng M, Chang B, Tian L, et al. Relationship between inflammatory markers and mild cognitive impairment in Chinese patients with type 2 diabetes: a case-control study. BMC Endocr Disord. 2019;19(1):73. PubMed CrossRef
- Sachdev PS, Lipnicki DM, Crawford J, et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PLoS One. 2013;8(3):e59649. PubMed CrossRef
- Hussenoeder FS, Conrad I, Roehr S, et al. Mild cognitive impairment and quality of life in the oldest old: a closer look. Qual Life Res. 2020;29(6):1675–1683. PubMed CrossRef
- Mank A, Rijnhart JJ, Van Maurik IS, et al. A longitudinal study on quality of life along the spectrum of Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):132. PubMed
- Angevaare MJ, Vonk JM, Bertola L, et al. Predictors of incident mild cognitive impairment and its course in a diverse community-based population. Neurology. 2022;98(1):e15–e26. PubMed CrossRef
- Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–1598. PubMed CrossRef
- Mukku SS, Varghese M, Bharath S, et al. Mild cognitive impairment – a hospital based prospective study. J Geriatr Ment Health. 2019;6(1):19–25.
- Schulz R, Cook TB, Beach SR, et al. Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. Am J Geriatr Psychiatry. 2013;21(1):14–25. PubMed CrossRef
- Cooper C, Sommerlad A, Lyketsos CG, et al. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323–334. PubMed CrossRef
- Luck T, Riedel-Heller SG, Luppa M, et al. A hierarchy of predictors for dementia free survival in old-age: results of the AgeCoDe study. Acta Psychiatr Scand. 2014;129(1):63–72. PubMed CrossRef
- Tricco AC, Soobiah C, Berliner S, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta analysis. CMAJ. 2013;185(16):1393–1401. PubMed CrossRef
- Matsunaga S, Fujishiro H, Takechi H. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 2019;71(2):513–523. PubMed CrossRef
- Campbell NL, Unverzagt F, LaMantia MA, et al. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29(4):873–893. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite



