LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2021;23(2):20f02749
To cite: Rustad JK, Stern TA, Eth S. A patient-centered approach to opioid use disorder. Prim Care Companion CNS Disord. 2021;23(2):20f02749.
To share: https://doi.org/10.4088/PCC.20f02749
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire
bWhite River Junction VA Medical Center, White River Junction, Vermont
cBurlington Lakeside VA Community Based Outpatient Clinic, Burlington, Vermont
dDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
eHarvard Medical School, Boston, Massachusetts
fDepartments of Forensic Psychiatry and Addiction Psychiatry, University of Miami Miller School of Medicine, Miami, Florida
gMiami VA Healthcare System, Miami, Florida
*Corresponding author: James K. Rustad, MD, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, New Hampshire 03756 ([email protected]).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
CME Objective
After studying this article, you should be able to:
- Take steps to avoid perpetuating racial stigma in the provision of treatment for opioid use disorder
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
 Credit Designation
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in April 2021 and is eligible for AMA PRA Category 1 Credit™ through April 30, 2023. The latest review of this material was April 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AbbVie, Acadia, Allergan, Eisai, Merck, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Have you ever struggled to provide comprehensive evaluations and management of your patients with opioid misuse? Have you been uncertain about whether you can involuntarily admit one of your patients with persistent opioid misuse? Have you been concerned about recurrent complications of opioid misuse in your patients despite your best efforts? If you have, the following case vignette, historical overview, and discussion should prove useful.
The current opioid epidemic is a public health emergency; it has resulted in abundant morbidity and mortality, lost economic productivity, and a breakdown of important social connections. This crisis affects everyone; therefore, it is essential for clinicians to understand the myriad biological, psychological, and social factors that contribute to opioid misuse. This article describes the neurobiological, psychological, psychosocial, and cultural determinants of opioid use disorder. Approaches to integrated care and public policy interventions that address the opioid crisis are also reviewed.
Case Vignette Part 1
Mr A is a 72-year-old white man with atherosclerotic cardiovascular disease, atrial fibrillation, heart failure with reduced ejection fraction (45%–50%), chronic obstructive pulmonary disease, hypertension, hyperlipidemia, and osteomyelitis of the left foot status post recent transmetatarsal amputation who was admitted for management of a left foot ulcer and heart failure exacerbation. At admission, he reported that he had not been taking his medications for the past 3 weeks, including furosemide, metoprolol, and apixaban. He ran out of his medications and reported being unable to get them filled. He was also recently discharged from the anticoagulation clinic due to repeatedly missing appointments. He reported daily heroin use (via intranasal route of administration but denied intravenous use). He had a history of cocaine use, although he denied any current use. He reported smoking marijuana about 1 to 2 times per week. During a prior medical admission, he was evaluated by the substance use disorder treatment team but was not interested in engaging in treatment. His urine toxicology screen at admission was positive for fentanyl and cocaine but negative for opiates or marijuana. Coronavirus disease 2019 (COVID-19) testing was negative, and his brain natriuretic peptide level was elevated at 2,000 pg/mL (normal range, 10–100 pg/mL).
Mr A was initiated on a symptom-triggered opioid withdrawal protocol with buprenorphine based on the Clinical Opiate Withdrawal Scale (COWS),1 an 11-item scale used to reproducibly rate common signs and symptoms of opioid withdrawal (eg, sweating, yawning, pupil dilation, and gastrointestinal symptoms like nausea and vomiting). Higher total scores on the COWS indicate more symptoms or more severe symptoms. Four hours after admission, Mr A received a score between 5 and 12 (mild withdrawal) and received 2 mg of buprenorphine sublingually. He then received 4 mg 5 hours after admission and 4 mg 9 hours after admission for scores that were between 13 and 24 (moderate withdrawal). The psychiatry consultation-liaison team was consulted to help evaluate and treat the patient. On clinical interview with the psychiatry team, Mr A reported severe symptoms of opioid withdrawal (including muscle aches, joint pain, and abdominal spasms) and was doubled over writhing in discomfort.
What Are the Psychosocial Determinants of Opioid Addiction?
Media coverage of the US opioid crisis has emphasized the “vector model,” which casts opioid “pain killers” as toxic medications, physicians as inadvertent facilitators of addiction, and profit-driven pharmaceutical companies as sinister promoters through a combination of pharmaceutical detailing (ie, a 1:1 marketing technique used by pharmaceutical companies to educate a physician about their products in hopes that the physician will prescribe these products more often) and direct-to-consumer advertising.2,3 Indeed, in the mid 1990s and early 2000s the medical community increasingly focused their attention on the subjective pain reports of their patients in accordance with the guidance of the Joint Commission. Pain was commonly referred to as “the fifth vital sign.”4 In addition, many communities became besieged by a proliferation of “pill mills” that wantonly afforded easy access to large quantities of opioids. In this climate, the frequency and dosing of prescribed opioids to treat pain dramatically increased. A tragic surge in morbidity and mortality followed, and the prescription of narcotics to manage chronic pain started to fall out of favor. Medical facilities across the country reduced or discontinued opioid prescriptions for their patients despite a paucity of data on whether such a rapid taper was safe, leaving many individuals caught in the crossfire of these contrasting prescribing philosophies.4
However, the factors that served as root causes for the misuse of opioids and other substances were often overlooked.5 Driving factors include a lack of economic opportunity, poor working conditions, eroded social capital in depressed communities, systemic racism, hopelessness, and despair. Individuals with opioid use disorder also report increased rates of being a victim of childhood maltreatment,6 and there has been a dose-response relationship between exposure to childhood maltreatment and accelerated progression from opioid use to dependence.7 Additionally, the earlier children are exposed to psychoactive substances, the more likely it is they will have problems (and more severe problems) with substance use disorder as adults.8
In the 1970s, Khantzian et al9 proposed “the self-medication hypothesis,” which posited the psychodynamic understanding of individuals with substance use disorder. Khantzian and colleagues9,10 conceptualized opioids as a means of coping with conflicts that encompassed ordinary human pain, disappointment, anxiety, loss, anguish, and other forms of suffering. Fricchione11 applied the human attachment theory of a British child psychoanalyst, John Bowlby, to his own thinking on substance use disorder: “As mammals our survival advantage is based on secure attachment behavior; and to the extent that addictive substances deflect us from social attachment supports, isolation and solipsism are accentuated and a subsequent loss of resiliency puts us at risk for escalating addiction and maladaptive behaviors.”(p175)
In Hari’s Ted Talk, “Everything You Think You Know About Addiction Is Wrong,”12 the journalist asserted that “the opposite of addiction is connection.” Hari further explored how disordered attachment and unhealthy environments can lead to and perpetuate substance use disorder in his book Chasing the Scream: The First and Last days of the War on Drugs.13 Hari described Billie Holiday’s challenging life experiences, including parental neglect and abandonment, poverty, sexual assault, prostitution, and incarceration. Following incarceration, Holiday became addicted to heroin. She believed that “it’s the one thing that makes me know there is a person called Billie Holiday.”13(p21)
In 1939, Holiday sang the iconic lyrics of “Strange Fruit,” which described the lynching of Black Americans, and it became a Civil Rights anthem. The authorities ordered Lady Day, the nickname saxophonist Lester Young gave to Holiday, to stop singing that song, but she refused. Shortly afterward, the Federal Bureau of Narcotics began a campaign of racist and systematic harassment of Billie Holiday, and the criminalization of her drug use led to her untimely death. Stanley Nelson’s documentary, Miles Davis: Birth of the Cool,14 supported the link between racial discrimination and substance use by detailing how Davis used heroin to cope with the discrimination he suffered as a Black man living under Jim Crow laws in the 1940s.15 Davis became disillusioned by American racism after spending time in France, where he could live and love without restrictions.
Racism insidiously affects all domains of the social determinants of health.16 Bailey and colleagues17 define structural racism as “the totality of ways in which societies foster racial discrimination through mutually reinforcing systems of housing, education, employment, earnings, benefits, credit, media, health care, and criminal justice.”(p1,453) Alexander describes the devastating effects of structural racism in drug policy on communities of people of color in her book The New Jim Crow: Mass Incarceration in the Age of Colorblindness.18 Since the start of the punitive US drug control policies called the “war on drugs” in 1971, people of color have disproportionately been targeted, arrested, and incarcerated through overtly racist policies (eg, “stop and frisk”).16 During the 1980s, stiffer criminal penalties were administered for crack cocaine (which was more likely to be used by Black people) than powder cocaine.19 Although Black Americans are not more likely than White Americans to use illicit drugs, they are 6–10 times more likely to be incarcerated for drug offenses.20 These systemically racist policies cause downstream effects that worsen economic, educational, and housing opportunities, which increase the incidence and consequences of substance use disorders such as opioid use disorder.21
Media coverage of the suburban and rural opioid epidemic of the 2000s symbolically divided the addiction to heroin of urban people of color from the prescription addiction to opioids of suburban and rural White people.19 Media portrayals of Black people who use substances as criminal deviants and White people who misuse substances as victims of a brain disease support the racialized deployment of the war on drugs. “Color blind” ideologies and the lack of specific discussion on race perpetuate the harmful effects of structural racism.19
Like drug policy, therapeutic intervention is also racially stratified due to disparities in access to buprenorphine (more flexibly prescribed than methadone) for people of color relative to White people.16,20 Racially targeted marketing for buprenorphine took place after its US Food and Drug Administration approval, focusing on suburban middle-class White people with opioid use disorders through the internet (rather than television or radio ads). Nearly 20 years after the introduction of buprenorphine into the market, treatment disparities based on race, ethnicity, and income continue as buprenorphine is most frequently prescribed for White persons and those with private insurance or use of self-pay.22 The national treatment-focused response to the opioid crisis prioritizes White individuals and categorizes it as a White epidemic.20 The “White drug war” serves as a microcosm for White privilege as a whole by creating a decriminalized, medicalized conceptualization for White people who use psychoactive substances, while more punitive systems remain for the use of substances by people of color.20
What Causes Opioid Use to Turn Into a Disorder?
Koob and Volkow23 conceptualized substance use disorder as dysregulation of motivational circuits with 3 stages: binging/intoxication, withdrawal/negative affect, and preoccupation/anticipation. The reward provided by the effects of psychoactive substances, the development of incentive salience, and drug seeking involves changes in dopamine and opioid peptides. Conditioned reinforcement of psychoactive substances has a profound effect on response to previously neutral stimuli (eg, seeing drug paraphernalia or the house where drugs are used) to which drugs become paired.
Evolutionary neurobiological adaptations lead people to seek potentially pleasurable and nurturant objects and to avoid painful or life-threatening situations. Reward, mediated by the brain reward circuitry,24 induces approach behavior.23 Incentive salience, defined as the motivation for reward derived from one’s physiologic state and previously learned associations about a reward cue, is relevant to drug use.
Those who develop substance use disorders evolve from a state of impulsivity (ie, using for pleasure/gratification) to one of compulsivity (ie, thereby reducing tension/anxiety), with the drive for drug-using behavior paralleling shifts in reinforcement from positive to negative.23 During the withdrawal/negative affect stage, negative emotional states are heightened, dysphoria arises, and stress response leads to decreases in the dopaminergic component of the reward system and in recruitment of stress-modulating neurotransmitters (eg, corticotropin-releasing factor and dynorphin).23 Antireward circuits become engaged as neuroadaptation occurs and produce aversive or stress-like states during withdrawal and protracted abstinence.25
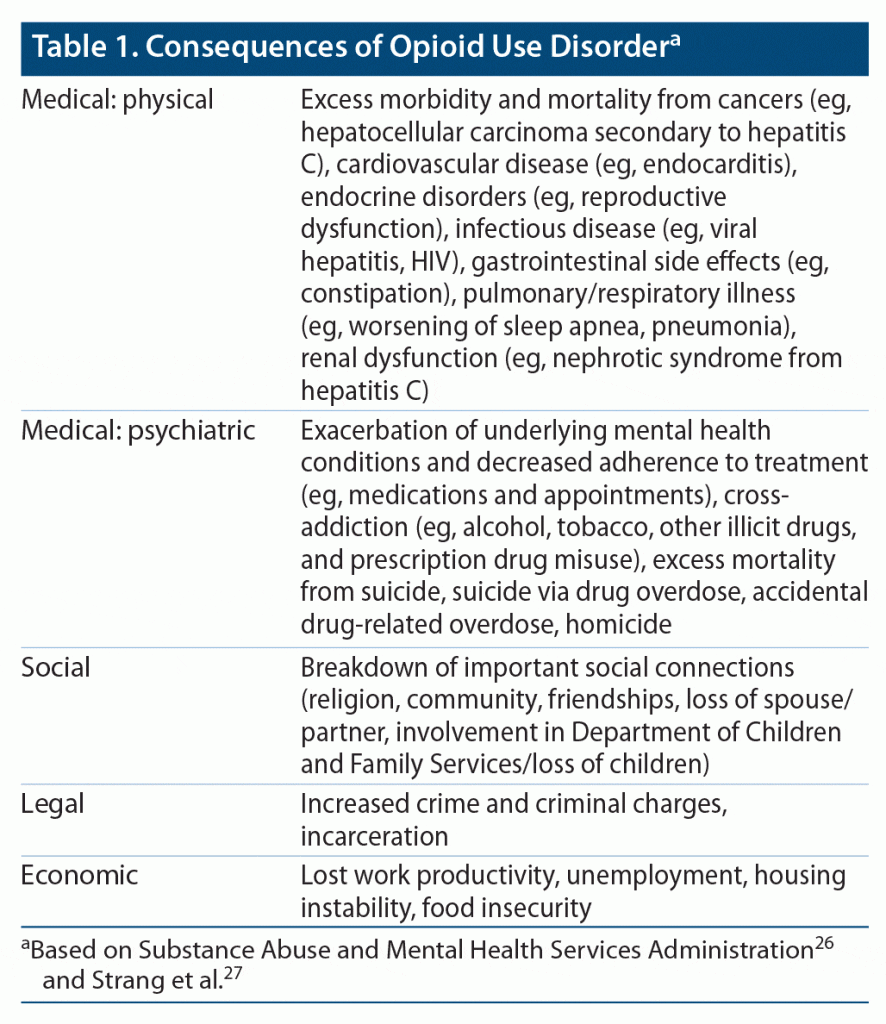
The preoccupation/anticipation stage has been thought to be a critical element in relapse, and it characterizes substance use disorders as chronic relapsing conditions.23 Executive control over incentive salience is necessary to maintain goal-directed behavior and flexibility of stimulus-response associations, in addition to distinguishing between potentially helpful and harmful attachments. Individuals with opioid use disorder lose control over incentive salience and continue use despite often dire consequences (Table 1).
Does Nature or Nurture Cause Opioid Addictions?
Genetic factors such as susceptibility genes, augmented by environmental factors, contribute to vulnerability to develop opioid use disorder and disease etiology.28 Relatives of probands with opioid use disorders were found to be 10 times more likely to have opioid-related disorders (adjusted odds ratio [OR] = 10.2, 95% CI, 3.2–32.6).29 Initiation of drug use may be more influenced by environmental factors, while the transition from use to dependence may be more influenced by genetic factors.28 Further, genetic factors play a strong role in the response to treatment of opioid addiction.30
To study environmental contributions to development of opioid addiction, psychologists Alexander and colleagues built 2 sets of homes for laboratory rats.13,31 One home was a solitary chamber, while the other home was a rat paradise (called “rat park”) complete with wheels, great food, and the companionship of other rats. The rats in the isolated environment used up to 25 mg of morphine per day, an amount consistent with other trials, while the rats in rat park used less than 5 mg per day.13,31 Adding further support to addictions as an adaptation to one’s environment, a study showed that during the Vietnam War almost half of a random sample (n = 943) of US Army soldiers tried narcotics and 20% reported addiction to opioids.13,32 Within a year of military discharge, usage and addiction rates decreased to the levels observed prior to their deployment.
What Are Commonly Misused Opiate and Opioid Drugs and How Are They Tested?
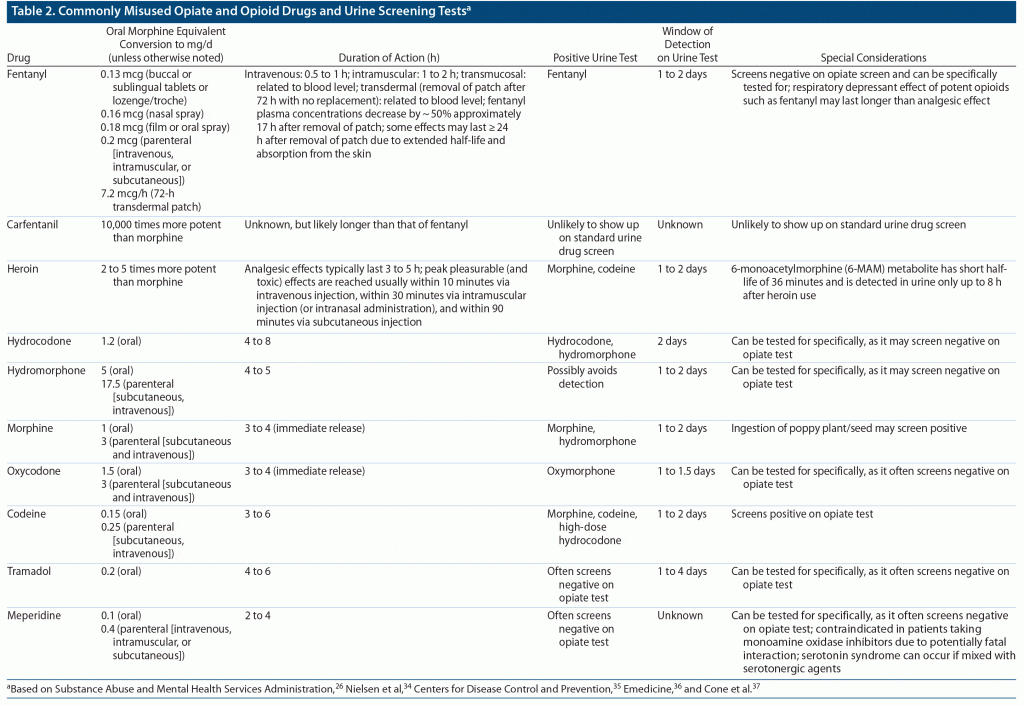
The term opiate refers to naturally occurring drugs (eg, morphine and codeine, which are naturally occurring alkaloids from the opium poppy seed [Papaver somniferum]).33 The term opioid refers to semisynthetic (eg, heroin) and completely synthetic agents (eg, methadone and buprenorphine), which have morphine-like actions (eg, analgesia, respiratory depression).
The most used specimen in mental health and substance use treatment settings continues to be urine.33 Urine tests are easy to administer, are inexpensive, and give instant results. However, they are easy to tamper or substitute, although adulteration of samples can often be detected by the laboratory. Urine tests have a narrow window of detection (often < 3 days). Blood tests detect recent drug use (over the past few hours), thus if an incident is suspected to be the result of drug use, blood samples are preferred to urine. However, blood tests are invasive and more difficult to collect and thus are less frequently used in clinical practice.
Most urine drug screens for opiates detect morphine (also the primary metabolite of heroin and codeine).33 Table 2 provides a review of commonly misused opiate and opioid drugs and information about urine screening tests for these substances.34–37 It should be noted that there are agents with potential to cause false positives (eg, codeine from cough syrup, poppy seeds, quinolones, rifampicin, verapamil), and positive tests warrant further confirmatory testing, especially if there are discrepancies between patient report and the laboratory results.
What Medications and Medical Settings Can Treat Opioid Use Disorder?
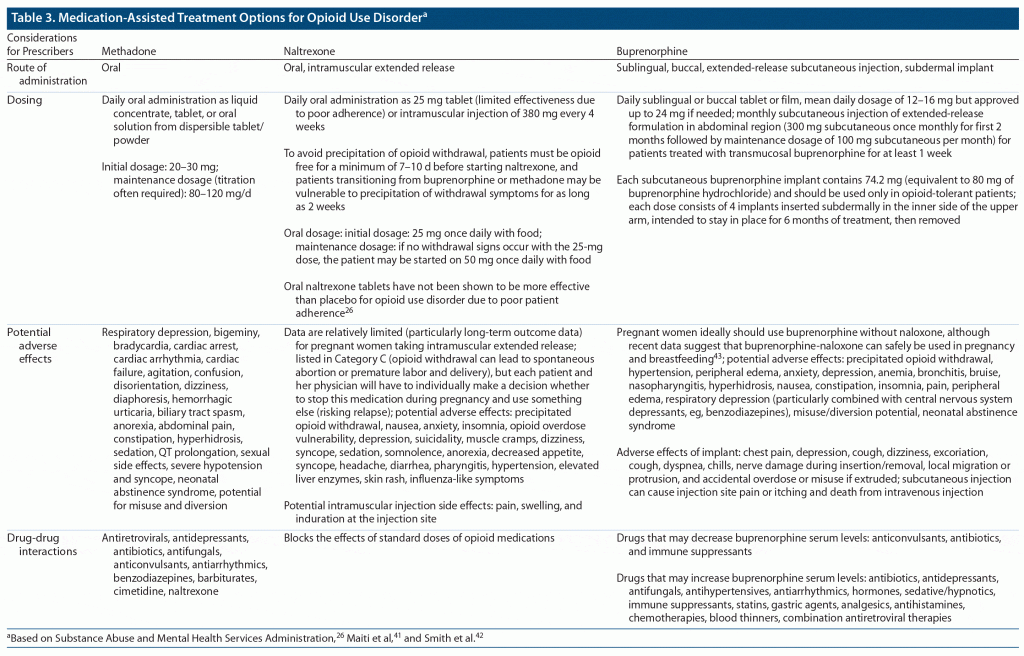
Opioids are μ-opioid receptor agonists, which cause activation of mesolimbic dopamine reward pathways and euphoria.38 Table 3 provides medication-assisted treatment options for opioid use disorder. Medication-assisted treatment is well established as a therapeutic strategy that decreases overdose and all-cause mortality among individuals with opioid use disorder.39 Methadone, a μ-opioid receptor agonist, is available only in federally regulated treatment facilities (eg, outpatient licensed substance use disorder treatment programs, often called “methadone clinics,” although buprenorphine is also frequently prescribed), except for its use in outpatient management of chronic pain. Buprenorphine, a partial μ-opioid agonist, κ-opioid receptor antagonist, and nociceptin receptor agonist, can be prescribed sublingually in a monthly administered long-acting injection or as a subdermal implant lasting 6 months. This medication requires a practitioner Drug Enforcement Administration (DEA) waiver to prescribe or dispense and should be started when a patient is withdrawing to avoid precipitated withdrawal symptoms. It should be noted that any physician (eg, internists or psychiatrists) in the inpatient setting can prescribe methadone or buprenorphine to prevent withdrawal during an admission if the patient has it prescribed as an outpatient and this information is verified. The only barrier may be if the hospital requires the DEA buprenorphine waiver, though the waiver should not be required for the maintenance of outpatient medications. Ready access to naloxone (a pure opioid antagonist that can reverse opioid overdoses) is a key public health strategy to help control the opioid overdose epidemic.40 Naltrexone, a μ-opioid receptor antagonist, can be administered orally or in a monthly long-acting injection that can be prescribed by any practitioner without a waiver. To avoid precipitation of opioid withdrawal, individuals using short-acting opioids and long-acting opioids should abstain for 7–10 days and at least 10–14 days, respectively, before beginning naltrexone. Patients transitioning to naltrexone from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as 2 weeks. The naltrexone long-acting injection can be particularly helpful for those who are not interested in taking either methadone or buprenorphine.38 However, oral naltrexone tablets have not been shown to be more effective than placebo for opioid use disorder due to poor patient adherence.26
How Can Collaborative Care and Integrated Care Treat Opioid Use Disorder?
Collaborative care models feature close collaboration between mental health practitioners and medical/nursing providers, while integrated care systematically coordinates general and mental/substance use health care. These models emphasize increasing access to care for those suffering from opioid addictions. In 2003, an office-based opioid treatment (OBOT) program using a collaborative care model with nurse care managers was started at Boston Medical Center. The program successfully treated patients with opioid use disorder while making efficient use of physicians’ time while prescribing buprenorphine.44 The expansion of OBOT with buprenorphine to 14 statewide community mental health centers increased the overall annual admissions into these programs from 178 to 1,210 over a 5-year period, clearly demonstrating the increased access to care provided by collaborative care models for opioid use disorders.45 The Vermont Hub-and-Spoke Model of Care for Opioid Use Disorder led to substantial increases in the state’s opioid use disorder treatment capacity by creating an integrated health system utilizing hubs (eg, methadone clinics and complex addiction treatment centers) and spokes (eg, nurse-counselor teams with prescribing physicians, pain management clinics, medical homes, corrections, and in-person services).46 The collaborative opioid prescribing (CoOp) program model developed and implemented at Johns Hopkins Hospital (Baltimore, Maryland) incorporates specialized addiction treatment with comprehensive wrap-around services (such as case management, peer recovery, occupational therapy, vocational training, family engagement, and links to transitional and recovery housing).47,48
What Public Policy Interventions Can Help Address Opioid Use Disorder?
Historically, US policy has relegated substance misuse as a societal problem best addressed through punishment.49 Facing a growing opioid epidemic and recognizing the failure of punitive approaches to substance use disorder, Portugal developed a National Drug Strategy (Comissão para a Estratégia Nacional de Combate á Droga [1998]).50 In July 2001, Portugal decriminalized possession of all categories of illicit drugs for up to 10 days’ supply.51 The overarching principles of the public health policy include prevention, harm reduction, treatment, social reintegration, supply reduction, and channeling minor drug offenders into the drug treatment system. Portugal made the decision to invest in job programs for those recovering from substance use disorder instead of investing in incarceration. The results have included reductions in problematic use, drug-related harms, and criminal justice system overcrowding. Moreover, major increases in drug use have not materialized.51 In the United States, law enforcement is mostly opposed to decriminalization, despite its benefits.52 Morally, the general public sentiment is against decriminalization as well,53 which constitutes another example of systemic racism due to the more severe impact of the war on drugs on people of color.
Establishing access to treatment is paramount in our battle against the opioid crisis. Sadly, the opening of drug treatment facilities in the United States is often met with public outcries fueled by fears of increased crime and social nuisance. Prevailing attitudes about substance use disorder include intolerance, stigma, and moralistic condemnation.49 However, the sentiments driving the so-called “not in my backyard” syndrome are not supported by evidence.54
Harm-reduction strategies, such as needle exchange programs and supervised injection facilities (SIFs), may seem counterproductive by providing a convenient means for those who are intent on continuing their intravenous (IV) drug use. SIFs, also called safe injection spaces and drug consumption rooms, are legally sanctioned facilities that provide a hygienic space for drug users to inject (previously obtained) drugs under the supervision of medically trained staff.49 SIFs provide clean needles to reduce infections, immediate medical intervention to reduce overdose death/complications, and access to care. They serve as a stabilizing force for one of the most marginalized populations. There are at least 98 SIFs operating in 66 cities around the world in 10 countries (Switzerland, Germany, the Netherlands, Norway, Luxembourg, Spain, Denmark, Greece, Australia, and Canada).55 SIFs do not encourage additional drug use, and naive users are not initiated by presence of consumption rooms.56 SIFs provide enhanced opportunities for health care workers to connect with people who inject drugs and refer them into primary care and substance use disorder treatment.57 Consistent use of the SIF (Insite) in Vancouver, Canada has been associated with safer practices (eg, less reusing of syringes and outdoor injecting), which can lead to a lower rate of transmission of infectious diseases, such as HIV and hepatitis C,58 and improvement of public order by reduction of discarded syringes.59 SIFs are cost effective and lead to enormous life-years gained for those suffering from opioid use disorder.56
Currently, in the United States, SIFs are in violation of federal criminal laws (using narcotics, maintaining a premise for purpose of narcotics use under the Controlled Substance Act).49 Other criticisms of SIFs include that they are an affront to federal control, governments should not facilitate drug use, and supervised injection sites do nothing to deter drug use or help individuals with opioid use disorder.60 Evidence from existing SIFs is not consistent with these concerns.61
Are There Involuntary Commitment Statutes That Can Be Used to Treat People With Substance Use Disorders?
Thirty-five states and the District of Columbia have a statute that provides a legal means to compel individuals with substance use disorder into treatment through a form of civil commitment.62 Vermont’s law only applies to those with substance use disorders, while Rhode Island’s law applies to only those suffering from alcohol use disorder. Many of these statutes contain criteria such as dangerousness to oneself or others, grave disability, lack of decision-making capacity, inability to take care of basic needs, and loss of control/addiction.62 Involuntary treatment of people with substance use disorders involves a legal process that can be initiated by desperate family members or loved ones.63 Civil commitment orders are generally issued in a formal court proceeding, wherein a judge weighs the evidence whether life-threatening behaviors justify temporarily depriving an individual of their liberty.64 Arguments in support of this approach include opportunities to improve diagnostic clarity and select appropriate treatment options, enhance motivation, use diversion to mental health care as an alternative to the criminal justice system, and provide a “safety net” for families.63 Arguments against civil commitment for those with substance use disorder include the deleterious impact of coercion on the therapeutic alliance and patient self-esteem and the resulting deprivation of individual freedom and autonomy. A common criticism of these procedures is the perception that enforced treatment is at its core a mechanism of state control of deviance rather than medical care.
A recent review65 documented that Florida (known as the Marchman Act for involuntary treatment of individuals with addiction) and Massachusetts (covered by Section 35 of the Mental Health Code, Mass. Gen. L. ch. 123 Section 35) annually committed thousands of individuals to evaluation and treatment (9,000 and 4,500 on average, respectively). The Massachusetts involuntary addiction treatment protocol is controversial, insofar as individuals may not receive an appropriate standard of medical care following confinement.66 The state provides no medication-assisted treatment at its facility for men involuntarily committed to treatment for substance use disorder. After achieving abstinence and with it a significantly decreased opioid tolerance in a compulsory program, these patients are at risk for an inadvertent overdose following discharge. This has become especially dangerous since 2012, when the maximum period of commitment was extended to 90 days (Act of October 27, 2011, ch. 142, Section 18, 2011 Mass. Acts 830, 855).
Overall, civil commitment procedures are a poor conceptual fit for individuals with substance use disorder, constituting an awkward hybrid of the judicial/legal and medical models.63 There are also troubling concerns that such orders may serve manipulative or punitive motives by family members.
How Might the COVID-19 Pandemic Affect Individuals With Opioid Use Disorder?
The COVID-19 pandemic is fueling the next wave of the opioid crisis.67 Drug overdose deaths in the United States previously set a new record high in 2019 per the Centers for Disease Control (70,980 projected overdose deaths, with over 50% of these deaths involving fentanyl and other synthetic opioids),68 and increases in opioid deaths are trending (as of this writing) upward in 2020 during the COVID-19 pandemic in the United States. For example, in the state of Vermont, opioid overdose deaths were up 36% in July 2020 compared to July 2019.69 Vulnerable individuals become more vulnerable during a pandemic.67 Individuals with opioid use disorder are often alienated from traditional news sources and are less likely to learn about their risk of infection and best practices. They are more likely to use opioids alone (where another person is not around to administer naloxone to reverse the overdose) during the pandemic due to social distancing and lockdown measures.69 They often suffer from financial insecurity, live in shelters or prison/jail, and have medical comorbidities with reduced access to health care and are less able to follow pandemic-related guidelines. Moreover, individuals with opioid use disorder can be skeptical of authority (eg, due to previous interactions with law enforcement). Disruption of drug supply chains can paradoxically cause overdoses to increase as supplies go down.67 Drugs are harder to purchase in this climate, and people may substitute drugs with which they are less familiar, thereby increasing risks of adverse effects. There is less access to treatment programs, and residential programs have cut down their number of beds. In addition, there are no in-person groups, and video sessions are difficult for some if they do not have a cell phone or are homeless. Telepsychiatry appointments also may make it harder to detect intoxication (eg, harder to see pupil constriction due to opioid intoxication).
On a more positive note, the easing of restrictions by the Substance Abuse and Mental Health Services Administration and the DEA to decrease COVID-19 infection risk and spread have allowed health care providers unprecedented freedom to prescribe medications for substance use disorder treatment by way of telemedicine (eg, take-home 28-day doses of methadone, new buprenorphine prescriptions after an initial telephone call).70 The DEA leveraged the public health emergency exception to the Ryan Haight Online Pharmacy Consumer Protection Act (which restricts the prescribing of controlled substances via telehealth with certain exceptions), which has helped improve access.71 Several states currently have reciprocity so that physicians do not have to get licensed in other states to treat patients there (though this is unlikely to be permanent). Many hope that these streamlined changes can continue after the pandemic to increase access and ability to meet patients “where they are at.” The easing of these restrictions and limitations could become the “new normal” as the pandemic subsides.
Case Vignette Part 2
The symptom-triggered administration of buprenorphine did not appear to be adequately treating Mr A’s opioid withdrawal symptoms. The psychiatry consultation-liaison team was consulted and recommended an immediate order of buprenorphine 4 mg and an additional standing dose of buprenorphine 4 mg sublingual tablets 3 times a day to treat Mr A’s uncomfortable symptoms of opioid withdrawal. He received a total dose of 26 mg of buprenorphine over the next 24 hours and robustly responded to this intervention. He was much more comfortable and interactive with the interview on hospital day 2 and voiced concerns about becoming “addicted” to buprenorphine, and the medication was gradually tapered as per his request. He did not want to engage with outpatient substance use disorder services, stating that he knew how to manage on his own. His hospitalization lasted for 2 weeks despite resolution of his foot ulcer pain and successful treatment of his heart failure, as it was difficult for social work to find placement for him for subacute physical rehabilitation. Admission requests to multiple local skilled nursing facilities were rejected due to his treatment with buprenorphine (the facilities reported inability to prescribe this medication in the physical rehabilitation setting even on a short-term taper). Following the end of his buprenorphine taper, admission requests were again denied, as the facilities expressed concerns about Mr A’s history of psychoactive substance misuse and his potential influence on other patients. Fortunately, the social work department was eventually able to find a nursing home in which Mr A could temporarily stay and complete physical rehabilitation.
Summary
The current opioid epidemic is a public health emergency that has been associated with a variety of poor medical outcomes, including increased risk of mortality. Psychodynamic and socioeconomic factors can clearly play a contributory role in the development of opioid use disorders, along with genetic and neurobiological factors. Structural racism against people of color has preserved punitive approaches and hampered the efforts of public health approaches, and future programs for opioid use disorder should take steps to avoid reproducing racial stigma and criminalization.16 Policy efforts should specifically address racial/ethnic and economic differences in treatment access and engagement.22 We described medication-assisted treatments and public policy interventions (eg, harm-reduction strategies, drug court) that have been shown to be effective in treating opioid use disorder and relieve patient suffering. Involuntary commitment statutes, although widely used in the United States, are a poor conceptual fit for substance use disorder. Individuals with opioid use disorder are more vulnerable to complications from the COVID-19 pandemic; however, streamlined changes such as telemedicine medication-assisted treatment appointments and lessened prescribing restrictions have increased access to care. Improved access to treatment through health care policy and education is most essential in our battle against the opioid crisis.
Submitted: July 10, 2020; accepted October 2, 2020.
Published online: April 8, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Financial disclosure: Dr Stern is an employee of the Academy of Consultation-Liaison Psychiatry and has received royalties from Elsevier and the Massachusetts General Hospital Psychiatry Academy. Drs Rustad and Eth have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None.
Disclaimer: Drs Rustad and Eth are employed by the United States Department of Veterans Affairs, but the opinions expressed in this article do not reflect those of the Department of Veterans Affairs.
Previous presentation: The material in this article is based on a Geisel School of Medicine at Dartmouth/Dartmouth-Hitchcock Department of Psychiatry Virtual Grand Rounds entitled “Understanding and Undermining the Opioid Crisis: A Patient-Centered Approach” presented by Dr Rustad on May 19, 2020.
Acknowledgments: The authors thank Loretta Grikis, MLS, AHIP, clinical librarian, and Marina Trefethen, PharmD, BCPP, mental health clinical pharmacy specialist, for assistance with literature searches. Ms Grikis and Dr Trefethen have no conflicts of interest related to the subject of this article.
Additional information: The case vignette is a composite of several patient cases. All information has been de-identified to protect anonymity.
Clinical Points
- Knowledge about psychosocial determinants of opioid misuse (eg, structural racism, lack of economic opportunity, social isolation, and trauma) can help clinicians provide patient-centered care for opioid use disorder and facilitate recovery.
- Ready access to naloxone (a pure opioid antagonist that can reverse opioid overdoses) is a key public health strategy to help control the opioid overdose epidemic.
- Individuals with opioid use disorder are more vulnerable to complications from the COVID-19 pandemic; however, streamlined changes such as telemedicine medication-assisted treatment appointments and lessened prescribing restrictions have increased access to care.
References (71)

- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253–259. PubMed CrossRef
- Madras BK. The surge of opioid use, addiction, and overdoses: responsibility and response of the US health care system. JAMA Psychiatry. 2017;74(5):441–442. PubMed CrossRef
- Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182–186. PubMed CrossRef
- Pomerance B. Yet another war: Battling for reasoned responses for veterans amid the opioid crisis. 2018; 11 GOV’T L. REV. 147, 173.
- National Academies of Sciences, Engineering, and Medicine. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: National Academies Press; 2017.
- Garami J, Valikhani A, Parkes D, et al. Examining perceived stress, childhood trauma and interpersonal trauma in individuals with drug addiction. Psychol Rep. 2019;122(2):433–450. PubMed CrossRef
- Larance B, Gisev N, Cama E, et al. Predictors of transitions across stages of heroin use and dependence prior to treatment-seeking among people in treatment for opioid dependence. Drug Alcohol Depend. 2018;191:145–151. PubMed CrossRef
- Trenz RC, Scherer M, Harrell P, et al. Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addict Behav. 2012;37(4):367–372. PubMed CrossRef
- Khantzian EJ, Mack JE, Schatzberg AF. Heroin use as an attempt to cope: clinical observations. Am J Psychiatry. 1974;131(2):160–164. PubMed CrossRef
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142(11):1259–1264. PubMed CrossRef
- Fricchione G. The neurocircuitry of attachment and recovery in Alcoholics Anonymous. Alcohol Treat Q. 2014;32(2–3):173–193. CrossRef
- Hari J. Everything you think you know about addiction is wrong (Ted talk). YouTube. July 9, 2015. Accessed December 23, 2018. https://www.youtube.com/watch?v=PY9DcIMGxMs
- Hari J. Chasing the Scream: The First and Last Days of the War on Drugs. New York, London: Bloomsbury USA; 2015.
- Davis J. “Miles Davis: Birth of the Cool” Connects Jim Crow Oppression to Davis’ Heroin Addiction. The Fix. Published April 17, 2019. Accessed May 10, 2020. https://www.thefix.com/miles-davis-birth-cool-connects-jim-crow-oppression-davis-heroin-addiction
- Gibbons FX, Etcheverry PE, Stock ML, et al. Exploring the link between racial discrimination and substance use: what mediates? what buffers? J Pers Soc Psychol. 2010;99(5):785–801. PubMed CrossRef
- Kunins HV. Structural racism and the opioid overdose epidemic: the need for antiracist public health practice. J Public Health Manag Pract. 2020;26(3):201–205. PubMed CrossRef
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. PubMed CrossRef
- Alexander M. The New Jim Crow: Mass Incarceration in the Age of Colorblindness. New York, NY: New Press; 2010.
- Netherland J, Hansen H. White opioids: pharmaceutical race and the war on drugs that wasn’t. Biosocieties. 2017;12(2):217–238. PubMed CrossRef
- Netherland J, Hansen HB. The war on drugs that wasn’t: wasted whiteness, “dirty doctors,” and race in media coverage of prescription opioid misuse. Cult Med Psychiatry. 2016;40(4):664–686. PubMed CrossRef
- Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. Lancet. 2017;389(10077):1464–1474. PubMed CrossRef
- Lagisetty PA, Ross R, Bohnert A, et al. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76(9):979–981. PubMed CrossRef
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. PubMed CrossRef
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6(5):391–399. PubMed CrossRef
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59(1):29–53. PubMed CrossRef
- Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63. Publication No. PEP20-02-01-006. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2020.
- Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. PubMed CrossRef
- Eirich A, Biermann T, Müller CP, et al. Association of CamK2A genetic variants with transition time from occasional to regular heroin use in a sample of heroin-dependent individuals. Psychiatr Genet. 2019;29(1):18–25. PubMed CrossRef
- Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–979. PubMed CrossRef
- Hurd YL, O’Brien CP. Molecular genetics and new medication strategies for opioid addiction. Am J Psychiatry. 2018;175(10):935–942. PubMed CrossRef
- Alexander BK, Beyerstein BL, Hadaway PF, et al. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav. 1981;15(4):571–576. PubMed CrossRef
- Robins LN, Helzer JE, Davis DH. Narcotic use in southeast Asia and afterward: an interview study of 898 Vietnam returnees. Arch Gen Psychiatry. 1975;32(8):955–961. PubMed CrossRef
- Abraham A, Luty J. Testing for illicit drug use in mental health services. Adv Psychiatr Treat. 2010;16(5):369–379. CrossRef
- Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–737. PubMed CrossRef
- Calculating Total Daily Dose of Opioids for Safer Dosage. Centers for Disease Control and Prevention. Accessed June 15, 2020. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
- Habal R.. Heroin Toxicity. Emedicine. December 16, 2018. Accessed June 18, 2020. https://emedicine.medscape.com/article/166464-overview
- Cone EJ, Dickerson S, Paul BD, et al. Forensic drug testing for opiates, V: urine testing for heroin, morphine, and codeine with commercial opiate immunoassays. J Anal Toxicol. 1993;17(3):156–164. PubMed CrossRef
- Volkow ND, Jones EB, Einstein EB, et al. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216. PubMed CrossRef
- Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868–1883. PubMed CrossRef
- Olsen Y, Sharfstein JM. The Opioid Epidemic: What Everyone Needs to Know. New York, NY: Oxford University Press; 2019.
- Maiti T, Das S, Ramasamy A, et al. An overview on medication-assisted treatment (MAT) for opioid dependence. J Opioid Manag. 2020;16(2):141–149. PubMed CrossRef
- Smith L, Mosley J, Johnson J, et al. Probuphine (buprenorphine) subdermal implants for the treatment of opioid-dependent patients. P T. 2017;42(8):505–508. PubMed
- Dooley J, Gerber-Finn L, Antone I, et al. Buprenorphine-naloxone use in pregnancy for treatment of opioid dependence: retrospective cohort study of 30 patients. Can Fam Physician. 2016;62(4):e194–e200.
- Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171:425–431. PubMed CrossRef
- LaBelle CT, Han SC, Bergeron A, et al. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts Collaborative Care Model in community health centers. J Subst Abuse Treat. 2016;60:6–13. PubMed CrossRef
- Brooklyn JR, Sigmon SC. Vermont hub-and-spoke model of care for opioid use disorder: development, implementation, and impact. J Addict Med. 2017;11(4):286–292. PubMed CrossRef
- Stoller KB. A collaborative opioid prescribing (CoOP) model linking opioid treatment programs with office-based buprenorphine providers. Addict Sci Clin Pract. 2015;10(suppl 1):A63. CrossRef
- Stoller KB, Stephens MC, Schorr A. Integrated Service Delivery Models for Opioid Treatment Programs in an Era of Increasing Opioid Addiction, Health Reform, and Parity. American Association for the Treatment of Opioid Dependence. July 13, 2016. http://www.aatod.org/wp-content/uploads/2016/07/2nd-Whitepaper-.pdf
- Leary AA. A safe harbor in the opioid crisis: how the federal government should allow states to legislate for safe injection facilities in light of the opioid public health emergency. Brooklyn Law Review. 2019;84(2):19.
- Comissão para a Estratégia Nacional de Combate á Droga. Estratégia Nacional de Luta Contra a Droga: Relatório da Comissāo para a Estratégia Nacional de Combate á Droga. 1998. http://www.sicad.pt/BK/Publicacoes/Lists/SICAD_PUBLICACOES/Attachments/48/ENcomissao.pdf
- Hughes CE, Stevens A. What can we learn from the Portuguese decriminalization of illicit drugs? Br J Criminol. 2010;50(6):999–1022. CrossRef
- Petrocelli M, Oberweis T, Smith MR, et al. Assessing police attitudes toward drugs and drug enforcement. Am J Crim Justice. 2014;39(1):22–40. CrossRef
- Hammond AS, Dunn KE, Strain EC. Drug legalization and decriminalization beliefs among substance-using and nonusing individuals. J Addict Med. 2020;14(1):56–62. PubMed CrossRef
- Furr-Holden CD, Milam AJ, Nesoff ED, et al. Not in my back yard: a comparative analysis of crime around publicly funded drug treatment centers, liquor stores, convenience stores, and corner stores in one mid-Atlantic city. J Stud Alcohol Drugs. 2016;77(1):17–24. PubMed CrossRef
- Schatz E, Nougier M. IDPC Briefing Paper—Drug Consumption Rooms: Evidence and Practice. SSRN. June 22, 2012. Accessed March 10, 2021. https://ssrn.com/abstract=2184810
- Drug Consumption Rooms: An Overview Of Provision And Evidence. European Monitoring Centre For Drugs And Drug Addiction. June 7, 2018. https://www.emcdda.europa.eu/topics/pods/drug-consumption-rooms_en
- Broadhead RS, Kerr TH, Grund J-PC, et al. Safer injection facilities in North America: their place in public policy and health initiatives. J Drug Issues. 2002;32(1):329–355. CrossRef
- Stoltz JA, Wood E, Small W, et al. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health (Oxf). 2007;29(1):35–39. PubMed CrossRef
- Wood E, Tyndall MW, Montaner JS, et al. Summary of findings from the evaluation of a pilot medically supervised safer injecting facility. CMAJ. 2006;175(11):1399–1404. PubMed CrossRef
- Stueck W. The arguments for and against Vancouver’s supervised injection site. The Globe and Mail. May 11, 2011. Accessed May 17, 2020. https://www.theglobeandmail.com/news/british-columbia/the-arguments-for-and-against-vancouvers-supervised-injection-site/article596153/
- Barry CL, Sherman SG, Stone E, et al. Arguments supporting and opposing legalization of safe consumption sites in the US. Int J Drug Policy. 2019;63:18–22. PubMed CrossRef
- National Judicial Opioid Task Force. Involuntary Commitment and Guardianship Laws: Persons with a Substance Use Disorder. National Center for State Courts; 2019.
- Rustad JK, Junquera P, Chaves L, et al. Civil commitment among patients with alcohol and drug abuse: practical, conceptual and ethical issues. Addict Disord Their Treat. 2012;11(3):136–145. CrossRef
- Satel SL, Farabee DJ. The role of coercion in drug treatment. In: Lowinson JH, Ruiz P, Millman RB, et al, eds. Substance Abuse: A Comprehensive Textbook. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:690–706.
- Christopher PP, Pinals DA, Stayton T, et al. Nature and utilization of civil commitment for substance abuse in the United States. J Am Acad Psychiatry Law. 2015;43(3):313–320. PubMed
- Bhalla IP, Cohen N, Haupt CE, et al. The role of civil commitment in the opioid crisis. J Law Med Ethics. 2018;46(2):343–350. PubMed CrossRef
- Parsley L. The pandemic may fuel the next wave of the opioid crisis. National Geographic. 2020. Obtained May 17, 2020. https://www.nationalgeographic.com/science/2020/04/coronavirus-pandemic-may-fuel-the-next-wave-of-the-opioid-crisis/
- Erdman SL. Drug overdose deaths jump in 2019 to nearly 71,000, a record high, CDC says. Cable News Network (CNN). July 16, 2020. Accessed September 22, 2020. https://www.cnn.com/2020/07/15/health/drug-overdose-deaths-2019/index.html
- Brouwer D. As Vermont Battled the Pandemic, Its Opioid Epidemic Worsened. Seven Days. September 16, 2020. Obtained September 22, 2020. https://www.sevendaysvt.com/vermont/as-vermont-battled-the-pandemic-its-opioid-epidemic-worsened/Content?oid=31226809
- 70. Facher L. COVID-19 has streamlined addiction medicine. Will the changes stick? Stat News. May 12, 2020. Accessed May 17, 2020. https://www.statnews.com/2020/05/12/coronavirus-addiction-medicine-reforms-future/
- Whaibeh E, Mahmoud H, Naal H. Telemental health in the context of a pandemic: the COVID-19 experience. Curr Treat Options Psychiatry. 2020;7(2):1–5. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite