LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2021;23(5):20f02852
To cite: Petriceks AH, Stern TA. Mild cognitive impairment, dementia, and the evaluation of patients who present with a concern about cognitive decline. Prim Care Companion CNS Disord. 2021;23(5):20f02852.
To share: https://doi.org/10.4088/PCC.20f02852
© Copyright 2021 Physicians Postgraduate Press, Inc.
aHarvard Medical School, Boston, Massachusetts
bDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
*Corresponding author: Aldis H. Petriceks, BA, Harvard Medical School, 25 Shattuck St, Boston, MA 02115 ([email protected]).
Have you ever felt uncertain about when and how to evaluate someone who complains of memory problems or confusion? Have you struggled to understand the various forms of cognitive impairment and dementia? Have you been unsure about whether to recommend memory-enhancing drugs or lifestyle changes for someone with cognitive impairment? If you have, then the following vignette and discussion should prove useful.
CASE VIGNETTE
Mr A, a 75-year-old man with a history of hypertension, hyperlipidemia, and diabetes mellitus, presented to an emergency department with an altered mental status, tachycardia, orthostatic hypotension, and other signs of severe dehydration. History acquired from his wife revealed several days of fever, malaise, and dry cough prior to onset of these symptoms. His wife also said that he had been episodically “forgetting to take his diabetes medications” over the past few months. In the emergency department, Mr A had a serum glucose level of 675 mg/dL and a serum osmolarity level of 340 mOsm/L and was negative for ketoacidosis. He was diagnosed with hyperosmolar hyperglycemic syndrome and was managed with isotonic intravenous (IV) fluids, with additional IV potassium and insulin.
During his hospital stay, while discussing the importance of adhering to his regimen of metformin and glipizide, the internal medicine resident asked Mr A why he has had trouble taking his medications. “I don’t know,” Mr A said, “I use a pillbox and have been placing all my medications there for the past 15 years. I never had any problems before 6 or 7 months ago. At first I’d look and see that a couple pills were missing here and there, just a couple days out of the week. Now I just forget how many pills I’m supposed to use while I’m putting them in every Sunday evening.”
“He’s forgetting other things, too,” his wife added. “He even gets lost while walking around the neighborhood before dinner. We’ve lived here for decades and that had never happened before. But one night he was gone for 2 hours! I had to call the police. They found him sitting on a park bench, looking very frustrated.”
The medical resident was concerned that Mr A may be suffering from dementia. However, the resident also wondered whether this could simply be “normal” cognitive decline. He was unfamiliar with the guidelines for classifying cognitive impairment in older adults, but was intent on clarifying these for himself, for Mr A’s family, and for his care team.
DISCUSSION
What Are the Definitions and Presentations of Mild Cognitive Impairment and Dementia?
Mild cognitive impairment (MCI) and dementia are distinct clinical syndromes, both characterized by a decline of cognitive function.1,2 Such a decline must be greater than the changes normally expected during the ageing process.
As Knopman and Petersen1 noted, there are 5 “domains” of cognition: (1) learning and memory, (2) language, (3) visuospatial function, (4) executive function, and (5) psychomotor abilities. MCI is defined, in part, by the decline in function of 1 or more of these domains. Dementia, on the other hand, is diagnosed by the decline in at least 2 of the domains.3 Diagnosis of either syndrome entails subjective and objective data from the medical history, mental status examination, and relevant neuropsychological testing.1 Each syndrome may arise from a wide range of etiologies, and further workup is indicated following diagnosis.
Clinicians may suspect cognitive impairment in an older patient during a medical visit or hospitalization or the patient (or a loved one) may raise the issue. These patients may have difficulty remembering recent events. They may have trouble finding words or rely heavily on clichés during spontaneous speech. They may struggle to complete their instrumental activities of daily living (IADLs). Patients may describe concerns about forgetting names, faces, and appointments. They may find themselves lost in familiar geographic areas. They might experience unusual changes in mood or cognition. If the patient has limited insight into their condition, he or she might contradict or disagree with such concerns when addressed by caregivers or clinicians.
Once it has been established that the patient is experiencing cognitive impairment, clinicians should distinguish between MCI and dementia. Unlike adults with dementia, those with MCI are able to maintain their daily functioning despite lower-than-normal performance on objective neuropsychological testing.2,4 Patients with deficits in only 1 cognitive domain are said to have “single domain” MCI, which may be classified by the domain affected.2 Prominent decline in memory, for instance, is classified as “amnestic MCI,” while declines in other domains are considered “nonamnestic MCI.”2 Patients experiencing a decline in multiple cognitive domains are said to have “multi-domain MCI,” which may also be classified as amnestic or nonamnestic, depending on the presence or absence of memory involvement.4 For instance, a patient with lower-than-normal performance in language and executive domains may be said to have “nonamnestic multidomain MCI.”
Dementia is a much broader, more complex, clinical syndrome. The diagnosis of dementia requires that a patient demonstrate lower-than-normal performance on objective neuropsychological testing of at least 2 cognitive domains. In addition, this lower performance must represent a decline from previous assessments and correlate with impairment of daily functioning.2 Patients may arrive at their primary care physician’s office concerned by an inability to remember daily appointments or tasks or with a gradual but noticeable recollection of details about recent events. It is typical for loved ones to accompany the patient to the visit or to have scheduled the visit deliberately because they have noticed this decline. For instance, an adult daughter sharing a home with her 80-year-old mother may be concerned that the latter continues to lose her way during short walks around their neighborhood and frequently stops speaking in the middle of sentences because she cannot “find the right words.” Or, an adult son may tell his father’s physician that the latter cannot recall the names of his grandchildren and that he struggles to plan daily tasks, such as doing the laundry, shopping for groceries, and eating meals. In these cases, the patient, loved ones, or both may express significant concern about the patient’s ability to function independently or semi-independently.
What Are the Common Causes of Dementia? What Should Be Considered in the Differential Diagnosis of Dementia?
Once a clinician has determined that a patient meets criteria for dementia, further assessment is needed to determine its specific etiology. While no cure exists for true dementia of any etiology, the varying causes for dementia entail differing natural histories, characteristics, and prognoses that will inform care and planning. Table 1 provides the proportion of total dementia cases for the irreversible dementia etiologies.5–9
Alzheimer’s disease (AD) is the most common etiology for dementia, accounting for approximately 60% to 80% of cases.9 The most common presentation is an initial decline in anterograde episodic memory.10 Patients commonly repeat questions or conversations with the same individuals, forget to pay their bills, and subjectively report short-term memory problems. Depressive symptoms—including hopelessness and a loss of purpose—are also common in the early stages of AD.2,10 These symptoms begin gradually (slow onset) and progress over months to years. As the disease progresses, patients typically experience greater impairment in semantic memory (ie, general knowledge, including the names of colors, the capitals of countries, or the meanings of words) and verbal fluency.10 Distant memories tend to remain more intact than recent memories. Visuospatial deficits may arise in the middle stages, with patients (or their loved ones on behalf of the patients) expressing concern over difficulty dressing, eating, or using household objects.2,10 Paucity of speech is an indicator of progression toward late-stage disease. Comprehension also declines over time. Rigidity, akinesia, and incontinence may develop during the late stages, and changes in personality and behavior often arise.
Vascular dementia, also known as multi-infarct dementia or cerebrovascular disease, is generally considered the second-leading etiology of dementia.5 Vascular dementia encompasses all presentations of dementia for which cerebrovascular pathology or impaired blood flow plays a primary contributing role. These presentations often come in 1 of 2 forms: (1) a patient develops symptoms of dementia after suffering a clinically confirmed stroke or (2) a patient without a confirmed history of stroke develops symptoms of dementia, and evidence of cerebrovascular injury is found on brain imaging.11 Both etiologies—but particularly poststroke dementia—often follow a “stepwise” decline in cognitive function. For instance, a patient might suffer a large ischemic stroke of their left middle cerebral artery, followed by a steep decline in executive function with the appearance of cortical signs (eg, aphasia, ataxia).12 The patient may regain certain aspects of cognitive function during recovery, but rates of cognitive decline tend to increase in poststroke populations, and approximately 1 in 10 patients develops dementia soon after a first stroke.13 Focal neurologic deficits serve as a key distinguishing feature between these patients and those with other forms of dementia.
Dementia with Lewy bodies (DLB) is another etiology to consider. It is distinguished by α-synuclein inclusions in neurons and general brain atrophy.2 Patients with DLB often experience impairment in cognitive processing speed, executive function, alertness, and attention.10 These symptoms begin gradually, progress over months to years, and fluctuate in severity.2 They are also accompanied by parkinsonism, in which patients show signs of bradykinesia, rigidity, stooped posture, and the slow and shuffling gait associated with Parkinson disease.2,10 Patients often report detailed visual hallucinations, such as a rabbit running across the room.
Frontotemporal dementia (FTD) should also be considered in an older patient with cognitive decline. Pathologically, FTD is defined by focal atrophy of the frontal and anterior temporal lobes, alongside histologic evidence of specific protein phosphorylations.2 Several subtypes of FTD exist; they are differentiated by the predominant regions of atrophy and their accompanying signs and symptoms. In the “semantic variant” FTD subtype, atrophy is concentrated primarily in the anterior temporal lobes.10 This often presents as a decline in semantic knowledge, leading to long and seemingly empty instances of speech. Patients may forget the names of common places, objects, or ideas and have a progressive difficulty understanding the meanings of words.14,15 In the more common “behavioral variant,” patients often present with a marked personality change, impairment in social interaction, emotional blunting, and other behavioral disturbances. Apathy, withdrawal, disinhibition, impulsivity, carelessness, and inappropriate behavior may all be present at various stages. Patients with FTD generally experience a slow onset of signs and symptoms that progress over months or years. Memory is intact in the early stages, but executive function (especially in attention and planning) is typically impaired.
Clinicians should also consider medical conditions, such as normal pressure hydrocephalus (NPH). Patients with NPH often present with impaired cognition, ataxia (in which the feet appear “glued to the floor”), and urinary incontinence.10 Executive function is often impaired, and symptoms may closely resemble those of vascular dementia.
Depression should also be considered in older patients presenting with new concerns of cognitive decline. There is notable overlap in the symptoms of depression and dementia in older adults; these include executive dysfunction, emotional changes, and psychomotor impairment. Differentiating between the 2 etiologies is complicated by the question of whether depressive symptoms are responsible for apparent cognitive decline or the dementia is responsible for depressive symptoms. In either case, the 2 conditions are often comorbid: in 1 multicenter study,16 between 22.5% and 54.4% of patients with AD also met criteria for major depressive disorder (MDD). A separate longitudinal study17 indicated that, in at least certain populations of adults older than age 65 years, each additional depressive symptom is associated with a significant increase in risk for AD over 7-year follow-up. This finding was supported by a prospective cohort study that found that depressed patients with memory concerns presenting to general outpatient clinics were significantly more likely (85%) than nondepressed patients with memory concerns (32%) to develop AD over a mean 3-year follow-up period.18 As such, clinicians caring for older patients with depression should be especially cognizant of new or worsening cognitive concerns.
Despite the organization of dementia into discrete etiologies, it should be emphasized that in many cases—perhaps the majority of cases—patients who are diagnosed with dementia suffer from multiple causes of cognitive impairment (Table 1). According to 1 study,8 “mixed etiology dementia” may account for more than half of all dementia cases. The specific clinical manifestations of any given patient with mixed etiology dementia will depend on the particular etiologies involved in their cognitive decline, as well as the relative time course of those etiologies and numerous other factors.
What Are the Potentially Reversible Causes of Cognitive Impairment That Resemble Dementia?
In addition to the numerous (and often comorbid) etiologies of irreversible dementia, there exist potentially reversible conditions whose presentations may closely mimic dementia. Despite their number and wide pathophysiologic range, however, these potentially reversible conditions are as yet of uncertain cumulative prevalence. One highly cited meta-analysis19 estimated that reversible causes were found in approximately 9% of dementia cases in the literature, but only 0.6% of cases were recorded to have reversed. Regardless of the exact prevalence, it is important to assess for these causes and prevent unneeded, prolonged suffering that could otherwise be addressed through medical therapy or cessation of offending agents.
Adverse medication effects comprise 1 major category of etiology for potentially reversible dementias.20 According to Kabasakalian et al,20 the patients at increased risk for medication-related cognitive impairment include those with multiple medical conditions, preexisting brain pathology, decreased renal clearance, previous episodes of adverse drug reactions, polypharmacy, and multiple prescribers. Anticholinergic drugs, antipsychotics, hypnotics, antiepileptics, tricyclic antidepressants, opioids and other sedatives, and amphetamines have all been described as causes of potentially reversible cognitive impairment.20 If a patient presents with concerns for MCI or dementia and is taking 1 or more of these medications—particularly if they have recently begun taking the drug or drugs—the clinician should consider medication as an etiology.
Nutritional deficiencies and other nutritional abnormalities also may contribute to cognitive impairment in older adults. Vitamin B1 (thiamine) deficiency may lead to Wernicke’s encephalopathy, characterized by altered mental status, ataxia, and ophthalmoparesis.20 Wernicke’s encephalopathy is typically associated with alcohol use disorder but also may be precipitated by numerous other metabolic and nutritional derangements, including those caused by dialysis, bariatric surgery, and parenteral nutrition.20 Vitamin B3 (niacin) deficiency may cause pellagra, which is marked by a triad of “diarrhea, dementia, and dermatitis.”20 Vitamin B12 (cobalamin) deficiency also may result in cognitive impairment and, depending on the chronicity of the deficiency, macrocytic anemia and impaired sensation to proprioception and vibration.20
Endocrine and electrolyte disorders constitute another category of etiology for potentially reversible dementias. Hypothyroidism may be associated with cognitive impairment in older adults, though this is controversial.20 Derangements in parathyroid hormone levels also may lead to cognitive impairment, typically through changes in free calcium.20 Similarly, several forms of malignancy may cause hypercalcemia-related psychiatric symptoms and cognitive impairment. Other electrolyte disorders—such as hypernatremia and hyponatremia—also may precipitate reversible cognitive impairment.20 Hepatic encephalopathy is another condition to consider in older patients with underlying liver disease or characteristic signs and symptoms. Patients with chronic kidney disease should be evaluated for potential uremic contributions pre-dialysis, as well as post-dialysis encephalopathy syndrome, depending on the time course from their most recent hemodialysis session.20
Neuropsychiatric causes should also be evaluated. Depression may often manifest similarly to MCI or dementia in older adults.20 In both cases, the patient may demonstrate difficulty performing ADLs and IADLs.21 However, patients with dementia may be less likely to self-report concerns about their own cognition, while patients with depression are more likely to endorse such concerns, while also describing consistently low or apathetic mood.21 In addition, the onset elicited in the history of a depressed patient may be in the range of weeks to months, whereas that of a patient with dementia will likely be months to years.21 Clinicians concerned for depression—which may be comorbid with dementia in older adults—should screen using the Geriatric Depression Scale22 or the 9-item Patient Health Questionnaire (PHQ-9).21,23 Patients who screen positive for depression may be treated pharmacologically, with psychotherapy, or with a combination of the two. Treatment of depression is of high importance in cognitively nonimpaired older adults, as depression itself is a risk factor for development of dementia.20 Other potentially reversible neuropsychiatric diagnoses to consider include delirium, NPH, intracranial bleeding, and trauma.19
In addition to these etiologies, clinicians should evaluate for infectious causes of cognitive impairment when relevant (eg, patient is febrile, recent history of infection). They should also consider various etiologies of anoxia, acute alcohol intoxication and alcohol use disorder, and brain tumors.19
This list of potentially reversible causes is nonexhaustive, but provides a broad overview of diagnostic considerations for clinicians evaluating initial concerns of cognitive impairment or dementia. Given the wide range of potentially reversible causes, clinicians should prioritize those conditions most pertinent to the clinical history, patient demographics, and associated signs and symptoms.
How Does One Evaluate MCI, Dementia, and the Etiologies of Dementia?
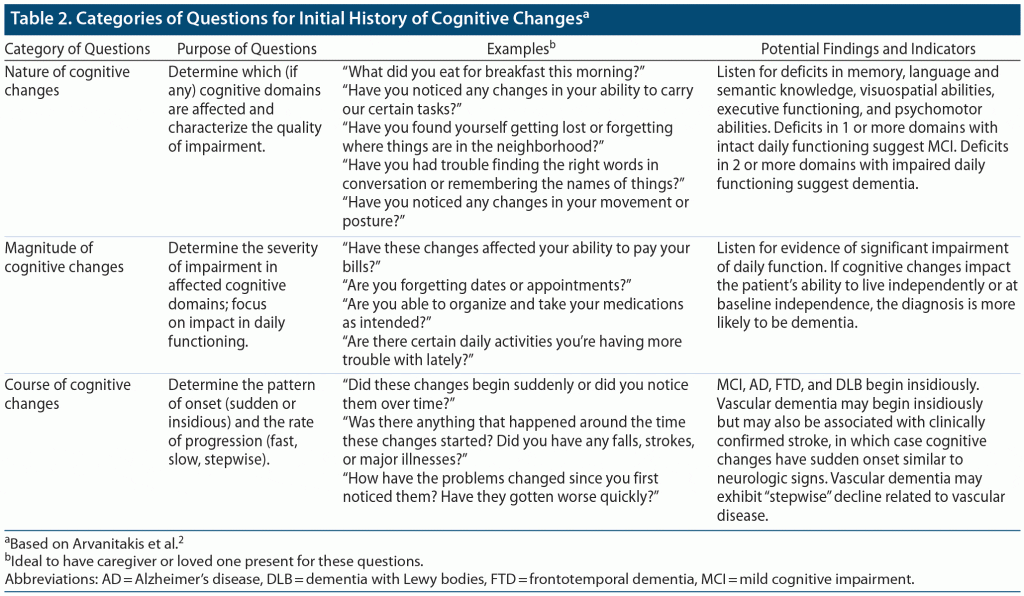
The evaluation of MCI or dementia involves 3 broad components. First, the clinician should perform a thorough medical history, obtaining information from both the patient and 1 or more loved ones or caregivers. The history should focus on cognition and daily functioning, with an emphasis placed on IADLs. Of particular importance is the assessment of the “nature,” “magnitude,” and “course” of the patient’s reported changes in cognition (Table 2).2
The medical history should also include questions about relevant medical conditions, family history, and medications. Risk factors for vascular disease—such as diabetes or hypertension—might predispose patients to vascular dementia.2 A wide range of medications (both prescription and nonprescription) may also contribute to cognitive impairment.24,25 Family histories may reveal strong patterns of inheritance, such as multiple generations of AD or a first-generation relative with early onset AD.
Second, clinicians should perform cognitive and neurologic examinations. Cognitive assessment for MCI or dementia often begins with use of a brief screening tool, such as the Montreal Cognitive Assessment (MoCA).26 The MoCA involves approximately 10 minutes of testing and is scored on a scale of 0–30. A score of 24 or lower suggests the need for further evaluation. If the examination is taking place within a scheduled comprehensive visit, a full mental status examination should also be conducted.27 The Mini-Mental State Examination (MMSE)28 is another test used to assess for cognitive function. A recent systematic review compared the relative accuracies of the MoCA and MMSE in detection of AD and MCI.29 Among the 34 articles included in the review, over 80% found the MoCA to be more accurate than the MMSE in the detection of MCI, while the 2 screening tools were similar in their detection of AD.29 Clinicians of any background are eligible to undergo training and receive certification to administer the MoCA. Certification occurs online through the developers of the MoCA, takes approximately 1 hour, and is valid for 2 years.
In the neurologic examination, focal deficits such as hemiplegia suggest a diagnosis of vascular dementia, particularly if the history reveals an onset that coincides with cognitive decline. Signs of parkinsonism (eg, gait ataxia, cogwheel rigidity) may suggest DLB. Lack of cooperation or presence of behavioral disinhibition may suggest FTD. Patients with AD are unlikely to present with motor or sensory deficits early in the disease course.
Finally, laboratory testing also may be included to assess for medical contributions to cognitive impairment. Patients with B12 deficiency or hypothyroidism may present with cognitive impairment and should be screened for low levels of serum B12, a complete blood count, and serum thyroid-stimulating hormone.30 Neuroimaging also may reveal structural abnormalities associated with specific diagnoses. The American Academy of Neurology recommends either magnetic resonance imaging or noncontrast computed tomography in the initial assessment of patients with dementia.31 Patients with AD may demonstrate cortical atrophy, especially in the hippocampus.32 Evidence of infarction or white matter lesions may indicate vascular dementia.2 Frontal or anterior temporal lobe atrophy suggests FTD. Ventricular enlargement suggests NPH. Further laboratory testing may be performed using positron emission tomography (PET) and cerebrospinal fluid (CSF) analysis.
Who Is at Risk for Cognitive Decline?
The risk factors for dementia include modifiable and nonmodifiable components. Modifiable risk factors include low education level, hearing loss, traumatic brain injury, hypertension, alcohol consumption greater than 21 drinks/week, obesity, smoking, depression, social isolation, physical inactivity, diabetes, and air pollution.13 Approximately 35% of dementia cases may be attributed to some combination of these factors.
Age is the strongest risk factor for dementia, with the incidence of AD doubling every 10 years among adults over the age of 60 years.33–36 Over 80% of adults with dementia are above the age of 75 years, and incidence rates appear to increase beyond 90 years of age.13 In contrast, dementia is rare in younger populations, with 2 recent studies suggesting a cumulative incidence rate of 11–13 cases per 100,000 person-years for those younger than age 65 years.13,37,38
Genetics also contributes to developing dementia. Individuals with a parental history of dementia are approximately twice as likely to suffer from AD or another form of dementia.13,39,40 However, this risk is inversely related to parental age at onset. FTD also exhibits some degree of heritability, and a family history of FTD may justify screening for common mutations associated with the disease.41
Medical conditions in early and middle life may also contribute to later cognitive decline or dementia. Obstructive sleep apnea is associated with an increased risk and earlier age at onset for cognitive decline, MCI, and AD.13,42,43 Chronic kidney disease is modestly associated with cognitive decline and dementia, with albuminuria being the most consistent indicator of risk in older adults.44 Finally, adults with MCI are approximately 3 times more likely to develop dementia in a 2–5 year timespan than their age-matched peers.45 It should be emphasized, however, that MCI is a heterogeneous clinical syndrome, and individual risk for dementia may differ widely among patients with this condition.
In summary, patients at highest risk for cognitive decline and dementia are those of increased age who demonstrate 1 or several of the modifiable risk factors for dementia, such as hypertension, obesity, or social isolation; who have a family history of dementia, particularly AD or FTD; and who have a medical history of obstructive sleep apnea, chronic kidney disease, MCI, or other conditions associated with cognitive decline or dementia. Clinicians should be especially observant in monitoring and screening for symptoms of MCI and dementia in these patients.
Does MCI Always Progress to Dementia? If So, How Frequently?
MCI and dementia—and particularly MCI and AD—exhibit a complex relationship. In some cases, MCI represents a preclinical or prodromal stage of AD, while in other cases MCI does not progress to dementia of any form.46 Nonetheless, older adults with MCI are at considerably higher risk for dementia than are those with normal cognitive function.47 Estimated rates of annual progression from MCI to dementia range from approximately 5%–20%, although 40%–70% of adults with MCI do not develop dementia within 10 years following diagnosis and 15%–20% show improvements in cognitive function within 1–2 years.46
These varying clinical courses complicate the prognosis of MCI. Known risk factors for progression to dementia include lower educational attainment, increased age, diabetes, stroke, and having the amnestic subtype of MCI. Beyond this, it is difficult to predict the likelihood and rate of progression to dementia in any given patient. This uncertainty may cause considerable frustration as patients and caregivers struggle to prepare for the future. For this reason, clinicians caring for those with MCI should conduct regular follow-up assessments of cognition and daily function. Serial MoCA assessments may help identify progressive cognitive decline due to early AD or a stepwise decline due to vascular dementia.
What Medications Can Cause or Exacerbate Cognitive Impairment?
Recent studies have demonstrated an association between use of anticholinergics and risk of cognitive impairment.24,25 Discontinuation of anticholinergic drugs has been associated with decreased risk of such impairment, suggesting that clinicians should consider deprescribing if possible and the necessity of these medications before prescribing.
Anxiolytics (eg, benzodiazepines) and codeine-containing analgesics might contribute to cognitive impairment, although these drugs have not been shown to increase risk for dementia itself.2,48 According to a review,46 digoxin, antihistamines, tricyclic antidepressants, skeletal muscle relaxants, and antiepileptics may also contribute to cognitive impairment, as may chronic hypotension that results from aggressive management of hypertension and hyperglycemia.
What Strategies Exist to Prevent or Decrease the Risk of Developing Dementia?
The prevention and slowing of dementia progression primarily targets modifiable risk factors.49 Clinicians should aim to promote a systolic blood pressure of 130 mm Hg from age 40 onward. Patients should be encouraged to wear hearing aids when necessary and counseled on effective measures for preventing hearing loss through adequate protection and avoidance of excess damaging noise. Whenever possible, patients should be counseled to avoid second-hand smoke and areas of dense smog. They should also be counseled to quit smoking if they are currently smoking cigarettes. Alcohol use should also be kept to a moderate amount or less. Risk for TBI should be addressed by preventing falls in the home and community, and mobility assessments may be useful to determine need for walking supports or home installments. Obesity should be addressed early on, ideally by midlife, through promotion of healthy lifestyle behaviors and, when appropriate, medical or surgical treatment.
Encouragement of physical activity is particularly important and should be emphasized throughout the life course. There is promising evidence that regular physical activity, and exercise in particular, significantly reduces the risk of cognitive decline. In 1 meta-analysis, the authors determined that subjects who performed a “high level of physical activity” showed a 38% lower risk of cognitive impairment during the follow-up period, and even subjects with only low- to moderate-level physical activity experienced a significant decrease in risk for cognitive decline, although the evidence suggests that increased levels of exercise yield greater reductions in risk.50 While the mechanisms of exercise-related cognitive protection are multiple and complex, some degree of protection is likely contributed through the improvement of cardiovascular health, and improvements beginning in middle adulthood may have lasting effects. This hypothesis is supported by recent findings that suggest that of all the forms of exercise studied for the purpose of preventing or slowing the progression of dementia, aerobic exercise demonstrates the strongest evidence of benefit.51 This hypothesis is also supported by findings that indicate that cardiovascular fitness in midlife significantly decreases risk of dementia later in life.52
Finally, clinicians may advocate for wider access to education and learning opportunities in early and middle life, which will serve to decrease the risk of dementia throughout the population.
What Nonmedical Interventions Exist to Manage Dementia Once It Arises?
Nonmedical interventions for dementia are somewhat limited by a current lack of efficacy evidence. However, most of these interventions are of low cost and risk and may thus be safely recommended.
Cognitive stimulation (eg, games, reading, playing or listening to music) has been demonstrated to improve the maintenance of cognition and to slow cognitive decline in adults with dementia.53,54 Forms of psychotherapy focusing on long-term memories and meaningful recollections from early life have also promoted psychological well-being in these patients.55 Aerobic exercise and strength training may support cognitive and physical function in adults with dementia.2,56 Engagement in social activities may provide additional cognitive and psychological benefit.2 Finally, caregiver training and education may help maintain quality of life in spouses and others who care for individuals with dementia, while also benefiting the cognitive function of patients with dementia.57
Are There Any Predictive Tests or Biomarkers for Dementia and MCI?
The respective pathophysiology of AD, DLB, and FTD likely include breakdowns in normal protein folding or degradation.58 AD is typically associated with misfolding of amyloid β (Aβ) and tau proteins, DLB is associated with intracellular aggregations of α-synuclein, and certain forms of FTD are associated with misfolding in the tau or TAR DNA-binding proteins.58 These “proteinopathies” inform much of the current and developing biomarker studies for dementia. In most cases, biomarker assessment is conducted through analysis of CSF.
In AD and MCI, levels of Aβ42 in the CSF are approximately 50% lower than those of (what are most often) age-matched controls, while CSF tau is markedly increased.58 While these changes are not specific and vary widely between individuals, increased CSF tau/Aβ42 ratios may prove useful in the early identification of AD.58 F2-isoproponates (F2-IsoPs) are quantitative indicators of lipid damage secondary to free radicals, and increased levels may be seen in the CSF of patients with early AD and MCI.58 Research has yet to demonstrate consistent CSF biomarkers for FTD, vascular dementia, or DLB.
PET may also aid in the diagnosis of dementia. Patients with AD often demonstrate bilateral decreases in metabolism in the temporoparietal lobes.2 When paired with the tracer fluorodeoxyglucose (FDG), PET yields a high sensitivity and specificity for AD. PET with FDG may also help differentiate AD from FTD, as the latter often shows an asymmetric pattern of decreased metabolism in the frontal lobes (for the behavioral variant) or decreased metabolism in the anterior temporal lobes (for the semantic variant).2
Which Medications Might Be Able to Slow Cognitive Decline or Improve Cognitive Function?
At present, medical management of dementia yields only moderate symptomatic benefit. However, a handful of medication classes with evidence-based and US Food and Drug Adminstration–approved indications for dementia exist.2
The first 3—donepezil, rivastigmine, galantamine—fall under the class of acetylcholinesterase inhibitors.2 These drugs slow the breakdown of acetylcholine at the synaptic cleft, promoting cholinergic neurotransmission. They have been associated with modest improvements in cognitive function and ADLs. They have similar efficacies and side effects (mostly gastrointestinal and sleep related) but differing applications. Donepezil is indicated for all stages of dementia and is covered by most health insurance plans. Rivastigmine and galantamine are indicated for mild to moderate dementia. For each of the 3, target doses are typically reached over a slow titration period of 4–8 weeks.2 Doses may be lowered to manage adverse effects, and clinicians may switch between medications in the same class (eg, from donepezil to galantamine). Well-tolerated medications can be monitored every 6–12 months. Clinicians must typically rely on caregiver reports when determining efficacy. According to a review,2 “A good response to a drug would result in the caregiver noticing a slight improvement in day-to-day life”(p1,596) of the patient, and benefits would not be expected on objective cognitive tests.
Moderate to severe dementia may be managed with memantine, an NMDA (N-methyl-D-aspartate) receptor agonist. Memantine may be taken on its own or in combination with an acetylcholinesterase inhibitor (typically donepezil). Side effects may include headaches or constipation.
What Is the Clinical Course of Advanced Dementia, and What Options Exist When Symptoms Become Severe?
Patients with advanced dementia face tremendous burdens of illness, disability, and mortality. In an 18-month investigation59 of patients with advanced dementia, over half of the 323 participants died before the study reached conclusion. Nearly one-quarter died within 6 months, and over 90% of those who died did so in a nursing home. Over 40% suffered from pneumonia during the study period, over 50% suffered a febrile episode, and over 85% had difficulty eating.59
Distressing symptoms were common during the 18-month study period. Over 45% of patients suffered symptoms of dyspnea, nearly 40% suffered from pain for more than 5 days each month, nearly 40% suffered from pressure ulcers, over 50% experienced agitation, and over 40% experienced aspiration.59 Burdensome interventions were common. Over 30% of patients received parenteral therapy, over 15% underwent hospitalization, nearly 10% were taken to an emergency department, and nearly 10% received tube feeding. Despite these burdensome interventions, the median life expectancy across the study was 1.3 years.59
The study59 also showed that holders of health care proxies who believed that the patients had less than 6 months to live, and who felt that they understood the clinical complications associated with advanced dementia, were less likely to consent to burdensome interventions in the final 3 months of life. As such, clinicians caring for patients with advanced dementia should work with these agents to determine goals of care, ensure that involved parties are clear on their perspectives, and document these perspectives for future reference. Numerous frameworks are available to provide clinicians with structures for their goals-of-care conversations.60 Clinicians should begin these conversations prior to the onset of life-threatening illness, or, in this case, prior to either the diagnosis or progression of dementia.
As dementia progresses, hospice nurses, social workers, volunteers, and other providers may add crucial value and comfort to the end-of-life process. Feeding assistance, cleaning and clothing, medication management, and family respite are only a few examples of meaningful care that can still be provided in the most severe cases of advanced dementia.
What Resources Are Available to Help Caregivers of Those With Cognitive Decline?
Under their “Aging and Health in American Data Briefs,” the Centers for Disease Control and Prevention (CDC) notes that the stresses of caregiving can “affect the caregiver’s life in a myriad of ways including his/her ability to work, engage in social interactions and relationships, and maintain good physical and mental health.”61 In a survey of those caring for individuals with dementia, the CDC found that 14.5% reported 14 or more mentally unhealthy days in the previous month; 17.6% reported 14 or more physically unhealthy days in the previous month; and 36.7% reported having fewer than 7 hours of sleep per night, on average.61 Caregivers were also at increased risk for having multiple chronic diseases.
Given these serious health impacts, it is important to support the people who care for persons with dementia. To that end, the National Institute on Aging (NIA) has organized a list of resources, telephone numbers, and websites provided by organizations focused on dementia care, healthy aging, and AD.62 These include educational resources on evaluation, treatment, caregiving, caregiver needs, and ongoing scientific research; locations of community services for older adults; lists of organizations providing direct home services such as home health care, meal services, adult day care services, and respite services; and websites for mental health resources and hospice organizations. Other articles on the NIA website offer advice on topics such as “Paying for Care” and “Taking Care of Yourself: Tips for Caregivers.” Clinicians caring for patients with AD should familiarize themselves with these resources.
What Happened to Mr A?
After reading about MCI, dementia, and the evaluation of cognitive decline, the medical resident took a thorough cognitive history and performed a bedside cognitive assessment. He found evidence of problems in memory and executive function. He also administered the MoCA, on which Mr A scored 23/30. Neuroimaging conducted during his hospital admission showed no evidence of a prior cerebral infarct or significant ischemic damage or other notable pathology.
Concerned about possible dementia, the resident contacted Mr A’s primary care provider and helped schedule a follow-up visit. He sat down with Mr A and his wife and explained that Mr A’s memory problems might be related to a larger cognitive issue. Both Mr A and his wife were very concerned and immediately asked if he had Alzheimer’s.
“I don’t know,” the resident said, “I’m so sorry for how scary this must be for you, on top of all you just went through. But I want you to know that, whatever might be going on, we will make sure that you are aware of how things are going, and that you are able to live your life in a meaningful way. I know these topics are frightening, but there are many ways we can promote healthy aging, and many ways we can support those who are having some more trouble than they used to.”
As the conversation drew to a close, Mr A and his wife, still visibly nervous, asked where they might find more information about cognitive decline, MCI, dementia, and the resources to support patients and families. The resident provided them with materials from the CDC and NIA and the phone number for the clinic of a geriatric psychiatrist should they wish to speak with a specialist.
Uncertain, but grateful, Mr A was discharged the next day and visited his primary care physician the following week. After extensive neuropsychological testing, laboratory tests, and review of neuroimaging, he was diagnosed with early stage AD. This was devastating news for him and his wife, but with the support of their primary care physician, they began planning for the immediate and long-term future, including home care, greater social involvement, regular cognitive and physical activity, and development of an advance directive. Their children grew more involved in support and caregiving. Saddened and aware of great challenges ahead, the family was nonetheless thankful for the dedication and expertise of their health care providers.
Submitted: October 27, 2020; accepted February 9, 2021.
Published online: September 16, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Clinical Points
- Cognition includes learning and memory, language, visuo-spatial function, executive function, and psychomotor skill; mild cognitive impairment is defined, in part, by the decline in function of 1 or more of these domains, and dementia is diagnosed by the decline in at least 2 of these domains.
- The history of a patient with suspected intellectual decline should focus on cognition and daily functioning, with an emphasis on instrumental activities of daily living and the nature, magnitude, and course of the reported changes in cognition.
- At present, medical management of dementia yields only moderate symptomatic benefit; however, a handful of medications with evidence-based and US Food and Drug Administration–approved indications for dementia exist.
- Caregivers of those with dementia are at increased risk for developing chronic diseases and should be offered support.
References (62)

- Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452–1459. PubMed CrossRef
- Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589–1599. PubMed CrossRef
- Geldmacher DS, Whitehouse PJ. Evaluation of dementia. N Engl J Med. 1996;335(5):330–336. PubMed CrossRef
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. PubMed CrossRef
- Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39(8):1542–1549. PubMed CrossRef
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–566. PubMed CrossRef
- Hogan DB, Jetté N, Fiest KM, et al. The prevalence and incidence of frontotemporal dementia: a systematic review. Can J Neurol Sci. 2016;43(suppl 1):S96–S109. PubMed CrossRef
- Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. PubMed CrossRef
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460. PubMed CrossRef
- Cooper S, Greene JDW. The clinical assessment of the patient with early dementia. J Neurol Neurosurg Psychiatry. 2005;76(suppl 5):v15–v24. PubMed
- Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131(11):1059–1068. PubMed CrossRef
- Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62(6):912–919. PubMed CrossRef
- Larson EB. Risk factors for cognitive decline and dementia. UpToDate website. https://www.uptodate.com/contents/risk-factors-for-cognitive-decline-and-dementia.
- Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329–3345. PubMed CrossRef
- Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386(10004):1672–1682. PubMed CrossRef
- Zubenko GS, Zubenko WN, McPherson S, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry. 2003;160(5):857–866. PubMed CrossRef
- Barnes LL, Wilson RS, Schneider JA, et al. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60(11):1777–1781. PubMed CrossRef
- Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–1293. PubMed CrossRef
- Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163(18):2219–2229. PubMed CrossRef
- Kabasakalian A, Finney GR. Reversible dementias. Int Rev Neurobiol. 2009:283–302.
- Gagliardi JP. Differentiating among depression, delirium, and dementia in elderly patients. Virtual Mentor. 2008;10(6):383–388. PubMed CrossRef
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
- Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. PubMed CrossRef
- Carrière I, Fourrier-Reglat A, Dartigues J-F, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317–1324. PubMed CrossRef
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
- Wilterdink JL. The mental status examination in adults. Accessed June 15, 2021. https://www.uptodate.com/contents/the-mental-status-examination-in-adults?search=DEMENTIA EVALUATION&topicRef=5083&source=see_link#references
- Schatz P. Mini-Mental State Exam. In: Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011:1627–1629., Available from http://link.springer.com/10.1007/978-0-387-79948-3_199.
- Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491–504. PubMed CrossRef
- Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. PubMed CrossRef
- Sabuncu MR, Desikan RS, Sepulcre J, et al; Alzheimer’s Disease Neuroimaging Initiative. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol. 2011;68(8):1040–1048. PubMed CrossRef
- Heiss W-D, Rosenberg GA, Thiel A, et al. Neuroimaging in vascular cognitive impairment: a state-of-the-art review. BMC Med. 2016;14(1):174. PubMed CrossRef
- Corrada MM, Brookmeyer R, Paganini-Hill A, et al. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67(1):114–121. PubMed CrossRef
- Corrada MM, Brookmeyer R, Berlau D, et al. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71(5):337–343. PubMed CrossRef
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, et al. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia. 2017;32(8):523–532. PubMed CrossRef
- Carone M, Asgharian M, Jewell NP. Estimating the lifetime risk of dementia in the Canadian elderly population using cross-sectional cohort survival data. J Am Stat Assoc. 2014;109(505):24–35. PubMed CrossRef
- Mercy L, Hodges JR, Dawson K, et al. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology. 2008;71(19):1496–1499. PubMed CrossRef
- Garre-Olmo J, Genís Batlle D, del Mar Fernández M, et al; Registry of Dementia of Girona Study Group (ReDeGi Study Group). Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology. 2010;75(14):1249–1255. PubMed CrossRef
- Green RC, Cupples LA, Go R, et al; MIRAGE Study Group. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. PubMed CrossRef
- Wolters FJ, van der Lee SJ, Koudstaal PJ, et al. Parental family history of dementia in relation to subclinical brain disease and dementia risk. Neurology. 2017;88(17):1642–1649. PubMed CrossRef
- Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126(Pt 9):2016–2022. PubMed CrossRef
- Osorio RS, Gumb T, Pirraglia E, et al; Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. PubMed CrossRef
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. PubMed CrossRef
- Deckers K, Camerino I, van Boxtel MPJ, et al. Dementia risk in renal dysfunction: a systematic review and meta-analysis of prospective studies. Neurology. 2017;88(2):198–208. PubMed CrossRef
- Peterson RC. Mild cognitive impairment: prognosis and treatment. UpToDate website. Accessed June 15, 2021. https://www.uptodate.com/contents/mild-cognitive-impairment-prognosis-and-treatment?search=DEMENTIA EVALUATION&topicRef=5083&source=see_link#H2
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551–2561. PubMed CrossRef
- Campbell NL, Unverzagt F, LaMantia MA, et al. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29(4):873–893. PubMed CrossRef
- Grossi CM, Richardson K, Fox C, et al. Anticholinergic and benzodiazepine medication use and risk of incident dementia: a UK cohort study. BMC Geriatr. 2019;19(1):276. PubMed CrossRef
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. PubMed CrossRef
- Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. PubMed CrossRef
- Alty J, Farrow M, Lawler K. Exercise and dementia prevention. Pract Neurol. 2020;20(3):234–240. PubMed CrossRef
- Hörder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: a 44-year longitudinal population study in women. Neurology. 2018;90(15):e1298–e1305. PubMed CrossRef
- Sánchez A, Maseda A, Marante-Moar MP, et al. Comparing the effects of multisensory stimulation and individualized music sessions on elderly people with severe dementia: a randomized controlled trial. J Alzheimers Dis. 2016;52(1):303–315. PubMed CrossRef
- Cheng S-T, Chow PK, Song Y-Q, et al. Mental and physical activities delay cognitive decline in older persons with dementia. Am J Geriatr Psychiatry. 2014;22(1):63–74. PubMed CrossRef
- Wang J-J. Group reminiscence therapy for cognitive and affective function of demented elderly in Taiwan. Int J Geriatr Psychiatry. 2007;22(12):1235–1240. PubMed CrossRef
- Pitkälä KH, Pöysti MM, Laakkonen M-L, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173(10):894–901. PubMed CrossRef
- Laakkonen M-L, Kautiainen H, Hölttä E, et al. Effects of self-management groups for people with dementia and their spouses-randomized controlled trial. J Am Geriatr Soc. 2016;64(4):752–760. PubMed CrossRef
- Sonnen JA, Montine KS, Quinn JF, et al. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7(8):704–714. PubMed CrossRef
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. PubMed
- Petriceks AH, Schwartz AWN. Goals of care and COVID-19: A GOOD framework for dealing with uncertainty. Palliat Support Care. 2020;18(4):379–381. PubMed CrossRef
- Centers for Disease Control and Prevention. Caregiving for family and friends — a public health issue. Accessed June 15, 2021. https://www.cdc.gov/aging/caregiving/caregiver-brief.html
- National Institute on Aging. Getting help with Alzheimer’s caregiving. Accessed June 15, 2021. https://www.nia.nih.gov/health/getting-help-alzheimers-caregiving
Please sign in or purchase this PDF for $40.
Save
Cite