ABSTRACT
Introduction: Aripiprazole once-monthly 400 mg (extended-release injectable suspension) is effective in long-term maintenance treatment of schizophrenia based on clinical studies. As study results may not reflect clinical practice, we compared treatment persistence with aripiprazole once-monthly 400 mg versus daily oral atypical antipsychotics in a naturalistic setting.
Methods: This was an observational, noninterventional study of patients (aged 18–35 years) with recent-onset schizophrenia (< 5 years post diagnosis) who initiated maintenance treatment with aripiprazole once-monthly 400 mg or any daily oral atypical antipsychotic during a schizophrenia-related hospitalization or within 3 months post hospital discharge (timeframe: July 13, 2017–July 31, 2019). Data were from patient files/obtained during routine visits. Patients were followed for ≤ 12 months or until all-cause treatment discontinuation (including lost to follow-up), whichever came first. Data were analyzed using a sample constructed with inverse probability of treatment weighting (IPTW).
Results: Among 357 patients (aripiprazole once-monthly 400 mg: 215, oral atypical antipsychotics: 142), all-cause treatment discontinuation occurred in 87 (41%) of aripiprazole once-monthly 400 mg, 68 (48%) of oral atypical antipsychotic patients over 52 weeks. In the IPTW sample, time to all-cause discontinuation was significantly different between both groups in favor of aripiprazole once-monthly 400 mg (hazard ratio = 1.46; 95% CI, 1.05–2.03; P = .023). Generalizability of results to the overall population with schizophrenia was limited due to incomplete overlap of patient characteristics between cohorts. The primary reason for treatment discontinuation in both groups was voluntary discontinuation by subject (aripiprazole once-monthly 400 mg: 11%; oral atypical antipsychotics: 8%).
Conclusions: In a naturalistic setting, younger patients with recent-onset schizophrenia treated with aripiprazole once-monthly 400 mg after hospitalization tended to discontinue treatment later than patients treated with daily oral atypical antipsychotics.
Trial Registration: ClinicalTrials.gov identifier: NCT03130465
Prim Care Companion CNS Disord 2021;23(5):20m02886
To cite: Such P, Bøg M, Kabra MS, et al. A noninterventional cohort study assessing time to all-cause treatment discontinuation after initiation of aripiprazole once monthly or daily oral atypical antipsychotic treatment in patients with recent-onset schizophrenia. Prim Care Companion CNS Disord. 2021;23(5):20m02886.
To share: https://doi.org/10.4088/PCC.20m02886
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Medical Affairs Psychiatry, H. Lundbeck A/S, Valby, Denmark
bDepartment of Value Evidence, H. Lundbeck A/S, Valby, Denmark
cOtsuka Pharmaceutical Europe Ltd, Wexham, United Kingdom
*Corresponding author: Anne C. de Jong-Laird, MD, Otsuka Pharmaceutical Europe Ltd, Gallions – 1st Floor, Wexham Springs, Framewood Rd, Wexham SL3 6PJ, United Kingdom ([email protected]).
Schizophrenia is a chronic mental illness characterized by positive symptoms such as delusions, conceptual disorganization, hallucinations, grandiosity, and paranoia; negative symptoms such as blunted affect, emotional withdrawal, apathy, and social deficits (including communicative skills); and general psychopathology in the forms of somatization, anxiety and depression, and cognitive and motor issues.1 The course of schizophrenia typically includes repeated relapses resulting in cumulative damage and diminution of functioning,2,3 including academic, social, and occupational functioning.4,5 The first 3 years after the first schizophrenic episode are considered to be a critical period during which much of this damage occurs.3,6 Early symptom control to prevent relapse is thus essential for avoiding/slowing this deterioration and preserving health-related quality of life (HRQOL).7
Predictors of relapse include younger age, onset of illness at earlier age, more severe symptoms, higher prevalence of substance use disorder, worse functional status (particularly early in the course of disease), and poor adherence.8–10 With regard to the latter predictor, rates of nonadherence are estimated to be as high as 75%, and it is estimated that patients with schizophrenia take only 51%–70% of their prescribed medication.11,12
The impact of adherence and the related marker of medication persistence—measured as time from initiation to discontinuation of treatment—on treatment outcomes is profound. In addition to relapse, poor adherence has been associated with increased risk of hospitalization, use of emergency psychiatric services, violence, arrests, diminished functioning, reduced HRQOL, and increased risk of substance abuse.12–14 Nonadherence in the first year of treatment has been linked with poor outcomes in the following 2 years,13 and treatment discontinuation after the first episode of psychosis has been shown to increase the risk of relapse by ~5 fold,15 underscoring the importance of early intervention to address issues related to adherence and persistence.
Long-acting injectable (LAI) formulations of antipsychotics (APs) are valuable treatment alternatives to oral agents, with the potential to improve adherence and persistence and reduce the risk of relapse by offering continuous medication delivery and transforming a once-daily decision to take medication into regularly scheduled, longer-interval occurrences under a physician’s care. LAIs offer consistent pharmacokinetics, and fewer peak-to-trough fluctuations have been shown to improve tolerability.16 In addition, studies have shown that patients’ attitudes toward treatment can improve if they do not have to take drugs on a daily basis17 and that monthly administration reduces monitoring requirements and allows treatment teams to focus on the HRQOL of patients and their personal goals, thus strengthening the therapeutic alliance.15
LAI APs have been shown to increase medication adherence and persistence relative to oral APs,18 as well as to reduce the risks of relapse and hospitalization, with the latter benefits likely attributable to improved adherence.11,19–21 In addition, there is a potential for cost reductions realized through reductions in hospitalizations and other health resource utilization.8,22
Aripiprazole once-monthly 400 mg (extended-release injectable suspension) is an atypical AP that may further improve adherence and persistence and provide additional protection against relapse.23–25 Traditional first- and second-generation (atypical) APs are full antagonists at D2 receptors, which makes them effective for treating positive symptoms. However, full D2-receptor antagonists generally have been reported to be less effective against negative symptoms (eg, social withdrawal and cognitive dysfunction) thought to contribute to functional impairments and nonadherence in patients with schizophrenia.26–28 By contrast, aripiprazole once-monthly 400 mg is a partial D2 receptor agonist,25 which offers the potential to yield greater aggregate (positive plus negative) symptom improvements and, consequently, greater improvements in functional outcome measures.
This potential has been borne out in randomized controlled trials (RCTs) wherein aripiprazole once-monthly 400 mg has been shown to be effective in long-term maintenance treatment of schizophrenia, demonstrating superiority to placebo and noninferiority to oral aripiprazole with respect to relapse prevention, with a favorable tolerability profile.29–31
Subsequently, in the 28-week Quality of Life with Abilify Maintena (QUALIFY) RCT,32 aripiprazole once-monthly 400 mg was associated with statistically significantly greater improvements in measures of HRQOL and functional status relative to paliperidone palmitate once monthly, eg, the Quality of Life Scale (QLS; primary endpoint), the Work Readiness Questionnaire,33,34 and the Personal and Social Performance Scale.35 Aripiprazole once-monthly 400 mg was also associated with less sexual dysfunction, lower prolactin levels, and less weight gain versus paliperidone palmitate once monthly.32,36 These findings are particularly significant because sexual dysfunction and weight gain can contribute to treatment dissatisfaction and discontinuation.37
RCTs have, however, yielded mixed results regarding potential advantages of LAIs compared with oral agents, which has been attributed to design elements that may inflate adherence and thereby improve outcomes in patients assigned to oral therapy.38 In addition, data suggesting improved functioning and tolerability in a research setting require confirmation in actual clinical practice. It should be noted that RCTs also tend to include a more homogenous population of patients due to exclusion of specific characteristics (eg, comorbidities, risk factors, concomitant medications),39 and, therefore, it is desirable to confirm evidence of beneficial effects in a more diverse patient population.
The present noninterventional study was designed to compare time to discontinuation and clinical outcomes of aripiprazole once-monthly 400 mg versus oral atypical APs in patients with recent-onset schizophrenia in a naturalistic setting (ie, in actual clinical practice), free from study design elements that might influence persistence with treatment.
METHODS
Study Design
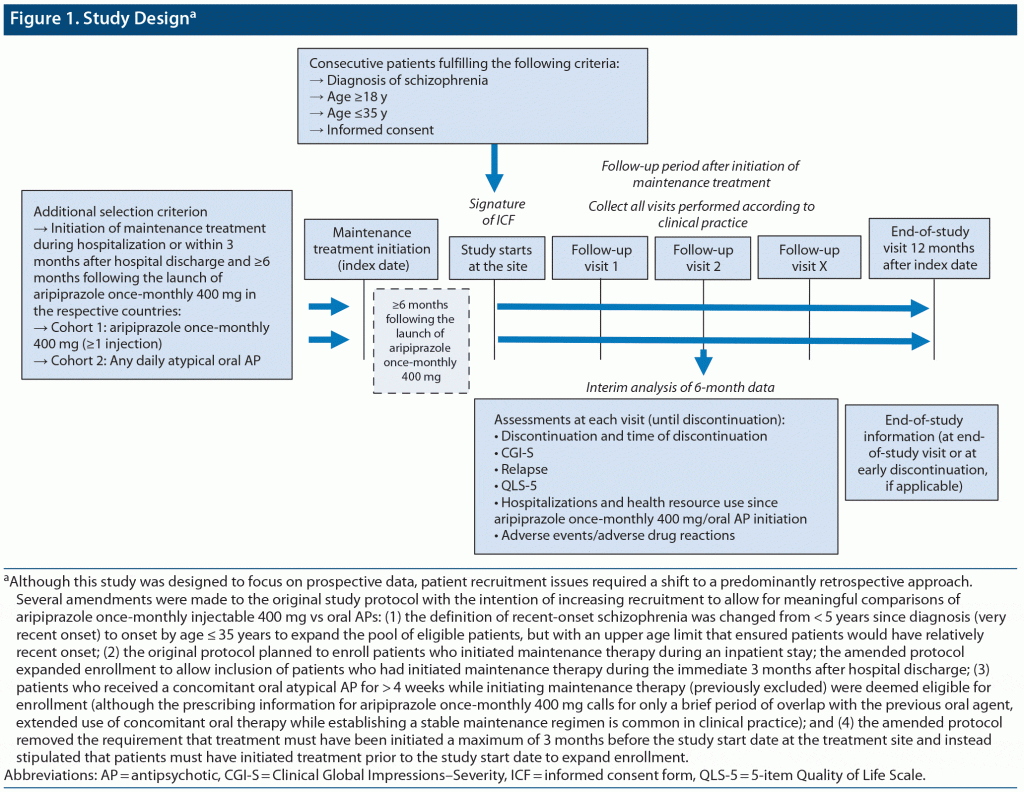
This was an observational noninterventional study comparing 2 cohorts of adult patients with recent-onset schizophrenia who initiated maintenance treatment with aripiprazole once-monthly 400 mg (cohort 1) or a daily oral atypical AP (cohort 2) in association with a schizophrenia-related hospitalization (during the hospitalization or the first 3 months after this hospitalization) (Figure 1). The study was conducted during the period from July 13, 2017, to July 31, 2019. Due to difficulties with patient recruitment, several amendments (detailed below) were made to the original study protocol with the intention of increasing recruitment to allow for meaningful comparisons of aripiprazole once-monthly 400 mg versus oral atypical APs.
Patients were enrolled at 51 sites in Italy, France, and Spain and were required to be aged 18–35 years, with recent onset of schizophrenia (the latter definition was changed from < 5 years since diagnosis [very recent onset] in the original protocol to onset by age ≤ 35 years to expand the pool of eligible patients, but with an upper age limit that ensured patients would have relatively recent onset). Diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria was required to be confirmed by the current investigator.
Patients were required to have initiated treatment before the study start at the site and ≥ 6 months following the launch of aripiprazole once-monthly 400 mg in their respective countries, with it having been prescribed in the usual manner in accordance with the terms of the marketing authorization. (The original protocol required that a patient’s treatment was initiated a maximum of 3 months before the study start date at the treatment site; the revised criteria expanded potential enrollment [by removing the limit on duration of prior therapy]). The 6-month post-launch stipulation (which was unchanged from the original protocol) was intended to mitigate possible period effects that may have occurred in the first patients treated with a new drug in a given region.
The date of initiation of maintenance therapy was considered to be the index date. For aripiprazole once-monthly 400 mg, this was the date of the first injection, whereas for oral atypical APs, it was defined by the physician as being when patients were believed to be sufficiently stable to be considered “in maintenance treatment.” The index date for each patient was required to be prior to the date the treatment site obtained the necessary approvals for participation in the study. Once approval was obtained, site staff preidentified patients for invitation to participate. The visit at which the patient was invited to participate and signed an informed consent form was the inclusion visit. The prescription of any medicinal product was clearly separated from the decision to include the patient in this noninterventional study.
Patients who received concomitant oral atypical APs for > 4 weeks while initiating maintenance therapy—who had been excluded by the original protocol—were deemed eligible under the amended protocol (although the prescribing information for aripiprazole once-monthly 400 mg calls for only a brief period of overlap with oral aripiprazole,40 extended use of concomitant oral therapy while establishing a stable maintenance regimen is common in clinical practice).
Patients treated in a daycare hospital were considered eligible for study inclusion if they were admitted to the hospital (full-time inpatient stay) for an acute psychotic episode and then discharged to a daycare hospital to achieve stabilization and initiate maintenance AP treatment. Patients treated in a daycare hospital were considered ineligible if they were treated exclusively in the daycare hospital since the first day (ie, no overnight stays), as they were likely to be subacutely psychotic.
Investigators for the current analysis were not involved in the treatment of patients at any participating site (ie, they simply reviewed and analyzed retrospective data from patient records). Treatment assignment to aripiprazole once-monthly 400 mg or an oral atypical AP was not decided in advance by a protocol, but rather fell within normal clinical practice at each treatment site. Patients initiated and continued treatment with aripiprazole once-monthly 400 mg or a daily oral atypical AP according to the normal clinical practice of the treating physician at each site.
Patients were followed for a maximum of 12 months or until all-cause treatment discontinuation (including loss to follow-up), whichever came first. Investigators collected data from patient files and during routine visits scheduled according to clinical practice at the site.
This study was performed in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, and all applicable legislation governing noninterventional studies. Appropriate informed consent was obtained from all participating patients. The study protocol and any amendments, informed consent forms, and any advertising used to recruit patients were approved by the appropriate institutional review board or independent ethics committee. The trial is registered in Clinical.Trials.gov (identifier: NCT03130465).
Outcome Variables
The primary outcome variable was time to all-cause treatment discontinuation for aripiprazole once-monthly 400 mg or oral atypical APs. Discontinuation was defined as interruption of treatment, or the replacement or addition of a new AP to aripiprazole once-monthly 400 mg or the oral AP, or loss to follow-up. Secondary clinical outcome variables relating to relapse, Clinical Global Impressions–Severity (CGI-S) scores, 5-item QLS (QLS-5) scores, and hospitalizations were planned but could not be captured for most patients (data not shown; complete list of secondary variables provided in the Supplementary Material). Exploratory outcome variables included patient preference regarding AP formulation and satisfaction with current AP treatment.
Safety
Safety outcomes included adverse events and adverse drug reactions.
Schedules for Data Collection
Inclusion visit and retrospective data collection. Although this study was designed to focus on prospective data, patient recruitment difficulties necessitated a protocol amendment, with a shift to a predominantly retrospective approach. The following data were scheduled to be obtained at the inclusion visit: CGI-S and QLS-5 scores by the clinician at the inclusion visit (if the patient was included while still in follow-up) and patient preference and satisfaction with their current AP formulation (if the patient was included while still in follow-up).
The following retrospective data were collected from patient files (all information available from the index date through the entire follow-up period and for the previous 5 years, when possible): demographic characteristics; clinical and treatment characteristics; CGI-S score; QLS-5 score; aripiprazole once-monthly 400 mg/oral atypical AP treatment details (eg, onset date, dosage, and injection dates), details about other schizophrenia therapies received at the index date, and changes in therapy up to the inclusion visit; relapse assessment; hospitalizations and health resource use related to schizophrenia; adverse events and adverse drug reactions; date of AP therapy discontinuation and reasons for discontinuation, if applicable; and patient flow between index date and inclusion visit.
Follow-up visits. The following information was collected at follow-up visits according to the clinical practice of the center: CGI-S and QLS-5 questionnaires by the clinician at each visit; changes in demographic characteristics, if applicable; changes in clinical and treatment characteristics, if applicable; changes in aripiprazole once-monthly 400 mg/oral atypical AP treatment details (eg, injection dates for aripiprazole once-monthly 400 mg, and pack-size/treatment dates and other details for oral atypical APs) and other schizophrenia therapies; relapse assessment; hospitalizations and health resource use related to schizophrenia between the previous and current visit; patient preferences and satisfaction with their assigned AP treatment formulation (assessed ~6 months after initiation of maintenance treatment); adverse events and adverse drug reactions; and end-of-study or early discontinuation information (including date of discontinuation and reasons for early discontinuation, if applicable).
Sample Size Calculation
Sample size calculations were based on the following considerations: in QUALIFY, 26.2% of young patients treated with aripiprazole once monthly had discontinued treatment at week 28,41 and in the Schizophrenia Trial of Aripiprazole,42 38.7% of the oral group had discontinued at week 26. Under these assumptions, a hazard ratio of 1.57 of discontinuation with oral atypical APs versus aripiprazole once monthly was estimated. Assuming a 1:1 recruitment ratio for the present study, an overall event rate of 32.1% was estimated. Using a multivariate Cox regression model with a 5% significance level (2-sided Wald test), and assuming a correlation of 0.2 between treatment cohort and other covariates, a sample size of 604 patients (302 in each group) was estimated to be needed to achieve 80% power.
Statistical Methods
Based on prior clinical experience,43 it was believed that the decision to prescribe aripiprazole once-monthly 400 mg or an oral atypical AP would have been influenced by patient characteristics and, therefore, the aripiprazole once-monthly 400 mg and oral AP cohorts could be expected to differ in clinically meaningful ways. An unadjusted comparison would thus not be able to provide unbiased evidence for the outcomes of interest, because such analyses would be subject to confounding by disease severity. To address this issue, an analysis was used to minimize potential confounding and allow the observational data collected at study sites to mimic some of the characteristics of data from RCTs that compare treatments in balanced groups.
The analysis of the primary objective (time to all-cause treatment discontinuation) was performed using a weighted sample constructed with inverse probability of treatment weighting (IPTW) methodology and analyzed in a Cox regression framework (key variables are denoted in Table 1). A propensity score was first estimated for each patient using logistic regression. The dependent variable was observed treatment (aripiprazole once monthly or oral atypical AP); independent variables comprised a prespecified list of baseline variables that were hypothesized to be associated with treatment assignment. Clinically important variables for potential inclusion in the model were first identified in the literature and through expert clinical advice. Variables considered for inclusion in the model were sex, time from schizophrenia diagnosis, number of previous APs within the 2 years prior to the index date, number of previous schizophrenia relapses within the 2 years prior to the index date, age (at time of maintenance treatment initiation), alcohol and drug abuse or dependence (at time of maintenance treatment initiation), living situation and family support (at time of maintenance treatment initiation), prior nonadherence (if “improve adherence to treatment” was checked as reason to initiate treatment at index date), recent (3 months prior to index date) depot AP use, and country. The final model was used to predict the probability of treatment assignment for each patient using their clinical baseline characteristics.
In the second step, a univariate Cox regression model was fitted to the weighted sample, using time to all-cause treatment discontinuation as the dependent variable and treatment cohort indicator (aripiprazole once-monthly 400 mg or daily oral atypical AP) as a covariate. Each patient was assigned a weight equal to the inverse of the predicted propensity, creating a pseudopopulation. In this population, the association between the confounding variables and treatment assignment was no longer present. Hazard ratios with 95% confidence intervals were calculated. Sensitivity analyses were conducted using an extended set of demographic and clinical variables and different sample weightings. All testing used 2-sided tests, with the criteria set at α = .05. Missing data for covariates were not imputed. Data for secondary variables were summarized using descriptive statistics due to low patient numbers.
RESULTS
Patient Disposition and Baseline Characteristics
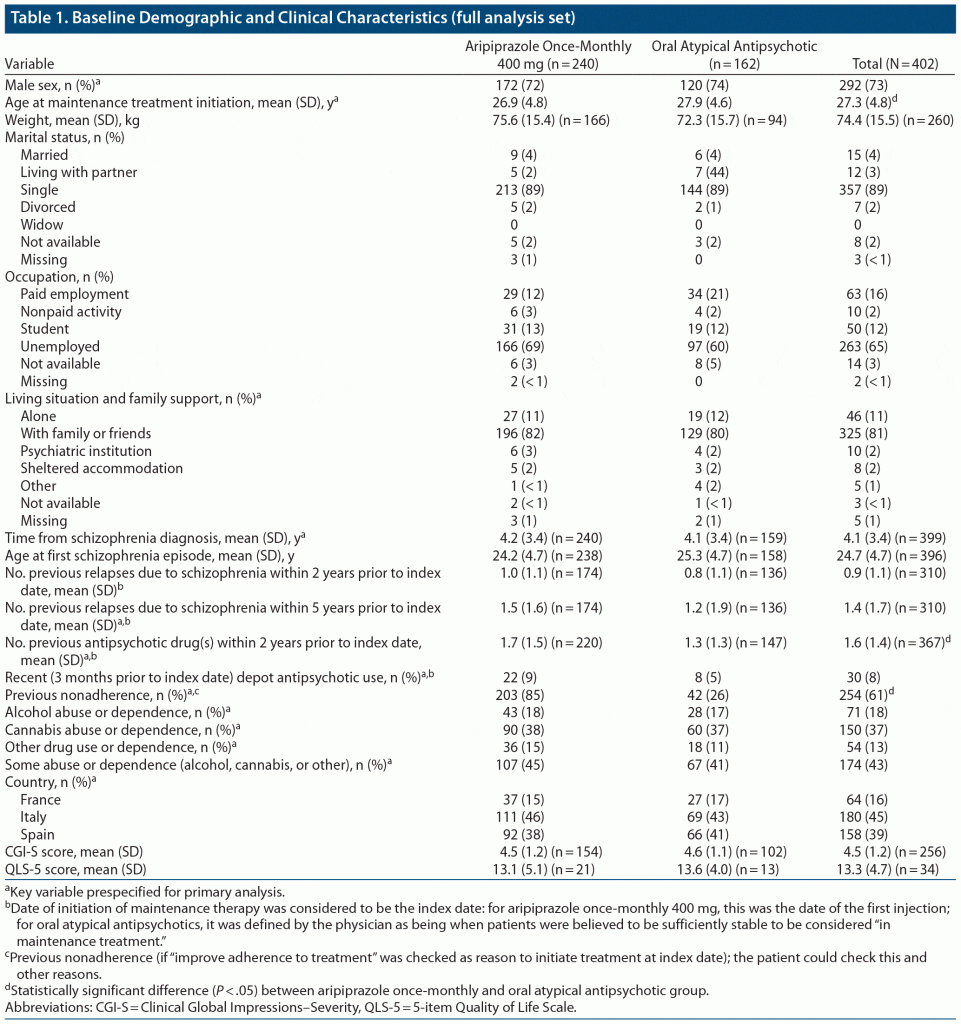
Of a total of 402 patients (185 in Italy, 166 in Spain, and 51 in France) treated with aripiprazole once-monthly 400 mg and oral atypical APs, 337 (83.8%) were followed retrospectively and 65 (16.2%) were followed prospectively. Of 357 patients for whom propensity scores were derived, 215 initiated treatment with aripiprazole once-monthly 400 mg and 142 with oral atypical APs; 297 (83.2%) of these patients were followed retrospectively and 60 (16.8%) were followed prospectively. Baseline clinical and demographic characteristics for the 2 treatment cohorts and the overall patient population are summarized in Table 1.
Primary Efficacy Analysis
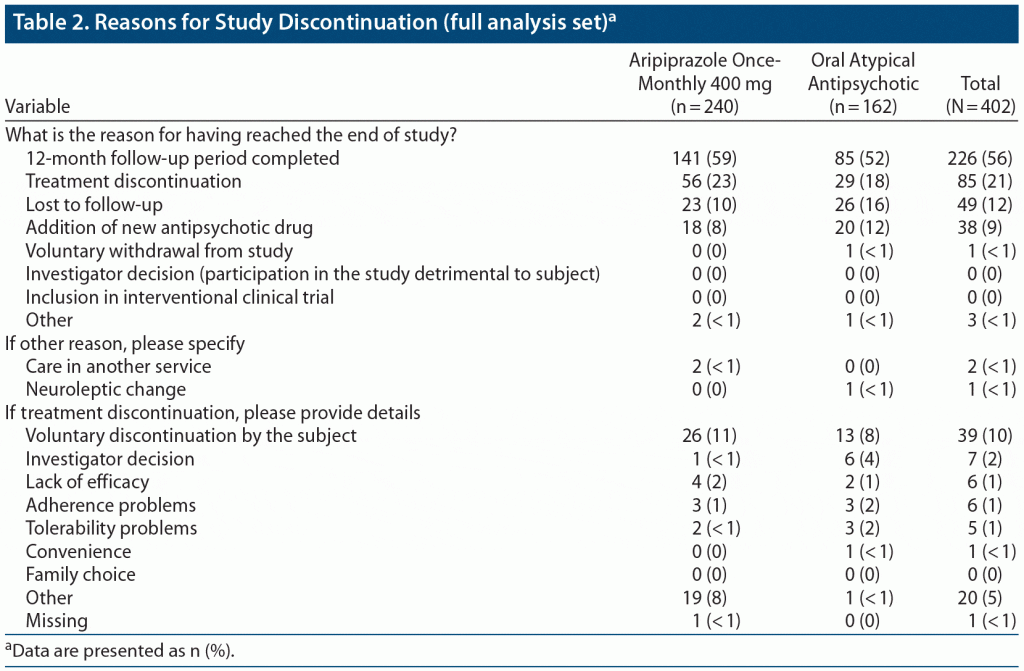
Reasons for study discontinuation, including reasons for treatment discontinuation, are listed in Table 2. In the unweighted sample, the primary reasons for study discontinuation included completion of the 12-month follow-up period (141 [59%] of aripiprazole once-monthly 400 mg patients and 85 [52%] on oral atypical APs), treatment discontinuation (56 [23%] and 29 [18%], respectively), lost to follow-up (23 [10%] and 26 [16%], respectively), and addition of a new AP drug (18 [8%] and 20 [12%], respectively). The main reason for treatment discontinuation in both groups was voluntary discontinuation by the patient (26 [11%] on aripiprazole once-monthly 400 mg and 13 [8%] on oral atypical APs). Other reasons for discontinuation included investigator decision (1 patient [< 1%] on aripiprazole once-monthly 400 mg and 6 [4%] on oral atypical APs), lack of efficacy (4 [2%] and 2 [1%], respectively), adherence problems (3 [1%] and 3 [2%], respectively), and tolerability problems (2 [< 1%] and 3 [2%], respectively).
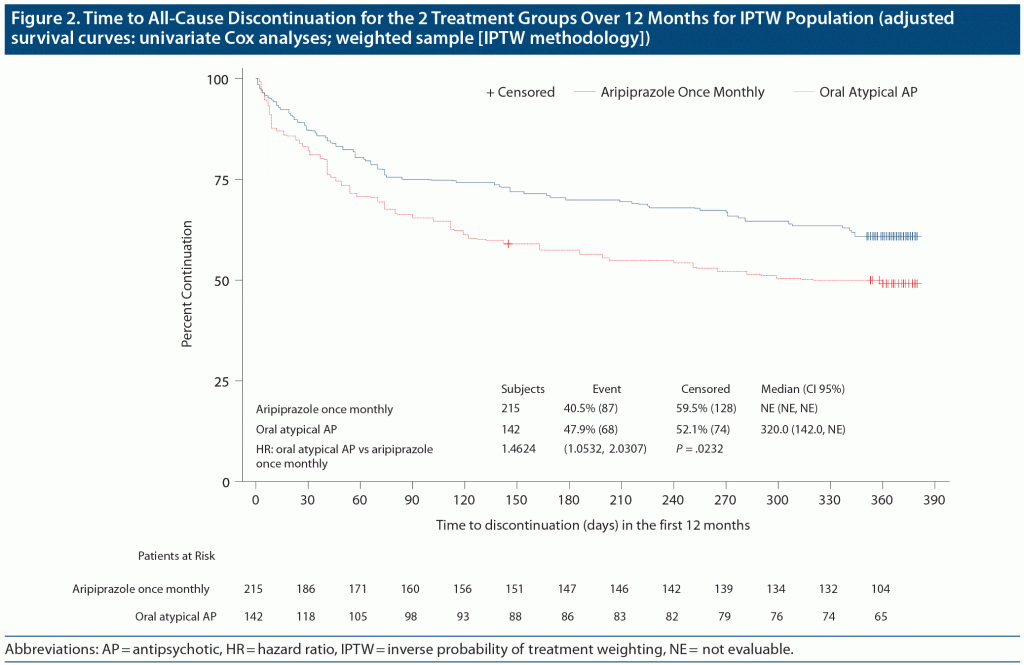
During the 52 weeks, all-cause treatment discontinuation in the IPTW sample occurred in 87 (40%) of the 215 patients treated with aripiprazole once-monthly 400 mg and 68 (48%) of the 142 treated with oral atypical APs (Figure 2). The time to all-cause discontinuation was significantly different between aripiprazole once-monthly 400 mg and oral atypical APs in favor of aripiprazole once-monthly 400 mg, with a hazard ratio of 1.46 (95% confidence interval, 1.05–2.03; P = .023); this finding was confirmed by sensitivity analyses with inclusion of additional clinical and demographic covariates in the model. Generalizability of the results to the full population of patients with schizophrenia, however, is limited due to incomplete overlap of patient characteristics in the aripiprazole once-monthly 400 mg and oral atypical AP cohorts, as well as the specific entry criteria of this study.
Secondary and Exploratory Outcome Variables
In all, 109 of 121 aripiprazole once-monthly 400 mg patients (90%) with available data reported being “satisfied” or “very satisfied” with their treatment after 6 months of maintenance therapy versus 47 of 70 oral atypical AP patients (67%, P < .001). Due to the aforementioned protocol adjustment/lack of sufficient prospective data, results are not reported for the secondary outcome variables.
Safety
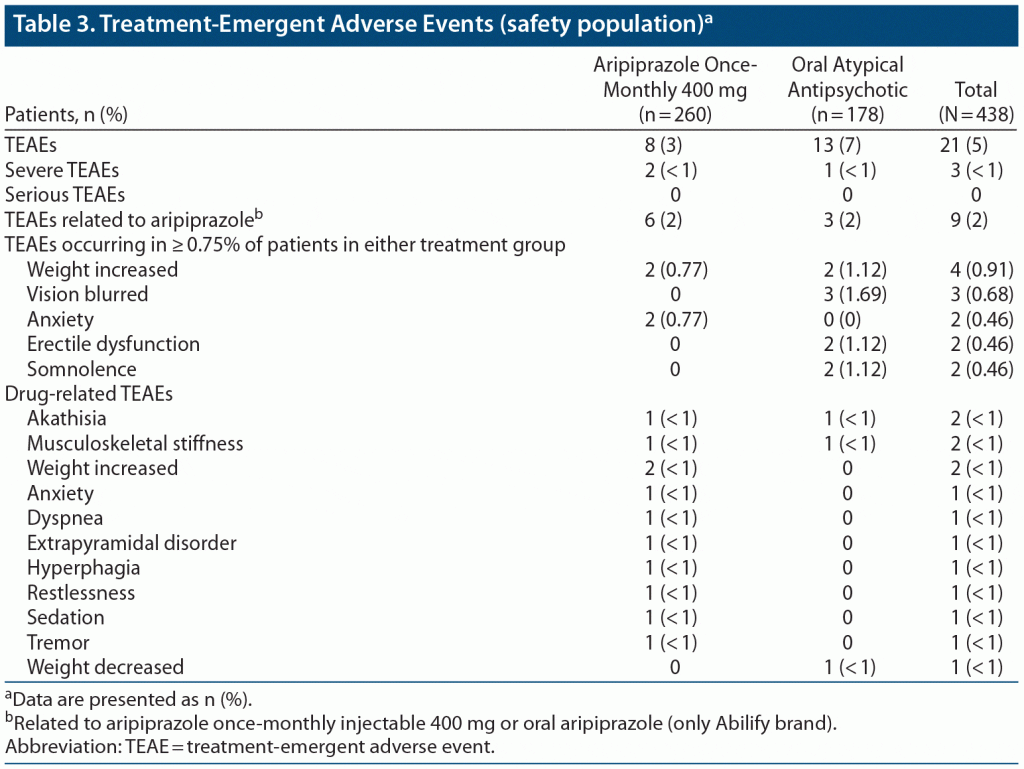
With respect to safety, there were too few adverse events in either treatment group to draw definitive conclusions. Adverse events in the aripiprazole once-monthly 400 mg group were mostly deemed to be related to treatment and were consistent with the safety findings of previous trials with this agent.32 The most common adverse events in the aripiprazole once-monthly 400 mg group were weight increase and anxiety, both of which occurred in 2 of 260 patients (< 1%) (Table 3). Severe adverse events were rare (2 patients [< 1%] in the aripiprazole once-monthly 400 mg group and 1 [< 1%] in the oral atypical AP group), and no serious adverse events were reported.
DISCUSSION
There are numerous challenges when assessing the relative value of LAIs versus oral atypical AP medications. Research conducted to date includes RCTs, mirror-image studies, and analyses of outcomes in actual clinical practice. RCTs comparing LAIs and oral atypical APs are conducted under tightly controlled conditions that may obscure positive effects of LAIs on adherence.38 In RCTs, patients on oral medications may receive reminders, insurance reimbursement, free medication, or routine assessments; these measures promote adherence and persistence and help ensure that patients have access to their medication. Further, a willingness to participate in an RCT may reflect a more positive predisposition toward treatment that could augment patient adherence with either treatment. In these trials, which are not representative of actual clinical practice, LAIs have not been shown to reduce the risk of relapse compared with oral APs.
Mirror-image studies20 comparing LAI versus oral atypical APs in the same patients in actual clinical settings have shown that LAIs are significantly superior to oral agents in preventing hospitalizations and reducing the length of hospital stays; however, there are limitations to this method of comparison as well. Mirror-image studies retrospectively examine outcomes in patients who switch from an oral agent to an LAI rather than the other way around; presumably, the reason for the switch could be inadequate response to the oral agent. This sequential design may introduce an expectation bias that affects outcomes after the switch.
To obtain more reliable data, direct comparisons of outcomes in patients treated with LAIs versus oral atypical APs in actual clinical practice have been performed. In an analysis of records of ~30,000 Swedish patients with schizophrenia, those treated with LAIs had substantially lower risks of rehospitalization and treatment failure (defined as rehospitalization, suicide attempt, treatment discontinuation/switch to another agent, or death) compared with oral agents.44 However, the vast majority of patients on an LAI were receiving risperidone (3,021 vs 1,042 patients on paliperidone palmitate once monthly), and none were on aripiprazole once-monthly 400 mg. Similarly, an observational study11 involving 2,588 consecutive patients following a first-time diagnosis of schizophrenia in Finland (mean age of 37.8 years) showed that depot agents were associated with a significantly lower risk of rehospitalization versus oral agents (adjusted hazard ratio = 0.36; 95% CI, 0.17–0.75; P = .007). Again, this study11 did not involve any patients on aripiprazole once-monthly 400 mg.
In an analysis of US claims data by Yan et al,45 treatment with aripiprazole once-monthly 400 mg was associated with significantly improved adherence and a significantly longer time to treatment discontinuation compared with an oral atypical AP. This analysis, however, included any patient with ≥ 1 inpatient or ≥ 2 outpatient claims for schizophrenia. It did not focus on benefits of early treatment and did not evaluate symptom management or functional status in the context of observed improvements in adherence.45
The present study was specifically designed to evaluate the benefits of aripiprazole once-monthly 400 mg compared with oral atypical APs with respect to treatment persistence and clinical outcomes in patients with early stage schizophrenia who were being treated in actual clinical practice rather than a research setting. We were unable to recruit sufficient numbers of patients on aripiprazole once-monthly 400 mg and oral atypical APs. As a result, the trial shifted from a prospective to a primarily retrospective approach, with an emphasis on treatment persistence rather than outcomes.
Results from the present analysis suggest that in a naturalistic setting, younger patients with recent-onset schizophrenia who were treated with aripiprazole once-monthly 400 mg tended to discontinue treatment later than those treated with oral atypical APs and that patient satisfaction was higher with aripiprazole once-monthly 400 mg versus oral atypical AP therapy. These findings are consistent with the concept that use of LAIs may be beneficial in early stage patients, who have been shown to be prone to early treatment discontinuation.11 There were insufficient data to draw conclusions regarding the secondary clinical variables or safety; however, there were no unexpected safety findings.
Regarding the primary endpoint, our results were aligned with those of Song et al,46 who reported that patients on aripiprazole once-monthly 400 mg had a significantly lower risk of discontinuing therapy versus those on oral atypical APs. Our results also aligned with a meta-analysis by Kishimoto et al47 and the observational trial by Tiihonen et al11 in early phase Finnish patients, both of which showed that LAI atypical APs were associated with significantly lower risk of all-cause discontinuation compared with oral atypical APs, and with the aforementioned findings of Yan et al.45 It is also worth noting that an observational study by Fagiolini et al48 that assessed treatment persistence in 261 Italian patients receiving aripiprazole once-monthly 400 mg found that 86% were persistent for at least 6 months; this compares with approximately two-thirds of the aripiprazole once-monthly 400 mg population who were persistent in our study at 6 months. The lower mean age of patients in our study (26.9 years vs ~40 years in the study by Fagiolini et al48) may have been a factor in the lower observed persistence.49,50
There were limitations of the present study. First, lack of prospective data/need for using primarily retrospective data resulted in underpowering of the treatment arms and made it impossible to investigate the secondary objectives. Second, the retrospective, observational study design may have introduced selection bias in the recruitment of patients, meaning that the patients included in this study may not be representative of the overall population of patients treated with aripiprazole once-monthly 400 mg or oral atypical APs. Incomplete overlap of patient characteristics in the aripiprazole once-monthly 400 mg and oral atypical AP cohorts, as well as the specific entry criteria of this study also limit generalizability of the results to the overall population of patients with schizophrenia. Third, the observational study design and lack of randomization may have increased the likelihood of confounding factors in interpretation of the results (this is especially a problem with retrospective studies, where there is a greater chance of missing data). It should be noted, however, that our study design included the use of IPTW methodology and sensitivity analyses, which allowed us to attempt to control for selection bias. Finally, reliance on retrospective information required that some data be inferred from medical records by participating clinicians, thus introducing the possibility of recall bias. Even given these limitations, observational studies such as this one are an important complement to RCTs due to their inclusion of a more heterogeneous population of patients39; moreover, they can serve to confirm the findings of RCTs in a naturalistic setting.
The present study provides real-world evidence of the potential advantages of the LAI aripiprazole 400-mg formulation versus oral atypical APs with respect to treatment persistence in patients with recent-onset schizophrenia, an effect that had not been demonstrated previously in RCTs. It also indicates higher patient satisfaction with LAI versus oral atypical AP therapy. These findings provide further evidence regarding the role of LAI APs early in treatment and point the way toward future research into the potential benefits of aripiprazole once-monthly 400 mg in this patient population.
Submitted: November 30, 2020; accepted March 30, 2021.
Published online: September 30, 2021.
Author contributions: All authors reviewed this manuscript in draft form and provided critical input during the development process, and all authors approved its submission for publication.
Potential conflicts of interest: Drs Such, Bøg, and Jørgensen are employees of H. Lundbeck A/S. Mr Kabra and Dr de Jong-Laird are employees of Otsuka Pharmaceutical Europe Ltd.
Funding/support: This study and the resultant publication were sponsored by Otsuka Pharmaceutical Europe Ltd and H. Lundbeck A/S. Editorial support, funded by Otsuka Pharmaceutical Development & Commercialization, Inc, was provided by BioScience Communications, New York, NY.
Role of the sponsor: The sponsors were involved in the study design and conduct, the analysis of the results, the generation of the manuscript, and the approval to submit the manuscript for publication.
Previous presentation: These findings were presented, in part, at the virtual European College of Neuropsychopharmacology Congress, September 12–15, 2020.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Aripiprazole once-monthly 400 mg is an extended-release, injectable atypical antipsychotic shown in previous clinical studies to be effective in long-term maintenance treatment of schizophrenia.
- In a naturalistic setting, younger patients with recent-onset schizophrenia treated with aripiprazole once-monthly 400 mg after hospitalization tended to have greater treatment persistence compared with patients treated with daily oral atypical antipsychotics; thus, the use of long-acting injectable agents could be considered in younger patients.
References (50)

- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Emsley R, Chiliza B, Asmal L, et al. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13(1):50. PubMed CrossRef
- Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. PubMed CrossRef
- Rosenheck R, Leslie D, Keefe R, et al; CATIE Study Investigators Group. Barriers to employment for people with schizophrenia. Am J Psychiatry. 2006;163(3):411–417. PubMed CrossRef
- Schenkel LS, Silverstein SM. Dimensions of premorbid functioning in schizophrenia: a review of neuromotor, cognitive, social, and behavioral domains. Genet Soc Gen Psychol Monogr. 2004;130(3):241–270. PubMed CrossRef
- Agius M, Goh C, Ulhaq S, et al. The staging model in schizophrenia, and its clinical implications. Psychiatr Danub. 2010;22(2):211–220. PubMed
- Stahl SM. Long-acting injectable antipsychotics: shall the last be first? CNS Spectr. 2014;19(1):3–5. PubMed CrossRef
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2. PubMed CrossRef
- Chen EY, Hui CL, Lam MM, et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ. 2010;341:c4024. PubMed CrossRef
- Köhler O, Horsdal HT, Baandrup L, et al. Association between Global Assessment of Functioning scores and indicators of functioning, severity, and prognosis in first-time schizophrenia. Clin Epidemiol. 2016;8:323–332. PubMed CrossRef
- Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. PubMed CrossRef
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The Expert Consensus Guideline Series: Adherence Problems in Patients With Serious and Persistent Mental Illness. J Clin Psychiatry. 2009;70(suppl 4):1–46, quiz 47–48. PubMed
- Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–460. PubMed CrossRef
- MacEwan JP, Forma FM, Shafrin J, et al. Patterns of adherence to oral atypical antipsychotics among patients diagnosed with schizophrenia. J Manag Care Spec Pharm. 2016;22(11):1349–1361. PubMed CrossRef
- Pietrini F, Albert U, Ballerini A, et al. The modern perspective for long-acting injectables antipsychotics in the patient-centered care of schizophrenia. Neuropsychiatr Dis Treat. 2019;15:1045–1060. PubMed CrossRef
- Sheehan JJ, Reilly KR, Fu DJ, et al. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. 2012;9(7–8):17–23. PubMed
- Pietrini F, D’Anna G, Tatini L, et al. Changes in attitude towards LAI antipsychotic maintenance treatment: a two-year follow-up study. Eur Psychiatry. 2018;53:58–65. PubMed CrossRef
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. PubMed CrossRef
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. PubMed CrossRef
- Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. PubMed CrossRef
- Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754–768. PubMed CrossRef
- Munday J, Greene M, Chang E, et al. Early initiation of long-acting injectable antipsychotic treatment is associated with lower hospitalization rates and healthcare costs in patients with schizophrenia: real-world evidence from US claims data. Curr Med Res Opin. 2019;35(7):1231–1239. PubMed CrossRef
- Casey AB, Canal CE. Classics in chemical neuroscience: aripiprazole. ACS Chem Neurosci. 2017;8(6):1135–1146. PubMed CrossRef
- Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385–3403. PubMed CrossRef
- Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251–267. PubMed CrossRef
- Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488–493. PubMed CrossRef
- Mas S, Gassó P, Fernández de Bobadilla R, et al. Secondary nonmotor negative symptoms in healthy volunteers after single doses of haloperidol and risperidone: a double-blind, crossover, placebo-controlled trial. Hum Psychopharmacol. 2013;28(6):586–593. PubMed CrossRef
- Saeedi H, Remington G, Christensen BK. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr Res. 2006;85(1-3):222–231. PubMed CrossRef
- Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–624. PubMed CrossRef
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–144. PubMed CrossRef
- Peters-Strickland T, Baker RA, McQuade RD, et al. Aripiprazole once-monthly 400 mg for long-term maintenance treatment of schizophrenia: a 52-week open-label study. NPJ Schizophr. 2015;1(1):15039. PubMed CrossRef
- Naber D, Hansen K, Forray C, et al. QUALIFY: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1–2):498–504. PubMed CrossRef
- Potkin SG, Loze J-Y, Forray C, et al. Multidimensional assessment of functional outcomes in schizophrenia: results from QUALIFY, a head-to-head trial of aripiprazole once-monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20(1):40–49. PubMed
- Potkin SG, Loze J-Y, Forray C, et al. Relationship between response to aripiprazole once-monthly and paliperidone palmitate on work readiness and functioning in schizophrenia: a post-hoc analysis of the QUALIFY study. PLoS One. 2017;12(8):e0183475. PubMed CrossRef
- Peters-Strickland T, Baker RA, Such P, et al. The effect of aripiprazole once-monthly on personal and social functioning: post hoc analyses of acute and long-term studies. Neuropsychiatr Dis Treat. 2019;15:1659–1669. PubMed CrossRef
- Potkin SG, Loze J-Y, Forray C, et al. Reduced sexual dysfunction with aripiprazole once-monthly versus paliperidone palmitate: results from QUALIFY. Int Clin Psychopharmacol. 2017;32(3):147–154. PubMed CrossRef
- Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20. PubMed CrossRef
- Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213. PubMed CrossRef
- Karcher H, Fu S, Meng J, et al; GetReal Consortium Work Package 2. The “RCT augmentation”: a novel simulation method to add patient heterogeneity into phase III trials. BMC Med Res Methodol. 2018;18(1):75. PubMed CrossRef
- Abilify Maintena (aripiprazole) [package insert]. Amsterdam, The Netherlands: Otsuka America Pharmaceutical, Inc; 2020.
- Naber D, Hansen K, Forray C, et al. Aripiprazole once-monthly and paliperidone palmitate in patients with schizophrenia stratified by age: Results from QUALIFY, a head-to-head study. Poster presented at the 28th annual European College of Neuropsychopharmacology (ECNP) Congress; August 31–September 1, 2015; Amsterdam, The Netherlands.
- Kerwin R, Millet B, Herman E, et al. A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry. 2007;22(7):433–443. PubMed CrossRef
- Verdoux H, Pambrun E, Tournier M, et al. Antipsychotic long-acting injections: a community-based study from 2007 to 2014 of prescribing trends and characteristics associated with initiation. Schizophr Res. 2016;178(1–3):58–63. PubMed CrossRef
- Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29,823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. PubMed CrossRef
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612–1625. PubMed CrossRef
- Song X, El Khoury AC, Brouillette M, et al. Treatment discontinuation of long-acting injectables or oral atypical antipsychotics among Medicaid recipients with schizophrenia. J Med Econ. 2019;22(11):1105–1112. PubMed CrossRef
- Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–619. PubMed CrossRef
- Fagiolini A, Aguglia E, Ballerini A, et al. Real-world effectiveness of long acting aripiprazole: treatment persistence and its correlates in the Italian clinical practice. Psychiatry Res. 2019;272:698–706. PubMed CrossRef
- Linden M, Godemann F, Gaebel W, et al. A prospective study of factors influencing adherence to a continuous neuroleptic treatment program in schizophrenia patients during 2 years. Schizophr Bull. 2001;27(4):585–596. PubMed CrossRef
- Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30(2):255–264. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite