Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(3):23f03680
Author affiliations are listed at the end of this article.
Have you ever wondered whether abnormally high vitamin D levels can cause mental status changes as well as somatic symptoms? Have you been uncertain about how to manage hypervitaminosis D? Have you been unsure about how patients do after an acute or chronic overdose of vitamin D? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Mr D, a 28-year-old man, presented to our emergency department (ED) with severe agitation, insomnia, weakness, constipation, and muscle jerks after nonsuicidal overdosing of vitamin D (which he began 4 years earlier to treat “pink eye”). The patient initially began taking 20,000 U of vitamin D, which slowly increased to 50,000 U, then 1,000,000 U, and at points even taking full bottles of over-the-counter (OTC) vitamin D in a single day, although the precise amount he took was variable day to day. He began taking it because he read online that vitamin D could be used to treat fungal infections. A Google search of “fungal infection vitamin D” yielded numerous seemingly reputable sources detailing a connection that likely contributed to his theory.
Mr D believed that he had suppressed his immune system with the vitamin D, which led to his numerous sores, and believed that carbohydrates in his diet “fed the yeast,” which led to his severely restricting his diet over many months until his body mass index reached 15.2 kg/m2. Prior unusual behaviors included injecting a melanin compound into his skin for a tanning effect “to look like Jersey Shore characters.” He also reported taking frequent Epsom salt baths and OTC magnesium after reading online that this might help with his perceived fungal infection. Notably, his magnesium levels were never noted to be elevated.
He described symptoms of obsessive-compulsive disorder (OCD) (ie, repetitive lock checking, compulsive extreme health-related behaviors and monitoring practices, and obsessive ruminations, primarily about health concerns and odd ideas about what causes his physical complaints including a belief that he has a disseminated yeast infection and that he can “sense” his blood magnesium level). On examination in the ED, he was a thin, tan man with pressured speech, grandiosity, flight of ideas, and tangential thoughts who repeatedly jumped up and down and kicked out his legs. However, he was oriented and cognitively intact.
His paternal grandfather had an unspecified kidney disease, and his paternal uncle developed renal failure in his 20s that required hemodialysis. His mother has anorexia nervosa with many psychiatric hospitalizations during his childhood, and his sister has bipolar affective disorder and OCD.
Two weeks prior to the current presentation, he was unable to walk due to profound weakness and had a workup at another hospital. Laboratory values revealed a creatinine level of 3.3 mg/dL (reference range, 0.5–1.5 mg/dL), hemoglobin of 7.8 g/dL (reference range, 12.8–17 g/dL), and ionized calcium of 4.0 mmol/L (reference range, 1.16–1.32 mmol/L). A computed tomography scan of his abdomen and pelvis revealed no evidence of stones or other acute intra-abdominal pathology. Health care providers at the outside hospital warned him that his use of vitamin D supplementation was damaging his kidneys, and he stopped taking it.
While in our ED, he denied bone pain or known fractures, polydipsia, or polyurea but was concerned about a diffuse rash (which he said felt like he had a plastic coating on his skin) that had hundreds of papules of various sizes and stages of healing and superficial erosions (all within his arm’s reach), which he thought was the result of a systemic candidiasis. Mr D also noted that he had intermittent, uncontrolled “jerking” of his legs throughout the day, frothy urine, and anxiety. Although he denied use of alcohol or tobacco, he reported taking marijuana every 2 hours. He described chronic daily heavy use of cannabis since adolescence, which he reported helped alleviate anxiety and provided him with his only sense of pleasure in life, as well as helping him be more productive and motivated at work.
In the ED, he was afebrile, hemodynamically stable but tachycardic, and affectively labile. Laboratory studies were notable for a hemoglobin level of 8.2 g/dL (reference range, 14–18 g/dL), an absolute neutrophil count of 8.0 (reference range, 2,500–6,000), creatinine of 2.95 mg/dL (reference range, 0.7–1.3 mg/dL), total calcium of 14.2 mg/dL (reference range, 8.7–10.2 mg/dL), ionized calcium of 1.84 mmol/L (reference range, 1.16–1.31 mmol/L), parathyroid hormone (PTH) of 9.1 picogram/mL (reference range, 10–55 picogram/ mL), and phosphorus of 5.2 mg/dL (reference range, 2.8–4.5 mg/dL). His urine drug screen was positive for cannabinoids. A chest X-ray was unremarkable, and his electrocardiogram (ECG) showed a normal sinus rhythm with nonspecific ST abnormalities. Mr D’s urinalysis was negative for protein and blood. Mr D was given 1 L of intravenous (IV) normal saline and calcitonin 400 U subcutaneously. He was evaluated by the dermatology department who ultimately felt that his hypo/ hyperpigmented lesions were due to posttraumatic changes after excessive self-excoriation. There was no evidence of a primary infectious, inflammatory, neurogenic, or metabolic cause of his pruritus.
WHAT IS VITAMIN D AND WHY IS IT IMPORTANT FOR OPTIMAL HEALTH?
Vitamin D is an essential prohormone that plays a crucial role in maintaining healthy bones and optimal calcium levels.1 Vitamin D deficiency leads to hypocalcemia and abnormalities in bone mineralization and is associated with an increased risk of cancer, autoimmune diseases, chronic obstructive pulmonary disease, and metabolic syndrome. Vitamin D can be obtained through dietary sources in the form of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol).2 However, most of “the sunshine vitamin”3 is synthesized when 7-dehydrocholesterol is exposed to ultraviolet (UV) B sun rays on the skin.2 Vitamin D3 supplementation (at recommended levels) may offer health benefits that include improvement in symptoms of depression and hypertension and fewer respiratory infections and a lower mortality rate.2
WHAT ARE NORMAL BLOOD LEVELS OF VITAMIN D AND WHAT DAILY DOSE IS RECOMMENDED FOR HEALTHY ADULTS?
The Endocrine Society, the National and International Osteoporosis Foundation, and the American Geriatric Society define vitamin D deficiency as a 25-hydroxy vitamin D (25 OH D) level <30 ng/mL.3 The Endocrine Society recommends a level of 40–60 ng/mL. To maintain this level, the Endocrine Society recommends an intake of 1,500–2,000 international units for all adults.
WHAT CONDITIONS CAN CONTRIBUTE TO EXCESSIVE BLOOD LEVELS OF VITAMIN D?
Individuals who take vitamin D for health problems (eg, osteoporosis and renal disease) may develop hypervitaminosis D due to accidental or intentional overuse.4 Similarly, medications that may cause lower serum calcium levels (eg, thiazide diuretics) may prompt individuals or providers to supplement vitamin D excessively. Alternatively, individuals with granulomatous disease (eg, tuberculosis, sarcoidosis, and fungal infections) may have elevated levels of 1,25-vitamin D, the active metabolite of vitamin D, due to hydroxylation occurring within granulomas.5
WHAT SHOULD THE EVALUATION OF HYPERCALCEMIA ENTAIL (AND WHY)?
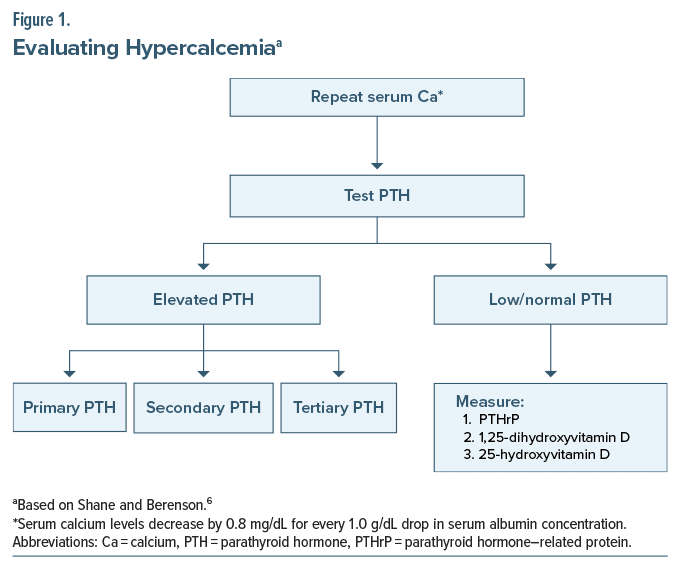
Primary, secondary, or tertiary hyperparathyroidism will appear in a similar fashion, as the signs and symptoms of hypercalcemia are similar regardless of their etiology. Therefore, clinical examination is the next important step in the evaluation, as some symptoms are specific to secondary or tertiary causes (eg, renal disease leads to hypertension, and urticaria is induced when the BUN is elevated) that could point the investigation in the proper direction. If PTH levels are normal and the clinical evaluation is unrevealing, the evaluation of alternative processes that could elevate calcium levels is necessary (Figure 1).6 Screening for malignancies, chronic granulomatous disorders, acromegaly, hyperthyroidism, adrenal insufficiency, and excessive vitamin D use are appropriate next steps.7 It is also important to review the patient’s medication list for OTC agents and herbal supplements, as well as a family history of heritable diseases such as multiple endocrine neoplasia syndromes, familial isolated hyperparathyroidism, or familial hypocalciuric hypercalcemia.8 Paget disease is rarely associated with elevated calcium levels, although it most often presents with a normal calcium level.
HOW QUICKLY SHOULD HYPERCALCEMIA BE CORRECTED (AND WHAT MIGHT HAPPEN WITH TOO SLOW OR TOO RAPID A CORRECTION)?
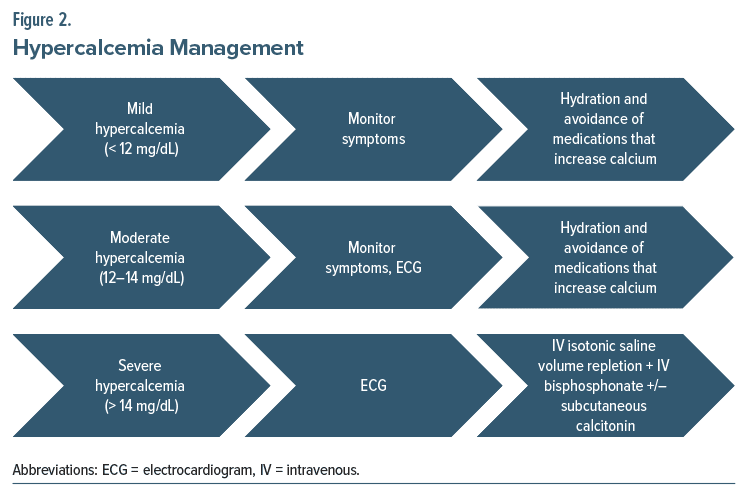
Treatment of hypercalcemia is largely dependent on the severity of the patient’s symptoms, the serum calcium levels, the rate of rise of serum calcium levels, and ECG findings.6 Moreover, serum calcium levels and symptom severity can be used to stratify the level of hypercalcemia into mild (calcium <12 mg/dL), moderate (12–14 mg/dL), and severe (>14 mg/dL) (Figure 2).6 Importantly, comorbid conditions, particularly renal failure and heart failure, can drastically impact the rate of urinary calcium clearance. Therefore, a urinary output goal of 100–150 mL/h is ideal, but it may necessitate the use of loop diuretics in patients with volume overload due to poor renal and/or heart function.9
WHICH CONDITIONS CAN INTERFERE WITH THE ABSORPTION OF VITAMIN D?
Impaired absorption and metabolism of vitamin D can occur in the setting of diseases of the small intestine, pancreas, and hepatobiliary and celiac network.10 Impaired vitamin D absorption and metabolism can also occur in inflammatory bowel diseases because of decreased luminal absorption. Bariatric surgery is another well-known cause of decreased vitamin D absorption, through bypass and disruption of the intestinal epithelium that are responsible for absorbing vitamin D.11 Fat malabsorption in the setting of pancreatic insufficiency impairs the absorption of fat soluble vitamins, including vitamin D, by disrupting chylomicron-facilitated absorption.10 Similarly, biliary dysfunction, either through decreased bile synthesis in the setting of cirrhosis or through impaired bile secretion, can also lead to impaired absorption of fat and fat-soluble vitamins through impaired chylomicron-facilitated absorption.12
WHAT ARE THE SIGNS AND SYMPTOMS OF ACUTE AND CHRONIC INGESTION OF EXCESSIVE AMOUNTS OF VITAMIN D AND WHY DO THEY DEVELOP?
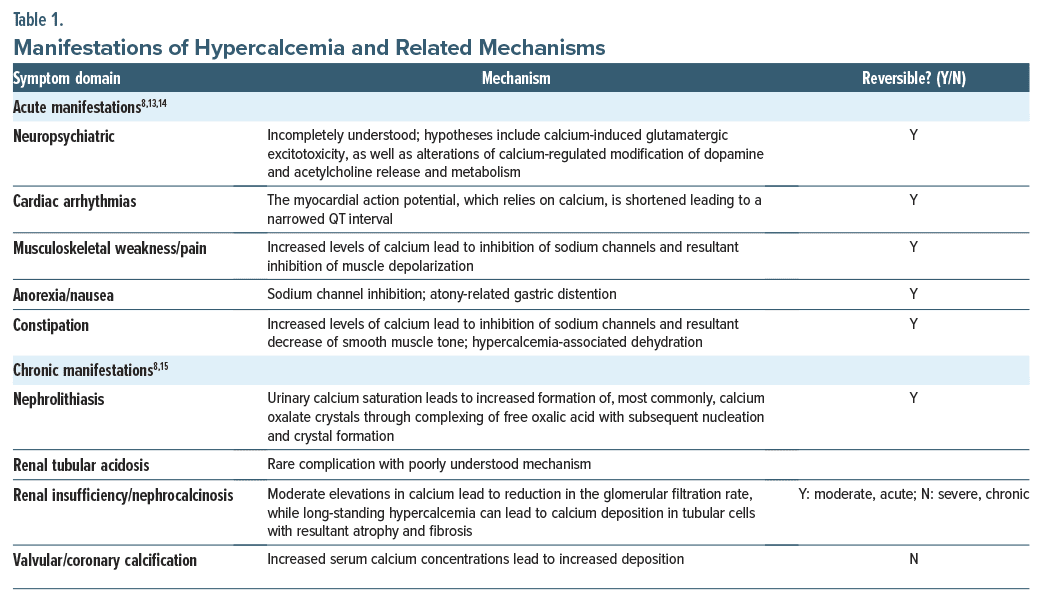
The signs and symptoms of excessive vitamin D levels are related to hypercalcemia.1 Hypercalcemia symptoms involve an altered mental status (eg, with confusion, lethargy, anxiety, and depression).8 Constipation, anorexia, nausea, and musculoskeletal pain may also arise, as can cardiac arrhythmias. Chronic hypercalcemia can lead to nephrolithiasis, renal tubular acidosis, and renal insufficiency. The mechanisms by which these manifestations occur are detailed in Table 1.8,13–15
WHICH SPECIALTIES ARE APT TO SEE PATIENTS WITH HYPERCALCEMIA AND WHY?
Hypercalcemia can have myriad presentations (such as fatigue, polydipsia, polyuria, confusion, lethargy, bone pain, nausea, vomiting, constipation, palpitations, fainting, and cardiac arrhythmias). These problems may bring patients to a primary care provider or an emergency medicine clinician. A constellation of these symptoms often leads clinicians to suspect hypercalcemia (eg, in patients with milk-alkali syndrome, from taking elevated doses of calcium plus vitamin D supplements). Clinicians in various specialties may see patients with a variety of underlying disorders that cause hypercalcemia. Given the common association of hypercalcemia with endocrine disorders (such as hyperparathyroidism and hyperthyroidism), endocrinologists commonly see those with hypercalcemia. Oncologists frequently see patients with hypercalcemia from bone metastases. Rheumatologists and infectious disease specialists see patients with sarcoidosis or bone infections and secondary hypercalcemia. Pediatricians, geriatricians, and physiatrists may see hypercalcemia in bed-bound or severely dehydrated patients. Nephrologists treat patients with chronic kidney disease or kidney stones associated with hypercalcemia.
WHICH CONDITIONS LOOK LIKE HYPERVITAMINOSIS D BUT ARE NOT?
Since hypervitaminosis D presents as hypercalcemia, other causes of hypercalcemia (such as hyperparathyroidism, malignancy [with bone loss/ metastases], sarcoidosis, and excess PTH-related protein) should be considered. A detailed history is also critical, as excessive vitamin D intake is often accidental. Elevated levels of vitamin D will confirm the presence of excess vitamin D.16 Although many physical and environmental factors can impact the body’s exposure to UV radiation and therefore its ability to synthesize previtamin D3, excessive exposure to UV radiation does not lead to hypervitaminosis D because the body regulates the amount of vitamin D produced by sun exposure.
HOW SHOULD HYPERVITAMINOSIS D BE MANAGED?
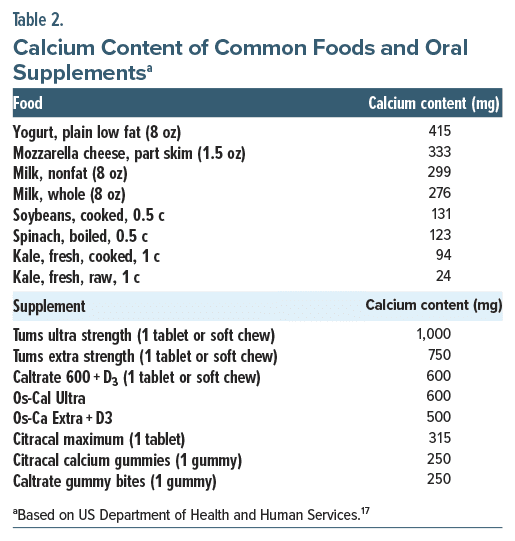
First-line treatments of acute vitamin D toxicity include (1) discontinuation of vitamin D supplementation and reduction of dietary calcium intake (calcium amounts of several common foods and supplements can be found in Table 2)17; (2) administration of isotonic sodium chloride solution (preferred due to hypercalcemia-related urinary salt-wasting and hypovolemia-related exacerbation of hypercalcemia) to correct dehydration and restore kidney function (and eventually the addition of loop diuretics [eg, furosemide, torsemide, and bumetanide], specifically in those with heart failure or renal failure), once volume has been restored; (3) replacement of sodium, potassium, chloride, and phosphate as hypoionic states may occur in the setting of volume repletion and can lead to further altered calcium homeostasis (with hypophosphatemia notably inducing hypercalcemia); (4) glucocorticoid therapy that decreases plasma calcium levels by reducing intestinal calcium absorption by decreasing transcellular active transport processes and increasing excretion of calcium in the urine; and (5) antiresorptive therapy with use of calcium, bisphosphonates, or both in cases when hypercalcemia results from increased osteoclastic bone resorption (due to vitamin D’s direct effect on bone tissue).1,15
ARE THE MENTAL STATUS MANIFESTATIONS OF ELEVATED LEVELS OF VITAMIN D A DIRECT EFFECT OF EXCESSIVE VITAMIN D LEVELS OR A CONSEQUENCE OF DOWNSTREAM SOMATIC SEQUELAE?
Neuropsychiatric symptoms of vitamin D toxicity are like those of other hypercalcemic states and include neuropsychiatric manifestations (eg, difficulty in concentration, confusion, apathy, drowsiness, depression, psychosis, and in more severe cases stupor and coma).1 Although the mechanisms by which these manifestations occur remain unclear, they are more likely to be related to downstream sequelae of an altered vitamin D, and related calcium, state rather than a result of a direct effect of vitamin D. Potential etiologies of neuropsychiatric symptoms of vitamin D toxicity include glutamatergic excitotoxity-related neuronal damage and calcium-related alterations to dopamine and acetylcholine release and metabolism, as well as psychological adjustment to the disease states and related social influences.13 Studies have shown that mean total cerebrospinal fluid (CSF) concentrations of 25-hydroxy vitamin D, a precursor to active 1α, 25- dihydroxy vitamin D used to indicate vitamin metabolic status because of its long half-life and high serum concentration, are generally 1.4-fold higher than serum concentrations. CSF concentrations are noted to show weakly positive, although significant, correlations with serum total, bioavailable, and free 25-hydroxy vitamin D, although total levels in CSF were comparable between those with neurological conditions and healthy controls.18
WHAT IS THE PROGNOSIS OF ACUTE OR CHRONIC HYPERVITAMINOSIS D?
Long-term complications of untreated hypervitaminosis D are related to the duration and severity of hypercalcemia. For example, renal function may recover fully or partially if hypercalcemia is managed promptly. On the other hand, long-standing hypercalcemia that causes vascular or soft tissue calcification is irreversible with cessation of vitamin D and correction of hypercalcemia. A complicating factor in the long-term outcomes is the long half-life of vitamin D (2–3 weeks) which means that hypercalcemia may persist even after vitamin D supplementation has been discontinued.19
DOES CALCIUM INFLUENCE THE EFFECTS AND METABOLISM OF, OR THE PROPENSITY OF DEPENDENCE TO, COMMONLY ABUSED SUBSTANCES?
Studies have established that prolonged exposure to various substances of misuse (including alcohol, opioids, stimulants [eg, methamphetamine and cocaine], and nicotine) leads to altered expression of L-type calcium channels, with associated upregulation of dihydropyridine calcium channel blocker binding sites. L-type calcium channels have been implicated in dependency through several mechanisms, including control of mesolimbic transmission associated with reinforcement and cue salience, as well as excitation of nucleus accumbens spiny neurons involved in reward-driven behaviors and prefrontal cortex neurons related to decision-making. Additional hypotheses recognize the development of dependence through calcium channel mediation of genomic-level expression patterns, as well as long-term potentiation and depression effects on synaptic plasticity.20 With this knowledge in mind, research has demonstrated that administration of calcium channel–blocking agents to opioid-sensitized mice attenuates the symptoms of morphine withdrawal induced through the use of naloxone.21 Interestingly, additional exploration of alcohol use disorder via the use of psychometric assessments and functional magnetic resonance imaging monitoring of alcohol cue-reactivity tasks demonstrated that higher plasma calcium concentrations at admission to detoxification treatment were linked to increased neuronal activity in brain areas involved in inhibition of cravings.22 The relationship between calcium levels and signaling and the effects on substance use disorder and misuse remains an active area of interest.
WHAT HAPPENED TO MR D?
Mr D received IV fluids and 4 doses of calcitonin 400 U (subcutaneously), but his calcium levels only normalized after receiving denosumab (a receptor activator of nuclear factor kappa-B ligand). For his normocytic anemia (with a differential diagnosis that included iron deficiency anemia and chronic kidney disease) and restless leg syndrome, he was started on darbepoetin and IV iron; this was continued as an outpatient. He experienced significant relief of his restless leg symptoms with initiation of intravenous iron treatments. His sinus tachycardia was attributed to anemia, and it improved with red blood cell transfusions. Mr D was started on topical ointments/ creams for skin barrier repair by a dermatology consultant. His creatinine remained around 3 mg/dL throughout his hospitalization.
From a mental health standpoint, Mr D was diagnosed with long-standing OCD and a cannabis use disorder; although a diagnosis of bipolar disorder was considered (due to his pressured speech, grandiosity, flight of ideas, and tangential thoughts), he did not meet criteria for bipolar I or II disorder. His OCD symptoms, especially his health-related ruminations, were felt to be a major contributing factor to his compulsive overuse of vitamin D supplements. Although he described heavy daily use of cannabis at home prior to his hospitalization, he did not manifest cannabis withdrawal symptoms or cravings despite his prolonged hospital stay. At the time of hospital discharge, he was resistant to recommendations to decrease his cannabis use, as he felt it was an essential part of his identity and habits for most of his life. His mental status improved during his medical hospitalization on olanzapine 10 mg every night at bedtime and lorazepam 1 mg 3 times a day as needed and extended-release zolpidem 12.5 mg every night at bedtime (which was eventually switched to 7.5 mg orally every night at bedtime). Although some antipsychotics, including olanzapine, can lower serum calcium levels and increase the risk of osteoporotic fractures in patients with previously normal calcium levels,23 a literature review did not find reports of increased complications (including cardiac or renal risks) from olanzapine in patients with hypercalcemia. Although the use of sedatives, such as lorazepam or zolpidem, in those with hypercalcemia might predispose to an increased risk of falls or confusion when somnolence is present, our agitated patient was not somnolent, and his mental status improved with the use of these agents. His odd beliefs about his health persisted, although he developed some insight into his abnormal behaviors (eg, he stated “I was an idiot for taking that much vitamin D”). He agreed to a referral for outpatient psychotherapy and medication management.
CONCLUSION
Vitamin toxicity is increasingly common given the emerging trend of high-dose OTC supplementation.24 Our case illustrates the dangers that can accompany non–evidence-based use and misuse of seemingly harmless substances, such as vitamin D.
Article Information
Published Online: June 13, 2024. https://doi.org/10.4088/PCC.23f03680
© 2024 Physicians Postgraduate Press, Inc.
Submitted: December 7, 2023; accepted February 29, 2024.
To Cite: Rustad JK, Spitz AZ, Wilson BC, et al. Neuropsychiatric complications of hypervitaminosis D: diagnosis, evaluation, and treatment.
Prim Care Companion CNS Disord. 2024;26(3):23f03680.
Author Affiliations: Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire (Rustad, Felde); White River Junction VA Medical Center, White River Junction, Vermont (Rustad, Felde); Burlington Lakeside VA Community Based Outpatient Clinic, Burlington, Vermont (Rustad); Dartmouth-Hitchcock Medical Center Internal Medicine Residency Program, Lebanon, New Hampshire (Spitz); Dartmouth Hitchcock Medical Center Adult Psychiatry Residency Program, Lebanon, New Hampshire (Wilson, Neu); Harvard Medical School, Boston, Massachusetts (Stern); Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts (Stern).
Corresponding Author: James K. Rustad, MD, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, One Medical Center Dr, Lebanon, NH 03756 ([email protected]).
Relevant Financial Relationships: Drs Rustad and Felde are employed by the United States Department of Veterans Affairs, but the opinions expressed in this article do not reflect those of the Department of Veterans Affairs. Dr Stern has received royalties from Elsevier for editing textbooks on psychiatry. The other authors report no relevant financial relationships.
Funding/Support: None.
Clinical Points
- Neuropsychiatric symptoms of vitamin D toxicity are like those of other hypercalcemic states and include neuropsychiatric manifestations (eg, difficulty in concentration, confusion, apathy, drowsiness, depression, psychosis, and in more severe cases stupor and coma).
- Long-term complications of untreated hypervitaminosis D include kidney stones, kidney damage, kidney failure, excess bone loss, calcification of arteries and soft tissues, and abnormal heart rhythms.
- Vitamin toxicity occurs more often given the emerging trend of high-dose over-the-counter supplementation; grave danger can accompany non–evidence-based use and misuse of seemingly harmless substances, such as vitamin D.
References (24)

- Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, et al. Vitamin D toxicity-a clinical perspective. Front Endocrinol (Lausanne). 2018;9:550. PubMed
- Nguyen T, Joe D, Shah AD. Forget the phosphorus: a case of hypervitaminosis D induced symptomatic hypercalcemia. Clin Nephrol Case Stud. 2021;9:1–3. PubMed
- Chauhan K, Shahrokhi M, Huecker MR. Vitamin D. In: StatPearls [Internet]. StatPearls Publishing;2023. April 9, 2023. https://www.ncbi.nlm.nih.gov/books/NBK441912/
- Pietrangelo A. “What’s to know about Hypervitaminosis D?”. Accessed November 23, 2023. https://www.medicalnewstoday.com/article/318415
- Wakeman M. A literature review of the potential impact of medication on vitamin D status. Risk Manag Healthc Policy. 2021;14:3357–3381. PubMed CrossRef
- Shane E, Berenson JR. Treatment of Hypercalcemia. UpToDate; 2023. Accessed April 5, 2024. https://www.uptodate.com/contents/treatment-of-hypercalcemia?search=hypercalcemia+management&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H1248513
- Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(suppl 2):S51–S59. PubMed CrossRef
- Walker MD, Shane E. Hypercalcemia: a review. JAMA. 2022;328(16):1624–1636. PubMed CrossRef
- Tonon CR, Silva TAAL, Pereira FWL, et al. A review of current clinical concepts in the pathophysiology, etiology, diagnosis, and management of hypercalcemia. Med Sci Monit. 2022;28:e935821. PubMed CrossRef
- Vitamin D Fact sheet for health professionals. Accessed November 23, 2023. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Peterson LA, Cheskin LJ, Furtado M, et al. Malnutrition in bariatric surgery candidates: multiple micronutrient deficiencies prior to surgery. Obes Surg. 2016;26(4):833–838. PubMed CrossRef
- Farooqui N, Elhence A, Shalimar. A current understanding of bile acids in chronic liver disease. J Clin Exp Hepatol. 2022;12(1):155–173. PubMed
- Calcaterra V, Pelizzo G, Pipolo A, et al. Hypercalcemia and neurological symptoms: a rare presentation of hyperfunctioning parathyroid adenoma in an adolescent. Front Surg. 2022;9:885188. PubMed CrossRef
- Chorin E, Rosso R, Viskin S. Electrocardiographic manifestations of calcium abnormalities. Ann Noninvasive Electrocardiol. 2016;21(1):7–9. PubMed
- Khoury N, Carmichael KA. Evaluation and therapy of hypercalcemia. Mo Med. 2011;108(2):99–103. PubMed
- Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99(4):1132–1141. PubMed CrossRef
- Calcium Factsheet for Health Professionals. US Department of Health and Human Services. Accessed December 3, 2023. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Lee DH, Kim JH, Jung MH, et al. Total 25-hydroxy vitamin D level in cerebrospinal fluid correlates with serum total, bioavailable, and free 25-hydroxy vitamin D levels in Korean population. PLoS One. 2019;14(3):e0213389. PubMed CrossRef
- Ramasamy I. Vitamin D metabolism and guidelines for vitamin D supplementation. Clin Biochem Rev. 2020;41(3):103–126. PubMed CrossRef
- Little HJ. L-type calcium channel blockers: a potential novel therapeutic approach to drug dependence. Pharmacol Rev. 2021;73(4):127–154. PubMed CrossRef
- Seth V, Upadhyaya P, Moghe V, et al. Role of calcium in morphine dependence and naloxone-precipitated withdrawal in mice. J Exp Pharmacol. 2011;3:7–12. PubMed CrossRef
- Bach P, Schuster R, Koopmann A, et al. Plasma calcium concentration during detoxification predicts neural cue-reactivity and craving during early abstinence in alcohol-dependent patients. Eur Arch Psychiatry Clin Neurosci. 2022;272(2):341–348. PubMed CrossRef
- Starki S, Loebis B, Husada M, et al. Calcium levels differences in men with schizophrenia treated with olanzapine and risperidone in Prof. Dr. M Ildrem Psychiatric Hospital Medan. Open Access Maced J Med Sci. 2021;9(T3):274–279.
- Mason N, Sweet LM, Wills Z, et al. Vitamin D abuse in pursuit of “gains” resulting in acute kidney injury. Mil Med. 2024;189(1–2):e417–e419. PubMed
Please sign in or purchase this PDF for $40.
Save
Cite