Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Stevens-Johnson syndrome (SJS) is a rare, potentially life-threatening hypersensitivity reaction involving the skin and mucous membranes.1,2 Medications associated with SJS have included anticonvulsants, particularly carbamazepine, lamotrigine, phenytoin, and valproate. Oxcarbazepine, an anticonvulsant structurally similar to carbamazepine, has been employed off-label to address affective instability in adult patients with bipolar disorder.
Oxcarbazepine-Induced Stevens-Johnson Syndrome
To the Editor: Stevens-Johnson syndrome (SJS) is a rare, potentially life-threatening hypersensitivity reaction involving the skin and mucous membranes.1,2 Medications associated with SJS have included anticonvulsants, particularly carbamazepine, lamotrigine, phenytoin, and valproate. Oxcarbazepine, an anticonvulsant structurally similar to carbamazepine, has been employed off-label to address affective instability in adult patients with bipolar disorder. We report a rare case of oxcarbazepine-induced SJS.
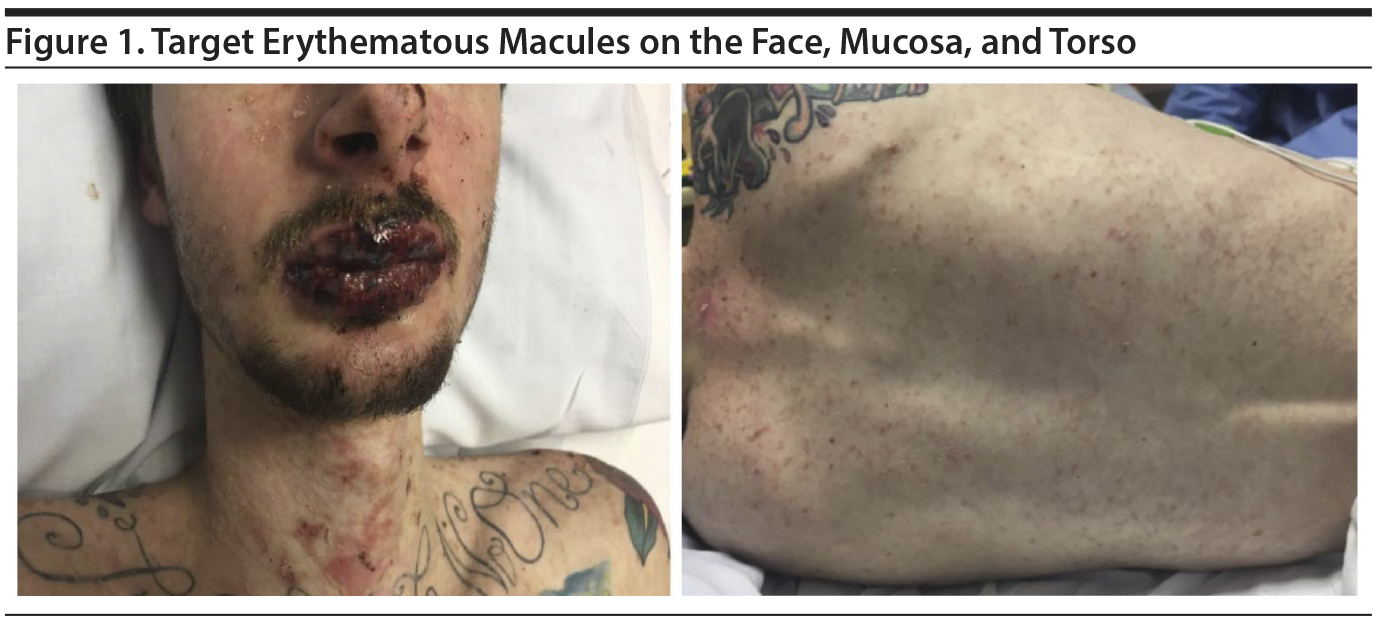
Case report. A 20-year-old white man with a history of oppositional defiant disorder and a bipolar disorder variant was treated with oxcarbazepine 300 mg bid; 2 weeks later, the dose was increased to 600 mg bid. After 9 days, he developed symptoms of fever, headache, myalgia, fatigue, and sore mouth and throat. Two days later, he presented to an emergency department with a painful red/purple symmetric rash over his face, lips, and neck (Figure 1).
On examination, he was febrile (39.4°C [102.9°F]) and tachycardic (122 beats/min), but normotensive. He was noted to have multiple, small, target macules, some of which developed into vesicles and bullae covering 10% body surface area. The core of the macules was vesicular, surrounded by erythema. His lips and oral cavity were characterized by deep red lesions hindering oral intake. Ophthalmology evaluation revealed inflammation, ie, punctate keratopathy without scarring or epithelial defects. Laboratory investigations revealed an elevated C-reactive protein (CRP) (49.35 mg/L; reference < 3 mg/L) and a leukocytopenia of 3.3 ח 109/L (reference range, 4.8-10.8 ח 109/L) with lymphopenia (25.7%; reference range, 16%-51%). Throat, blood, and urine cultures failed to reveal an infectious etiology.
Because the rash progressed distally over the upper torso, arms, and scrotum over ensuing days, the patient was transitioned to a burn treatment center for management. The oxcarbazepine was discontinued. He was provided with intravenous hydration and initiated on a course of intravenous IgG (immunoglobulin G), with complete resolution of his symptoms in 1 week.
Stevens-Johnson syndrome can be triggered by medication, malignancy, or infection. Although known to be associated with certain anticonvulsant agents, oxcarbazepine-induced SJS has been rarely reported.3,4 Typically, symptoms develop abruptly within 2-8 weeks after drug initiation or dose increase, as illustrated in the present case. Although definitive diagnosis is based upon biopsy and histologic examination, the diagnosis can be inferred from the sudden onset of the aforementioned clinical manifestations appearing on two or more mucosal surfaces.5 Laboratory investigations, eg, an elevated CRP or leukocytopenia, can be supportive of an SJS diagnosis but are not confirmatory. These results, in addition to cultures, can aid in ruling out other potential causes of SJS.
Differences in the metabolism of carbamazepine and oxcarbazepine, although structurally similar, may account for differences in the rates, and perhaps the degree of severity, of SJS between them. Specifically, cytochrome P450 formation of the epoxide metabolite of carbamazepine is thought to trigger immune reactions precipitating SJS. By contrast, oxcarbazepine is rapidly converted by conjugation to a 10-hydroxy-carbazepine metabolite that is less apt to trigger immune reactions.3 However, cross-reactivity between carbamazepine and oxcarbazepine has been reported. Some evidence suggests that the presence of variants of the HLA-B*15:02 allele is associated with a greater risk of SJS, at least among Asian populations treated with carbamazepine or oxcarbazepine.6 The cost-effectiveness of HLA screening for risk of SJS among the general population has not yet been established.7
The incidence of oxcarbazepine-associated SJS is estimated to range between 0.5-6 cases/million per year.8 This case was the first, to our knowledge, of oxcarbazepine-induced SJS reported in the psychiatric literature. Primary care physicians need to be aware of the risk of oxcarbazepine-induced SJS, particularly among Asian populations or in persons who demonstrated hypersensitivities to carbamazepine. Patient monitoring for subtle prodromal symptoms, especially in the first weeks of medication initiation or dose increase, is warranted to prevent progression of this potentially life-threatening condition.
References
1. Mockenhaupt M. Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management. Semin Cutan Med Surg. 2014;33(1):10-16. PubMed CrossRef
2. Frey N, Bodmer M, Bircher A, et al. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia. 2017;58(12):2178-2185. PubMed CrossRef
3. Sharma SR, Sharma N, Yeolekar ME. Oxcarbazepine-induced Stevens Johnson syndrome: a rare case report. Indian Dermatol Online J. 2011;2(1):13-15. PubMed CrossRef
4. Wal P, Wal A, Pandey U, et al. Genetic predisposition to oxcarbazepine induced Stevens-Johnson syndrome. Indian J Crit Care Med. 2011;15(3):173-175. PubMed CrossRef
5. Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. PubMed CrossRef
6. Phillips EJ, Sukasem C, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin Pharmacol Ther. 2018;103(4):574-581. PubMed CrossRef
7. Chen CB, Hsiao YH, Wu T, et al; Taiwan Severe Cutaneous Adverse Reaction Consortium. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. 2017;88(1):78-86. PubMed CrossRef
8. Dogan EA, Usta BE, Bilgen R, et al. Efficacy, tolerability, and side effects of oxcarbazepine monotherapy: a prospective study in adult and elderly patients with newly diagnosed partial epilepsy. Epilepsy Behav. 2008;13(1):156-161. PubMed CrossRef
aDepartment of Psychiatry, University at Buffalo, Jacobs School of Medicine and Biomedical Sciences, New York
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Consent was received from the patient to publish the case report and photographs, and information has been de-identified to protect anonymity.
Published online: December 20, 2018.
Prim Care Companion CNS Disord 2018;20(6):18l02304
To cite: Khalid K, Kwak BS, Leo RJ. Oxcarbazepine-induced Stevens-Johnson syndrome. Prim Care Companion CNS Disord. 2018;20(6):18l02304.
To share: https://doi.org/10.4088/PCC.18l02304
© Copyright 2018 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite