Objective: To compare patient characteristics, medical comorbidities, health care utilization, and health care costs among patients with bipolar disorder who initiated lurasidone versus other atypical antipsychotics in usual clinical practice.
Methods: A retrospective analysis of administrative claims data was conducted using the US Optum Research Database (December 30, 2012, through February 27, 2014). Adult, commercially insured patients with bipolar disorder with an atypical antipsychotic prescription between June 28, 2013, and November 30, 2013, were included. The lurasidone cohort first included any patients with a lurasidone prescription; remaining patients were assigned to their first atypical antipsychotic (aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone). Preindex patient characteristics comparisons to lurasidone were conducted with t tests (continuous variables) and χ2 or Fisher exact tests (categorical variables).
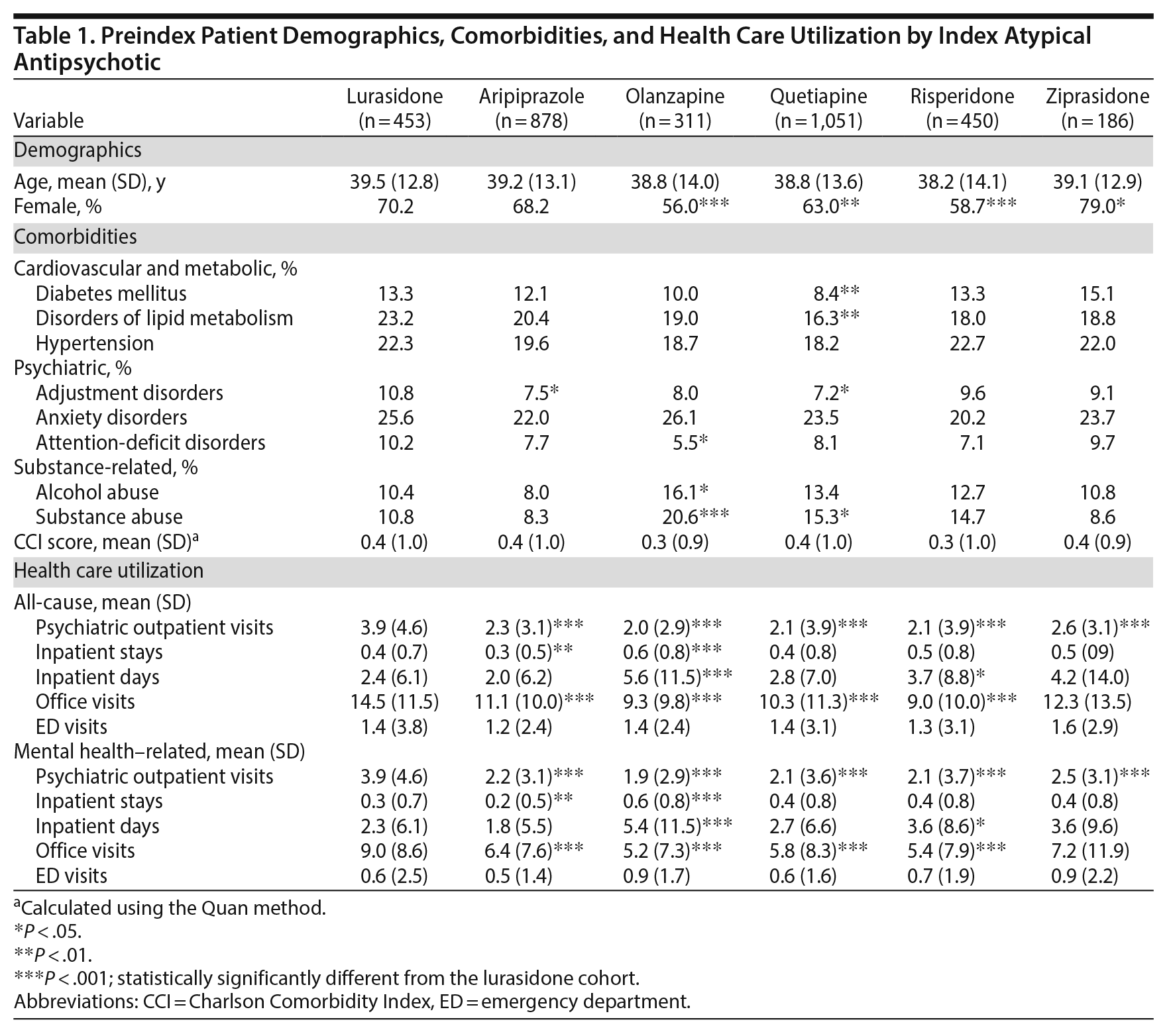
Results: A total of 3,329 patients were included in this database analysis. A higher percentage of the lurasidone cohort (31.1%) had bipolar depression compared with the other cohorts (23.5%−28.0%). The lurasidone cohort had a statistically significantly higher percentage of patients with prior diabetes mellitus (13.3%) and lipid metabolism disorders (23.2%) than did the quetiapine cohort (8.4% and 16.3%, P < .01). In addition, the lurasidone cohort had significantly more prior antipsychotic polypharmacy (23.0% vs 6.7%−12.9%, P < .01) and atypical antipsychotic use (55.6% vs 11.8%−26.3%, P < .01) than other cohorts. The lurasidone cohort had a statistically significantly higher mean number of prior all-cause and mental health office visits (P < .001) and higher mean prior pharmacy costs than most cohorts (P < .01).
Conclusions: Lurasidone-treated patients with bipolar disorder tended to have a more complex clinical profile, comorbidities, and prior treatment history compared to patients initiated with other atypical antipsychotics in this claims database study. This pattern of treatment may have reflected the overall clinical profile of lurasidone, the role perceived for lurasidone in the therapeutic armamentarium by practitioners, and the recent introduction of lurasidone into clinical practice during the study period.
Objective: To compare patient characteristics, medical comorbidities, health care utilization, and health care costs among patients with bipolar disorder who initiated lurasidone versus other atypical antipsychotics in usual clinical practice.
Methods: A retrospective analysis of administrative claims data was conducted using the US Optum Research Database (December 30, 2012, through February 27, 2014). Adult, commercially insured patients with bipolar disorder with an atypical antipsychotic prescription between June 28, 2013, and November 30, 2013, were included. The lurasidone cohort first included any patients with a lurasidone prescription; remaining patients were assigned to their first atypical antipsychotic (aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone). Preindex patient characteristics comparisons to lurasidone were conducted with t tests (continuous variables) and χ2 or Fisher exact tests (categorical variables).
Results: A total of 3,329 patients were included in this database analysis. A higher percentage of the lurasidone cohort (31.1%) had bipolar depression compared with the other cohorts (23.5%−28.0%). The lurasidone cohort had a statistically significantly higher percentage of patients with prior diabetes mellitus (13.3%) and lipid metabolism disorders (23.2%) than did the quetiapine cohort (8.4% and 16.3%, P < .01). In addition, the lurasidone cohort had significantly more prior antipsychotic polypharmacy (23.0% vs 6.7%−12.9%, P < .01) and atypical antipsychotic use (55.6% vs 11.8%−26.3%, P < .01) than other cohorts. The lurasidone cohort had a statistically significantly higher mean number of prior all-cause and mental health office visits (P < .001) and higher mean prior pharmacy costs than most cohorts (P < .01).
Conclusions: Lurasidone-treated patients with bipolar disorder tended to have a more complex clinical profile, comorbidities, and prior treatment history compared to patients initiated with other atypical antipsychotics in this claims database study. This pattern of treatment may have reflected the overall clinical profile of lurasidone, the role perceived for lurasidone in the therapeutic armamentarium by practitioners, and the recent introduction of lurasidone into clinical practice during the study period.
Prim Care Companion CNS Disord 2017;19(3):16m02066
https://doi.org/10.4088/PCC.16m02066
© Copyright 2017 Physicians Postgraduate Press, Inc.
aThe University of New Mexico, Department of Psychiatry and Behavioral Sciences, Albuquerque, New Mexico
bSunovion Pharmaceuticals Inc, Marlborough, Massachusetts
cOptum, Eden Prairie, Minnesota
dVertex Pharmaceuticals Inc, Boston, Massachusetts
eSunovion Pharmaceuticals Inc, Fort Lee, New Jersey
*Corresponding author: Daisy Ng-Mak, PhD, 84 Waterford Dr, Marlborough, MA 01752 ([email protected]).
Bipolar disorder is a persistent, serious psychiatric condition with a 2% to 4% lifetime prevalence among adults in the United States.1 Major depressive episodes associated with bipolar disorder constitute the most common syndromic state of bipolar disorder, imposing a large illness burden.2-4 The consequences of bipolar disorder include substantial direct and indirect cost burden on patients, families, the health care system, and society.4-7 Overall, the total economic burden associated with bipolar disorder in 2009 in the United States was estimated at $151 billion, with indirect costs of $120.3 billion and direct treatment costs of $30.7 billion.8
Bipolar disorder is known to be associated with a high prevalence of lifetime comorbidity with other psychiatric disorders.1 Individuals with bipolar disorder have nearly 5 times the age-, race-, and sex-adjusted risk of cardiovascular diseases9 and are significantly more likely to have comorbid cardiometabolic conditions than the general population.5 A meta-analysis9 of clinical trials found that almost all antipsychotics are associated with weight gain and increased body mass index after prolonged exposure. The metabolic problems associated with antipsychotic use may be further exacerbated by lower physical activity levels among individuals with bipolar disorder than healthy individuals,10 especially during periods of depression.11 In a retrospective hospital database analysis12 of 124,803 inpatients with bipolar disorder, 27% had 1 metabolic comorbidity, 17% had 2, and 17% had 3 or more. Each additional cardiovascular or metabolic comorbidity was estimated to increase patients’ risk of 30-day readmission by 6.4%.13
In addition to differences in efficacy profile, medications used for the treatment of bipolar disorder have varying safety profiles, including risk for weight gain and metabolic disturbance. In terms of atypical antipsychotics, the 2013 Canadian Network for Mood and Anxiety Treatments and International Society for Bipolar Disorder treatment guidelines for bipolar disorder14 recommend aripiprazole, asenapine, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone or combination therapy of an atypical antipsychotic (aripiprazole, asenapine, olanzapine, quetiapine, or risperidone) and a mood stabilizer (lithium or divalproex) for bipolar patients with manic episodes. For bipolar patients with an acute depressive episode, the only atypical antipsychotics recommended for first-line treatment are quetiapine and olanzapine plus a selective serotonin reuptake inhibitor, while lurasidone or lurasidone and a mood stabilizer (lithium or divalproex) are recommended as second-line treatment.14 Aripiprazole and ziprasidone are explicitly not recommended for treatment of bipolar depression.14 More recently, Florida’s 2015 Medicaid best-practice guidelines15 recommended lurasidone or quetiapine monotherapy as first-line bipolar depression treatment and cited lurasidone as having a more favorable metabolic profile than quetiapine.
Currently, the US Food and Drug Administration (FDA)-approved treatments for acute bipolar depression treatment are olanzapine-fluoxetine combination, quetiapine (immediate and extended release), and lurasidone (monotherapy and adjunctive therapy with lithium or valproate).16 Of these, lurasidone is the only atypical antipsychotic approved (in June 2013) both as monotherapy and as adjunctive therapy for treatment of adults with major depressive episodes associated with bipolar I disorder.17 A previous real-world study18 described lurasidone dosage and associated adherence patterns in bipolar disorder patients initiated on lurasidone. However, patient characteristics, health care resource utilization, and cost burden associated with use of lurasidone versus other atypical antipsychotics in patients with bipolar disorder have not been previously examined. The aim of this study was to describe and compare background characteristics, comorbidities, prior health care utilization, and costs for patients with bipolar disorder who initiated lurasidone versus other atypical antipsychotics in usual clinical practice.
METHODS
Study Design and Database
This retrospective cohort study used administrative claims data from December 30, 2012, through February 27, 2014, from the Optum Research Database (ORD) (Optum, Eden Prairie, Minnesota). The ORD is a proprietary research database with claims on over 150 million unique individuals and contains medical and pharmacy claims data linked to enrollment information from a large, US health plan. The use of claims data allowed for noninterfering observation of typical clinical practice. This study did not require institutional review board waiver or approval, because no identifiable health information protected by the Health Insurance Portability and Accountability Act of 199619 was accessed or extracted.

- In this claims database analysis, the proportion of patients with bipolar depression treated with lurasidone was higher than for other atypical antipsychotic agents, consistent with its approved US Food and Drug Administration indication for this population.
- In this study, lurasidone-treated patients tended to have a more complex clinical profile, medical comorbidities, and prior treatment history compared to patients initiated with other atypical antipsychotics, suggesting that lurasidone was utilized in a more difficult-to-treat bipolar disorder patient population.
- These findings may reflect the overall clinical profile of lurasidone, the role perceived for lurasidone in the therapeutic armamentarium by practitioners, and the recent introduction of lurasidone into clinical practice during the study period.
Inclusion/Exclusion Criteria
Patients included in this study were commercial health plan members at least 18 years old with at least 1 prescription claim for an atypical antipsychotic (asenapine, aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, olanzapine/fluoxetine, paliperidone, quetiapine, risperidone, or ziprasidone) during the identification period (June 28, 2013, through November 30, 2013). The identification period for this study was selected beginning with the date that lurasidone was approved for the treatment of bipolar depression (June 2013) and ending with the last available complete pharmacy claims data in the claims database (February 2014) at the time of data extraction. All patients with a claim for lurasidone were assigned to the lurasidone cohort with the date of the first lurasidone claim as the index date. The remaining non-lurasidone treated patients were then assigned to other cohorts based on the first observed atypical antipsychotic claim, with the date of the first claim as the index date and the medication filled as the index atypical antipsychotic.
Eligible patients were also required to have (1) continuous health plan enrollment for at least 6 months preindex and 3 months postindex, (2) a single claim for an atypical antipsychotic on the index date, (3) newly initiated the index atypical antipsychotic, (4) evidence of bipolar disorder diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 296.0x-296.1x, 296.4x-296.81, 296.89) during the preindex period or on the index date, and (5) no diagnoses for schizophrenia (ICD-9-CM code: 295.xx) during the preindex period or on the index date. Patients were excluded if they had missing sex or geographic region information or had index claims for asenapine, clozapine, iloperidone, olanzapine/fluoxetine, and paliperidone (due to the small sample sizes in these cohorts).
Bipolar Disorder Episode Type
Patients were assigned to 1 bipolar disorder episode type based on diagnostic codes during the preindex period or on the index date. The bipolar diagnosis code on the claim on, or closest to, the index date was used to identify the bipolar disorder episode type. The categories were bipolar depression (ICD-9-CM code: 296.5x), bipolar mania (ICD-9-CM codes: 296.0X, 296.1X, 296.4X, 296.81), bipolar mixed (ICD-9-CM code: 296.6x), bipolar unspecified (ICD-9-CM codes: 296.7, 296.80), and bipolar other (ICD-9-CM code: 296.89). In the case of multiple distinct bipolar disorder diagnoses, the current episode type was classified based on the following prioritization: depression, mania, mixed, unspecified, and other.
Comorbidities and Substance Abuse
During the preindex period, the Charlson Comorbidity Index score was calculated from medical claims data.20-22 Prior mental health comorbidities, cardiovascular and metabolic comorbidities, and substance abuse were obtained from medical claims data. Prior mental health comorbidities (adjustment disorders, anxiety disorders, and attention-deficit disorders) were identified as 2 or more claims with the respective diagnoses at least 14 days apart (ICD-9-CM codes in Supplementary Table 1). Prior cardiovascular and metabolic comorbidities of hypertension, disorders of lipid metabolism, and diabetes were identified among patients using the Clinical Classification Software from the Agency for Healthcare Research and Quality.23 Substance abuse was coded if the patient had a claim with a code for substance or alcohol abuse (Supplementary Table 1).
Medication Prescription Patterns
Medication prescription patterns were identified based on pharmacy claims. The index atypical antipsychotic therapy was categorized as monotherapy, adjunctive therapy, or atypical antipsychotic polypharmacy. Polypharmacy was coded for patients who did not meet adjunctive therapy criteria and had at least 1 claim for any other atypical antipsychotic within the first 60 postindex days. Adjunctive therapy was categorized if patients did not meet polypharmacy criteria but met all of the following criteria: ≥ 1 claim for lithium or valproate (valproic acid) within 30 days before the index date, ≥ 1 claim for lithium or valproate for at least the first 14 days of the postindex period, and ≥ 1 claim for lithium or valproate 15 to 60 days after the index date. All other patients were considered to be receiving atypical antipsychotic monotherapy.
Prior atypical antipsychotic use was defined as 1 or more preindex period claim for any nonindex atypical antipsychotic. The number of distinct prior atypical antipsychotics was calculated based on patients having at least 1 claim. Patients with at least 1 preindex claim for a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor (medication class definitions in Supplementary Table 2) were identified as antidepressant users.
Health Care Utilization and Costs
Health care utilization for office visits, inpatient stays (and days), emergency room visits, and outpatient visits with psychiatrists prior to atypical antipsychotic initiation were identified and counted. Mental health-related health care utilization and costs were measured from claims with ICD-9-CM codes 290.XX-319, and mental health pharmacy claims were those for psychotropic medications (Supplementary Table 1). All-cause health care utilization refers to care that was provided for any reason, including both mental health and non-mental health medical care. Health care costs reflect the combined health plan and patient-paid amounts. Costs were inflation-adjusted to 2013 US dollars using the medical care component of the Consumer Price Index from the US Bureau of Labor Statistics.24
Statistical Methods
Descriptive statistics included counts and percentages for dichotomous and categorical variables, means and standard deviations for continuous variables, and 99% confidence intervals (CIs). Results were stratified by index atypical antipsychotic, and comparisons were made between the lurasidone cohort and each other cohort. Statistical significance testing between the lurasidone cohort and comparator cohorts was conducted using 2-tailed Student t test for continuous variables (with a Sattherthwaite adjustment for unequal variances when needed). A χ2 test or Fisher exact test (for low cell counts) was performed for categorical variables. To account for large sample sizes and the number of comparisons, differences between the lurasidone cohort and other comparator cohorts were considered statistically significant with P < .01 (α = 0.05/5 sets of comparison = 0.01). SAS 9.2 (SAS Institute, Cary, North Carolina) was used for all analyses.
RESULTS
Patients
The database contained information on 73,565 patients with 1 or more atypical antipsychotic prescription claims during the identification period. Inclusion and exclusion criteria identified 3,459 patients. Patients treated with asenapine, clozapine, iloperidone, olanzapine/fluoxetine, or paliperidone were excluded because of the small cohort size (n = 130). The final index atypical antipsychotic cohorts who met the criteria included 3,329 patients: lurasidone (n = 453, 13.6%), aripiprazole (n = 878, 26.4%), olanzapine (n = 311, 9.3%), quetiapine (n = 1,051, 31.6%), risperidone (n = 450, 13.5%), and ziprasidone (n = 186, 5.6%). The patient flow through selection criteria can be seen in Figure 1.
Preindex patient characteristics by cohort are provided in Table 1. The lurasidone cohort had a significantly higher percentage of females in comparison to the olanzapine, quetiapine, and risperidone cohorts (P < .01). Although the Charlson Comorbidity Index score was similar across the cohorts, patients in the lurasidone cohort appeared to have a higher prevalence of several psychiatric and cardiovascular and metabolic comorbidities (Table 1), but most were not statistically significant. A statistically significantly higher proportion of lurasidone cohort patients had prior diagnoses of diabetes mellitus (13.3% vs 8.4%) and disorders of lipid metabolism (23.2% vs 16.3%) compared to the quetiapine cohort (P < .01). Prior substance abuse was significantly less likely in lurasidone (10.8%) than in olanzapine (20.6%) patients (P < .001).
Bipolar Disorder Episode Type
As seen in Figure 2, the lurasidone cohort had a significantly different distribution of bipolar episode type than most other index atypical antipsychotic cohorts (P < .01). Depression (31.1%) and mixed (16.1%) episodes appeared more common in the lurasidone cohort compared to the olanzapine (26.7% and 13.5%) and quetiapine (23.5% and 11.6%) cohorts, while bipolar mania appeared less common in the lurasidone cohort (13.3%) than the olanzapine (23.8%) and risperidone (20.7%) cohorts.
Medication Prescription Patterns
During the preindex period, the lurasidone cohort (52.8%) was significantly more likely to receive an antidepressant compared to the quetiapine (44.9%, P < .01) and risperidone (42.2%, P < .01) cohorts but not the aripiprazole (49.3%, P = .23), olanzapine (43.4%, P = .011), or ziprasidone (48.9%, P = .38) cohorts. Adjunctive treatment with lithium or valproate was rare among all cohorts and ranged from 5.0% for both quetiapine and aripiprazole to 7.7% for olanzapine cohorts, respectively. Conversely, polypharmacy occurred statistically significantly more often in the lurasidone cohort (23.0%) versus all other cohorts (6.7%−12.9%, P < .01).
Prior to the index date, more than half of the lurasidone cohort (55.6%) had pharmacy claims for 1 or more atypical antipsychotics, and 16.6% had claims for at least 2 antipsychotics. In all other cohorts, 26.3% or less of patients had claims for preindex atypical antipsychotics, and most only had a claim for a single atypical antipsychotic (Figure 3). These findings do not indicate whether or not prior antipsychotics were used simultaneously or sequentially.
Health Care Utilization and Costs
The prior health care resource use of each atypical antipsychotic cohort is reported in Table 1. With the exception of the ziprasidone cohort, the mean number of all-cause office visits was significantly higher for the lurasidone cohort compared to all other cohorts (14.5 vs 9.0−11.1, P < .001). The lurasidone cohort also had a notably higher mean number of outpatient visits with a psychiatrist than all other cohorts (3.9 vs 2.0−2.6).
Figure 4 shows prior all-cause and mental health-related health care costs for each atypical antipsychotic. Mean all-cause total health care costs were similar across all cohorts (ranging from $9,698 to $14,877); olanzapine had the highest costs. The lurasidone cohort had a lower mean all-cause inpatient cost than the olanzapine cohort ($3,475 vs $7,671, P < .01) and numerically lower all-cause emergency department cost ($669 vs $916−$1,108) than all other cohorts except aripiprazole. The lurasidone cohort also had lower mean mental health-related overall ($7,494 vs $11,060), inpatient ($3,229 vs $7,075), and emergency department ($336 vs $659) costs than the olanzapine cohort. The mean prior mental health-related pharmacy and office visit costs among the lurasidone cohort were statistically significantly higher than those of all other cohorts.
DISCUSSION
In this retrospective claims study, patients with bipolar disorder who were treated with lurasidone had the highest observed rates of prior cardiovascular and metabolic risk factors compared to other atypical antipsychotic cohorts. Relative to patients treated with quetiapine, lurasidone-treated patients had statistically significantly higher preindex rates of diabetes (13.3% vs 8.4%, P < .01) and disorders of lipid metabolism (23.2% vs 16.3%, P < .01). In addition, patients initiating lurasidone were more likely (31.1%) to have bipolar depression than both quetiapine (23.5%) and olanzapine (26.7%) cohorts. When compared to other atypical antipsychotic cohorts (prior to or at the time of atypical antipsychotic initiation), patients initiating lurasidone had significantly more antipsychotic polypharmacy, a higher mean number of all-cause and mental health-related office visits, and greater mean pharmacy costs at index. Mean preindex all-cause health care costs were similar across all cohorts.
Reasons for the higher rate of prior cardiovascular and metabolic comorbidities reported among the lurasidone-treated patients compared to patients treated with other atypical antipsychotics are unclear. However, one plausible explanation is the possibility that physicians may consider lurasidone to be a reasonable treatment option for those with cardiovascular and metabolic comorbidities given its potentially favorable metabolic tolerability profile.25-27 Lurasidone has been suggested as an alternative for patients experiencing clinically relevant weight gain on other atypical antipsychotic therapies.28 Notably, despite the known metabolic burden associated with olanzapine and quetiapine treatment,29 8.4% and 10.0% of patients with diabetes were initiated on treatment with quetiapine and olanzapine, respectively.
The bipolar disorder episode type distribution was significantly different between the lurasidone cohort and the other antipsychotics in a manner that appeared consistent with the FDA-approved indications—the cohort prescribed lurasidone, which is indicated for bipolar depression but not bipolar mania, had the highest percentage of patients classified as bipolar depression and lowest percentage classified as bipolar mania. Bipolar depression is generally more difficult to treat than other types of bipolar episodes,30 and recent treatment guidelines15 recommend lurasidone monotherapy as a first-line treatment for bipolar depression. Consistent with the underlying randomized clinical trials, a recent network meta-analysis31 found that ziprasidone and aripiprazole are not effective for treating patients with bipolar depression. Patients treated with lurasidone were also significantly more likely than those treated with quetiapine or risperidone to have had prior antidepressant treatment, which may be another indicator for bipolar depression but also could be a marker for patients with bipolar disorder that was previously diagnosed and treated as unipolar depression.32
Approximately 20% of patients in this study had evidence of prior atypical antipsychotic use, including some who had received multiple antipsychotics. Patients in the lurasidone cohort used more atypical antipsychotics during the preindex period than other cohorts. Prior antipsychotic use was also found to be associated with the likelihood of using higher doses of lurasidone according to a recent claims database study.18 Adherence to atypical antipsychotics is often poor, and discontinuation and switching are relatively common in both bipolar and schizophrenic patients,33,34 often because of adverse events.34 Prior antipsychotic use may also be a disease severity marker and, as the most recently introduced atypical antipsychotic in this study, lurasidone may have been reserved for nonresponders to other atypical antipsychotics.35 In addition, due to formulary restrictions, the use of lurasidone may have been limited in some cases to patients who had failed other medications.
Notably, the adjunctive use of lithium or valproate in patients with bipolar disorder was low in all cohorts (5.0%-7.7%). In contrast, adjunctive mood stabilizer use was previously reported among 47% of patients with bipolar disorder treated with atypical antipsychotics.36 The lower rates of adjunctive lithium and valproate use reported here may have been in part due to the stringent operational definition of adjunctive therapy used in this study: all patients were required to be using lithium or valproate before, during, and after the date atypical treatment was initiated.
The current study supports and extends the findings of a prior commercial claims database analysis of patients with bipolar disorder initiated on lurasidone.18 Taken together with the prior study, our findings suggest that patients initiating lurasidone may have a more complex clinical profile as it relates to the presence of medical comorbidities, concomitant medication use, and health resource utilization as compared with patients initiating treatment with other oral antipsychotics.
Limitations
The results of this study must be interpreted with appropriate consideration of limitations of retrospective database analyses. Claims data are collected primarily for reimbursement purposes, not research, and are subject to coding errors. The presence of a claim for a filled prescription does not indicate appropriate medication adherence. We did not identify the pattern of use of antipsychotics among pregnant women. Given the source of the claims database used, the ability to generalize the observed results may be limited to patients covered by commercial insurance. The commercial insurance sample is less likely to include individuals who meet age or disability requirements for Medicare and less likely to include individuals who meet income requirements for Medicaid. The database used in this analysis did not contain direct information regarding the reasoning for the choice of atypical antipsychotic or the circumstances surrounding the discontinuation of previous therapies. In an attempt to maximize the number of patients observable in the lurasidone cohort, all patients using lurasidone were identified first, and then the remaining patients were assigned to the other cohorts based on their index atypical antipsychotic. Some differences observed in prior atypical antipsychotic utilization between the lurasidone and other cohorts may be at least partially a function of the hierarchical assignment. As in any noncontrolled pharmacologic study, associations do not indicate causality.37
CONCLUSIONS
In this claims database study, patients treated with lurasidone were more likely to have bipolar depression compared with other atypical antipsychotic-treated patients, consistent with lurasidone’s FDA-approved indications and newer treatment guidelines. Lurasidone-treated patients with bipolar disorder tended to have a more complex clinical profile, medical comorbidities, and prior treatment history compared to patients initiated with other atypical antipsychotics. This pattern of treatment may have reflected the overall clinical profile of lurasidone, the role perceived for lurasidone in the therapeutic armamentarium by practitioners, and the recent introduction of lurasidone into clinical practice during the study period. Investigation of patterns of use of lurasidone and other atypical antipsychotics can provide insight and understanding of how these medications are utilized in real-world settings.
Submitted: October 27, 2016; accepted April 5, 2017.
Published online: June 1, 2017.
Author contributions: Dr Halpern directed the data analysis. All authors were involved in the study conception and design, interpretation of results, drafting of the manuscript, and critical revision of the manuscript. All authors approved the final version of the manuscript for submission to the Primary Care Companion for CNS Disorders.
Potential conflicts of interest: Dr Tohen was a full-time employee at Lilly (1997-2008). He has received honoraria from or consulted for Abbott, Actavis, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Sunovion, Forest, Gedeon Richter, Roche, Elan, Alkermes, Allergan, Lundbeck, Teva, Pamlab, Wyeth, and Wiley Publishing. His spouse was a full time employee at Lilly (1998-2013). Drs Ng-Mak, Rajagopalan, and Loebel are employees of Sunovion Pharmaceuticals, Inc. Dr Halpern is an employee of Optum. Dr Chuang was an employee of Sunovion Pharmaceuticals, Inc at the time of the study.
Funding/support: This research was funded by Sunovion Pharmaceuticals, Inc.
Role of the sponsor: The authors who were employees of Sunovion Pharmaceuticals Inc were involved in the final decision to publish study results, and the sponsor reviewed the manuscript prior to submission, but publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Acknowledgment: Michael D. Stensland, PhD, of Agile Outcomes Research Inc, Rochester, Minnesota, provided technical writing support, which was funded by Sunovion Pharmaceuticals Inc. Dr Stensland reports no other conflicts of interest related to the subject of this article.
Additional information: The data for this article are from the Optum Research Database (ORD; Optum, Eden Prairie, Minnesota). The ORD is a proprietary research database with claims data for over 150 million unique individuals and contains medical and pharmacy claims data linked to enrollment information from a large US health insurer. Further information about the data set can be requested through Dr Ng-Mak.
Supplementary material: See accompanying pages.
REFERENCES
1. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552. PubMed doi:10.1001/archpsyc.64.5.543
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. PubMed doi:10.1001/archpsyc.59.6.530
3. Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531-535. PubMed doi:10.1111/j.1399-5618.2007.00467.x
4. Calabrese JR, Hirschfeld RMA, Frye MA, et al. Impact of depressive symptoms compared with manic symptoms in bipolar disorder: results of a US community-based sample. J Clin Psychiatry. 2004;65(11):1499-1504. PubMed doi:10.4088/JCP.v65n1109
5. Fagiolini A, Forgione R, Maccari M, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord. 2013;148(2-3):161-169. PubMed doi:10.1016/j.jad.2013.02.001
6. Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007;9(1-2):183-196. PubMed doi:10.1111/j.1399-5618.2007.00430.x
7. Kleine-Budde K, Touil E, Moock J, et al. Cost of illness for bipolar disorder: a systematic review of the economic burden. Bipolar Disord. 2014;16(4):337-353. PubMed doi:10.1111/bdi.12165
8. Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. J Affect Disord. 2011;129(1-3):79-83. PubMed doi:10.1016/j.jad.2010.08.030
9. Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. PubMed doi:10.1371/journal.pone.0094112
10. Janney CA, Fagiolini A, Swartz HA, et al. Are adults with bipolar disorder active? objectively measured physical activity and sedentary behavior using accelerometry. J Affect Disord. 2014;152-154:498-504. PubMed doi:10.1016/j.jad.2013.09.009
11. Sylvia LG, Friedman ES, Kocsis JH, et al. Association of exercise with quality of life and mood symptoms in a comparative effectiveness study of bipolar disorder. J Affect Disord. 2013;151(2):722-727. PubMed doi:10.1016/j.jad.2013.07.031
12. Correll CU, Ng-Mak DS, Stafkey-Mailey D, et al. Cardiometabolic comorbidities, readmission, and costs in schizophrenia and bipolar disorder: a real-world analysis. Ann Gen Psychiatry. 2017;16:9. PubMed doi:10.1186/s12991-017-0133-7
13. Hassan K, Rajagodpalan D, Stafkey-Mailey D, et al. Impact of metabolic comorbidities on inpatient cost and rehospitalization rates for patients diagnosed with schizophrenia. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2013;16(3):A59. doi:10.1016/j.jval.2013.03.1570
14. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) Collaborative Update of CANMAT Guidelines for the Management of Patients With Bipolar Disorder: Update 2013. Bipolar Disord. 2013;15(1):1-44. PubMed doi:10.1111/bdi.12025
15. 2015 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. Florida Agency for Health Care Administration. The University of South Florida, Florida Medicaid Drug Therapy Management Program for Behavioral Health website. http://www.medicaidmentalhealth.org. Accessed May 2, 2017.
16. Ketter TA, Miller S, Dell’ Osso B, et al. Balancing benefits and harms of treatments for acute bipolar depression. J Affect Disord. 2014;169(suppl 1):S24-S33. PubMed doi:10.1016/S0165-0327(14)70006-0
17. Franklin R, Zorowitz S, Corse AK, et al. Lurasidone for the treatment of bipolar depression: an evidence-based review. Neuropsychiatr Dis Treat. 2015;11:2143-2152. PubMed
18. Sajatovic M, Ng-Mak D, Solem CT, et al. Dosing patterns and medication adherence in bipolar disorder patients treated with lurasidone: a US retrospective claims database analysis. Ther Adv Psychopharmacol. 2016;6(6):355-368. PubMed doi:10.1177/2045125316672135
19. United States Congress. Patient Protection and Affordable Care Act. Sect. 3025, 111-148. Government Publishing Office website. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. March 23, 2010.
20. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. PubMed doi:10.1093/aje/kwq433
21. Bayliss EA, Ellis JL, Shoup JA, et al. Association of patient-centered outcomes with patient-reported and ICD-9-based morbidity measures. Ann Fam Med. 2012;10(2):126-133. PubMed doi:10.1370/afm.1364
22. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed doi:10.1097/01.mlr.0000182534.19832.83
23. Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS). Rockville, MD: US Agency for Healthcare Research and Quality; 2014.
24. US Bureau of Labor Statistics. Measuring price change for medical care in the CPI. Bureau of Labor Statistics website. http://www.bls.gov/cpi/cpifact4.htm#1a. Accessed March 27, 2016.
25. De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. PubMed doi:10.1038/nrendo.2011.156
26. Baker RA, Pikalov A, Tran Q-V, et al. Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull. 2009;42(1):11-31. PubMed
27. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233. PubMed doi:10.1016/j.schres.2010.07.012
28. Kemp DE. Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 2014;169(suppl 1):S34-S44. PubMed doi:10.1016/S0165-0327(14)70007-2
29. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339-347. PubMed doi:10.1002/wps.20252
30. Hirschfeld RM. Bipolar depression: the real challenge. Eur Neuropsychopharmacol. 2004;14(suppl 2):S83-S88. PubMed doi:10.1016/j.euroneuro.2004.03.001
31. Ostacher M, Ng-Mak D, Patel P, et al. Lurasidone compared to other atypical antipsychotic monotherapies for bipolar depression: a systematic review and network meta-analysis [published online March 7, 2017]. World J Biol Psychiatry. PubMed doi:10.1080/15622975.2017.1285050
32. Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord. 2014;169(suppl 1):S12-S16. PubMed doi:10.1016/S0165-0327(14)70004-7
33. Chen W, Deveaugh-Geiss AM, Palmer L, et al. Patterns of atypical antipsychotic therapy use in adults with bipolar I disorder in the USA. Hum Psychopharmacol. 2013;28(5):428-437. PubMed doi:10.1002/hup.2326
34. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
35. Kane JM. Addressing nonresponse in schizophrenia. J Clin Psychiatry. 2012;73(2):e07. PubMed doi:10.4088/JCP.11076tx2c
36. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86. PubMed doi:10.1176/appi.ps.201100092
37. Tohen M. Bias and other methodological issues in follow-up (cohort) studies. In: Fava M, Rosenbaum G, eds. Research Designs and Methods in Psychiatry. Amsterdam: Elsevier; 1992:119-125.
This PDF is free for all visitors!
Save
Cite