Objective:To characterize impairments in daily life experienced by pharmacologically treated adults with attention-deficit/hyperactivity disorder (ADHD) versus adults without ADHD and to identify unmet needs in ADHD treatment from the perspective of adults with ADHD.
Methods: Adults with ADHD taking prescription medication for ≥ 6 months and adults without ADHD agreed to participate in a cross-sectional online survey during December 2016. Participants with ADHD were stratified by their current ADHD medication: long-acting (LA) once daily, short-acting (SA) ≤ 2 times/d, and augmenters (AU; LA > 1 time/d, SA > 2 times/d, or LA plus SA).
Results: A total of 616 adults with ADHD (SA: n = 166, LA: n = 201, AU: n = 249) and 200 adults without ADHD completed the survey. Even with treatment, adults with ADHD reported substantial impairments in their everyday life, particularly at home, at school/work, and in their social life and relationships. Participants with ADHD experienced impairments throughout the day, especially in the afternoon and evening. Signs or symptoms were reported when the ADHD medication was wearing off, resulting in negative effects (including school work, homework, work responsibilities, household responsibilities, emotional responses, mood, and relationships) on the daily life of adults with ADHD.
Conclusions: Adults with ADHD, despite receiving medication, experienced impairments and challenges in many aspects of their daily life. Adults with ADHD described various unmet needs, especially those relating to the duration of treatment effect. When optimizing treatment for adults with ADHD, it is important that the treatment regimen is sufficient to meet the needs of the patient throughout the day.
‘ ‹
aheadofprint = ‘article’;
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Unmet Treatment Needs in Adults With Attention-Deficit/Hyperactivity Disorder

ABSTRACT
Objective: To characterize impairments in daily life experienced by pharmacologically treated adults with attention-deficit/hyperactivity disorder (ADHD) versus adults without ADHD and to identify unmet needs in ADHD treatment from the perspective of adults with ADHD.
Methods: Adults with ADHD taking prescription medication for ≥ 6 months and adults without ADHD agreed to participate in a cross-sectional online survey during December 2016. Participants with ADHD were stratified by their current ADHD medication: long-acting (LA) once daily, short-acting (SA) ≤ 2 times/d, and augmenters (AU; LA > 1 time/d, SA > 2 times/d, or LA plus SA).
Results: A total of 616 adults with ADHD (SA: n = 166, LA: n = 201, AU: n = 249) and 200 adults without ADHD completed the survey. Even with treatment, adults with ADHD reported substantial impairments in their everyday life, particularly at home, at school/work, and in their social life and relationships. Participants with ADHD experienced impairments throughout the day, especially in the afternoon and evening. Signs or symptoms were reported when the ADHD medication was wearing off, resulting in negative effects (including school work, homework, work responsibilities, household responsibilities, emotional responses, mood, and relationships) on the daily life of adults with ADHD.
Conclusions: Adults with ADHD, despite receiving medication, experienced impairments and challenges in many aspects of their daily life. Adults with ADHD described various unmet needs, especially those relating to the duration of treatment effect. When optimizing treatment for adults with ADHD, it is important that the treatment regimen is sufficient to meet the needs of the patient throughout the day.
Prim Care Companion CNS Disord 2019;21(3):18m02397
To cite: Brown TE, Romero B, Sarocco P, et al. The patient perspective: unmet treatment needs in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2019;21(3):18m02397.
To share: https://doi.org/10.4088/PCC.18m02397
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Keck School of Medicine, University of Southern California, Los Angeles, California
bPatient Centered Outcomes, ICON, Gaithersburg, Maryland
cCytokinetics Inc, San Francisco, California. Formerly of Global Health Economics & Outcomes Research, Shire, Lexington, Massachusetts, a member of the Takeda group of companies
dEisai US, Woodcliff, New Jersey. Formerly of Global Medical Affairs, Shire, Lexington, Massachusetts, a member of the Takeda group of companies
ePatient Centered Outcomes, ICON, San Francisco, California
*Corresponding author: Thomas E. Brown, PhD, 500 S Sepulveda Blvd, Suite 218, Manhattan Beach, CA 90266 ([email protected]).
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder, with impairments persisting into adulthood in some patients.1-3 Adult ADHD imposes a substantial burden,4,5 affecting many areas of the individual’s life.6,7 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), ADHD is distinguished by a persistent pattern of inattention, hyperactivity, and/or impulsivity that interferes with functioning in ≥ 2 settings.8 Therefore, for clinicians to diagnose and determine ADHD severity in adults, it is imperative to confirm functional impairments. Consistent with the importance of functional impairment for ADHD diagnosis, outcome studies9-14 indicate that adults with ADHD report more work, school, and social life impairments than matched controls without ADHD.
Consideration of functional impairments is critical not only for diagnosing ADHD but also for ADHD treatment and management. Management options for adult ADHD include pharmacologic and psychosocial interventions.15,16 Although psychosocial therapy is considered an essential component of ADHD treatment,17 pharmacotherapy is a mainstay of treatment for adult ADHD. There are many short-acting (SA) and long-acting (LA) formulations of stimulants and nonstimulants approved for treating adult ADHD.18 Despite these pharmacologic options, some patients continue to experience symptoms and functional impairments.19-22 However, the degree and type of impairments experienced in daily life by adults with ADHD undergoing treatment are not well characterized.
Studies in other therapeutic areas indicate that differences in perception exist between patients and clinicians regarding patient symptoms and impairments,23-28 highlighting the importance of collecting information directly from the patient. Outside of the clinical trial setting, there is a relative lack of patient-reported burden of ADHD in adults. The objectives of this study were to characterize the type and degree of daily life impairments in adults with ADHD receiving pharmacotherapy versus adults without ADHD and to identify unmet needs in ADHD treatment from the perspective of adults with ADHD. Examination of the relative impact of ADHD in those who were medicated versus not medicated was not a goal of this study. Therefore, a nonmedicated ADHD group was not included in the study.
METHODS
This cross-sectional online survey, which was conducted in December 2016, included adults diagnosed with ADHD currently taking prescription ADHD medications and adults without ADHD. Adults with ADHD were stratified by treatment (SA: participants taking an SA medication ≤ 2 times/d; LA: participants taking a once-daily LA medication; augmenters [AU]: participants augmenting their current ADHD treatment by taking an SA medication > 2 times/d, an LA medication > 1 time/d, or an SA plus LA medication). The study was reviewed and approved by a central institutional review board (Salus IRB, Austin, Texas) and performed in accordance with the ethical standards of the Declaration of Helsinki.29 All participants provided informed consent before initiating the survey. Each participant received the equivalent of $3 in panel points.
Survey Development
The survey was developed using a patient blog-forum analysis, a targeted literature search, interviews with clinical experts (n = 2) who were psychiatrists specializing in adult ADHD, and 1-on-1 qualitative interviews with adults with ADHD (n = 31). Qualitative interviews were conducted to understand the symptoms and life challenges associated with ADHD. Participants were interviewed by trained interviewers using a semistructured guide to gather information regarding participants’ experiences with ADHD, focusing on identifying burden and unmet needs. Survey items for the study were generated using the qualitative interview data.
The draft survey was tested on a small sample of adults with ADHD (n = 5) using cognitive interviewing methodology. Online survey content was finalized based on this feedback.
Online Survey
CLINICAL POINTS
- Adults with attention-deficit/hyperactivity disorder (ADHD) who were taking prescription ADHD medication for at least 6 months reported substantial impairment across multiple aspects of their everyday life compared with adults not diagnosed with ADHD, despite reporting benefits from their medication.
- Adults with ADHD who were taking prescription ADHD medication for at least 6 months experienced impairment throughout the day, especially in the afternoon and evening, despite reporting benefits from their medication.
- When optimizing treatment for adults with ADHD, it is important that the treatment regimen be sufficient to meet the needs of the patient throughout the day and into the evening.
Participants with ADHD completed the 30-minute survey (Appendix A: Burden Survey for ADHD Population). Survey questions examined participants’ experiences with ADHD treatment and their everyday activities in multiple areas (work/school, home, and social life/interpersonal relationships). Participants with ADHD also completed questions related to clinical history, diagnosis, and experience with ADHD and ADHD treatment history. Non-ADHD participants completed a 15-minute version of the survey (Appendix B: Burden Survey for Normative Population) that included questions related to everyday activities and sociodemographic characteristics.
Participant Selection
Participants were recruited in the United States through research participant panels, from which those interested in participating in research received invitations for studies or surveys. The recruitment goal was 600 adults with ADHD (200/subgroup) and approximately 200 adults without ADHD.
Participants with ADHD were eligible if the following criteria were met at screening: patient-reported ADHD diagnosis; ≥ 18 years old; currently taking ≥ 1 prescription ADHD medication (either a stimulant or a nonstimulant), with treatment initiated ≥ 6 months before screening; no patient-reported diagnosis of a mental health disorder that would affect the ability to complete the survey (eg, dementia, schizophrenia); adequate written and oral fluency in English; Internet access; willing to provide informed consent; and able to comply with study procedures. The same criteria were used for non-ADHD participants, with the exceptions that non-ADHD participants did not have an ADHD diagnosis and could not be taking ADHD medication.
Statistical Analyses
Descriptive statistics were calculated for all questions. Mean ± SD or standard error (SE) is reported for continuous data. Percentages are reported for categorical data. Inferential analyses of sociodemographic and clinical characteristics consisted of χ2 tests for categorical variables and analysis of variance (ANOVA) for continuous variables. χ2 tests were also used to assess challenges in daily life, challenging parts of the day, and the effects of medication wearing off. Satisfaction with medication was assessed using ANOVA, with post hoc Tukey tests. Statistical significance was set at P < .05.
Sample size calculations were not performed. A standardized sample of 200 participants per group, with a total sample of 600 participants, was considered large enough for statistical comparisons while minimizing the constraints of large sample acquisition.
RESULTS
Participant Characteristics
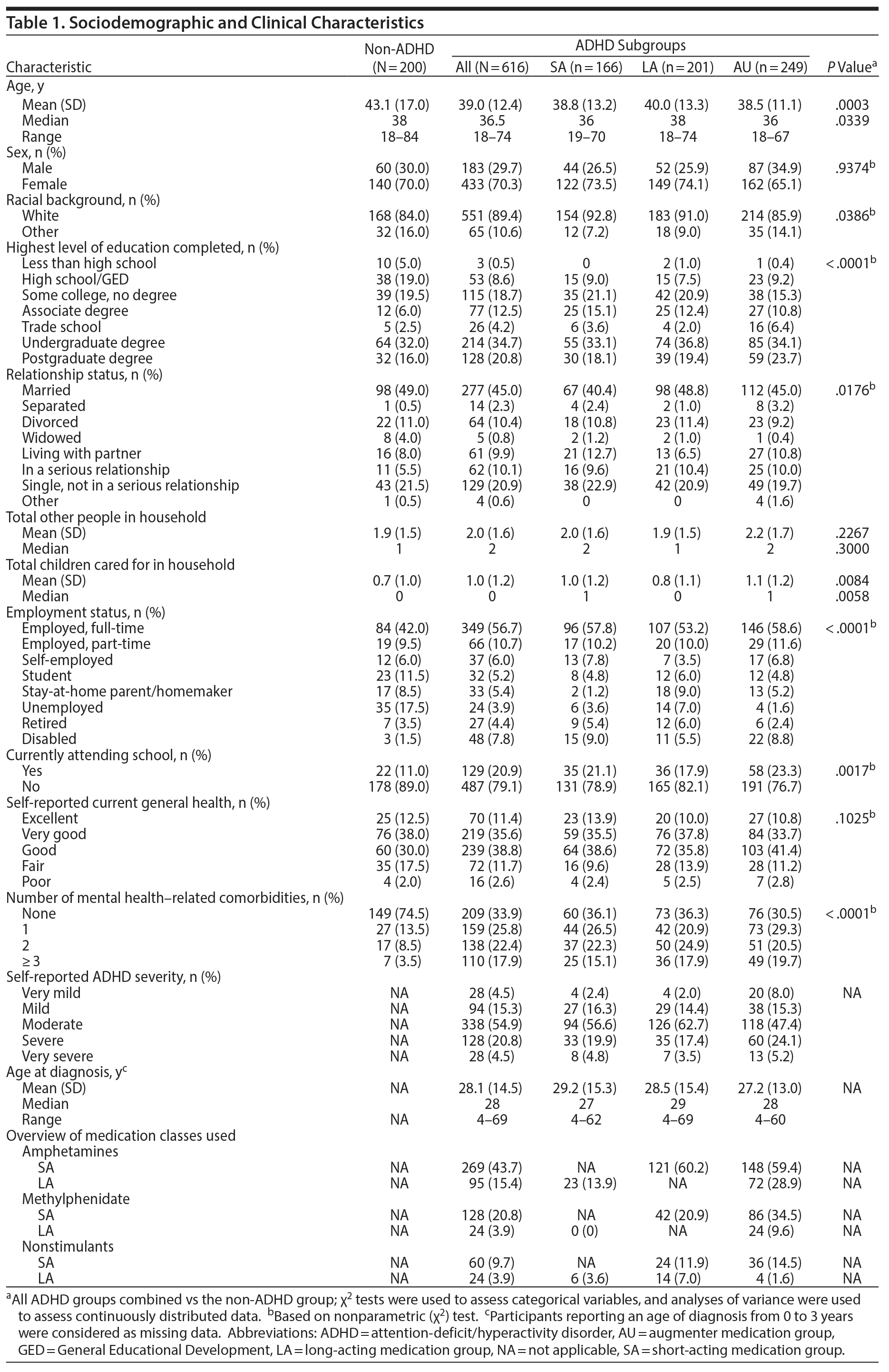
The survey was completed by 616 adults with ADHD (SA: n = 166, LA: n = 201, AU: n = 249) and 200 adults without ADHD. Demographic and clinical characteristics are reported in Table 1. Most participants were female. Participants with ADHD were significantly younger than non-ADHD participants (P = .0003). Significantly greater percentages of ADHD participants than non-ADHD participants were white (P = .0386) and reported higher education levels (P < .0001), being employed (P < .0001), attending school/classes (P = .0017), and having more comorbidities (P < .0001). The mean number of children per household was significantly higher in ADHD compared with non-ADHD participants (P = .0084).
Across ADHD subgroups, ADHD symptom severity was significantly different (P = .0122). The AU subgroup was less likely to report having moderate ADHD and more likely to report having mild, severe, or very severe ADHD than the LA subgroup (P = .0036). Stimulants were used more frequently than nonstimulants, and SA formulations were used more frequently than LA formulations. Further, amphetamine-based stimulants were used more frequently than methylphenidate-based stimulants.
Impairment: ADHD Versus Non-ADHD
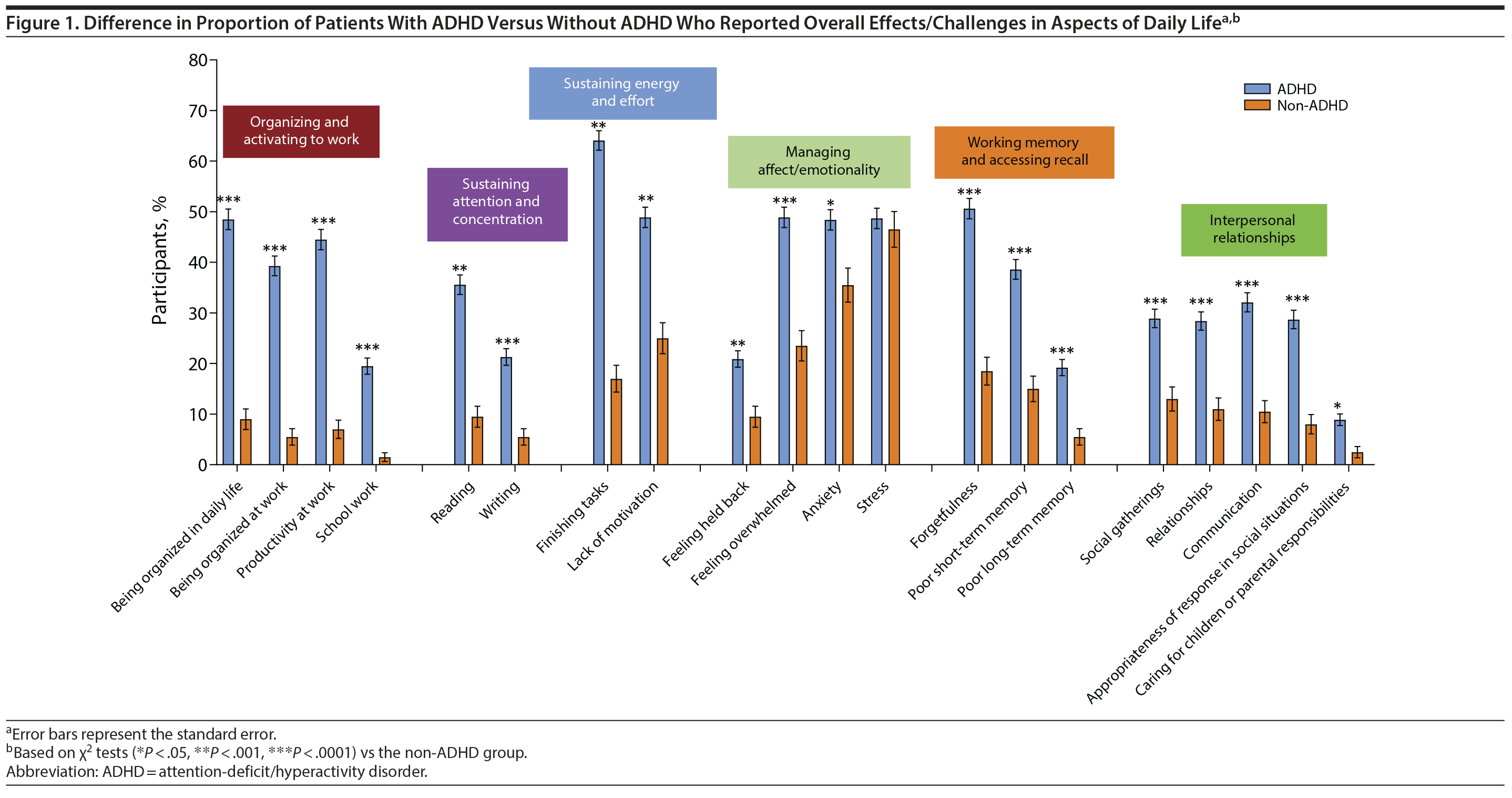
Everyday life. Significantly greater percentages of ADHD participants compared with non-ADHD participants reported impairment in organizing and activating to work, sustaining attention and concentration, sustaining energy and effort, managing affect/emotionality, working memory and accessing recall, and dealing with interpersonal relationships (Figure 1).
Home. Significantly greater percentages of ADHD than non-ADHD participants reported difficulties in sustaining attention and concentration (completing a task: 58.6% vs 15.5%, P < .0001), organizing/activating to work (paying bills on time: 28.4% vs 12.0%, P < .0001; keeping the household organized: 52.8% vs 19.0%, P < .0001; managing household chores: 48.1% vs 19.0%, P < .0001), sustaining energy and effort (caring for pets: 11.0% vs 5.0%, P = .0116), and dealing with interpersonal relationships (caring for children or handling parental responsibilities: 11.7% vs 3.0%, P = .0003).
Social life. Significantly greater percentages of ADHD than non-ADHD participants reported impairment in sustaining attention and concentration (difficulty focusing when with friends/family: 29.2% vs 6.0%, P < .0001; difficulty holding conversations: 31.7% vs 10.0%, P < .0001), organizing and activating to work (late to social events: 26.3% vs 6.5%, P < .0001), sustaining effort (not able to sit through a movie: 19.0% vs 7.0%, P < .0001), accessing working memory and recall (tended to forget names: 37.5% vs 23.0%, P = .0002; forget to call friends: 29.9% vs 12.5%, P < .0001), managing affect/emotionality (felt awkward: 37.5% vs 23.0%, P = .0002), and dealing with interpersonal relationships (difficulty making friends: 21.1% vs 14.5%, P = .0405; difficulty maintaining friendships: 21.9% vs 11.0%, P = .0007).
School/work. Significantly greater percentages of ADHD than non-ADHD participants reported difficulties with school/work, particularly in focusing on work (50.4% vs 10.4%, P < .0001) and school tasks (45.7% vs 18.2%, P = .0187); being organized (42.3% vs 11.3%, P < .0001) and productive (34.3% vs 11.3%, P < .0001) at work; and completing tasks on time at work (28.3% vs 5.2%, P < .0001).
Relationships. Significantly greater percentages of ADHD than non-ADHD participants reported difficulties in their relationships (interpersonal communication: 27.1% vs 11.5%, P < .0001; frustrating [35.1% vs 9.0%, P < .0001] and irritating [26.8% vs 8.0%, P < .0001] friends and family). A significantly greater percentage of ADHD participants acknowledged challenges in maintaining friendships (21.6% vs 10.0%, P = .0003) and being in romantic relationships (16.1% vs 9.5%, P = .0217).
Impairment Among ADHD Subgroups
Everyday life. Significantly greater percentages of participants in the SA and LA subgroups versus the AU subgroup indicated they had difficulty finishing tasks (72.3% and 69.7% vs 54.2%, respectively, P = .0002 and P = .0008). A significantly greater percentage of participants in the LA versus the AU subgroup (56.2% vs 41.4%, P = .0017) reported difficulty being organized in daily life; the difference between the SA and AU subgroups approached significance (50.0% vs 41.4%, P = .0831). Significantly greater percentages of participants in the LA subgroup (58.7%) reported a lack of motivation versus the AU subgroup (41.0%, P = .0002); the difference between the LA and SA (58.7% vs 48.8%) subgroups approached significance (P = .0578). Forgetfulness was reported by a significantly greater percentage of participants in the LA (60.7%) than the AU (47.4%, P = .0049) and SA (43.4%, P = .0009) subgroups.
Home. A significantly greater percentage of participants in the LA versus the AU and SA subgroups reported difficulties managing household chores (55.7% vs 43.8% [P = .0117] and 45.2% [P = .0444], respectively) and sleeping (48.3% vs 29.7% [P < .0001] and 37.3% [P = .0358]).
School/work. A significantly greater percentage of participants in the AU subgroup than the SA subgroup reported difficulty taking notes (43.1% vs 17.1%, P = .0101).
Social life and relationships. There were no significant differences among the ADHD subgroups regarding challenges in their social life or relationships.
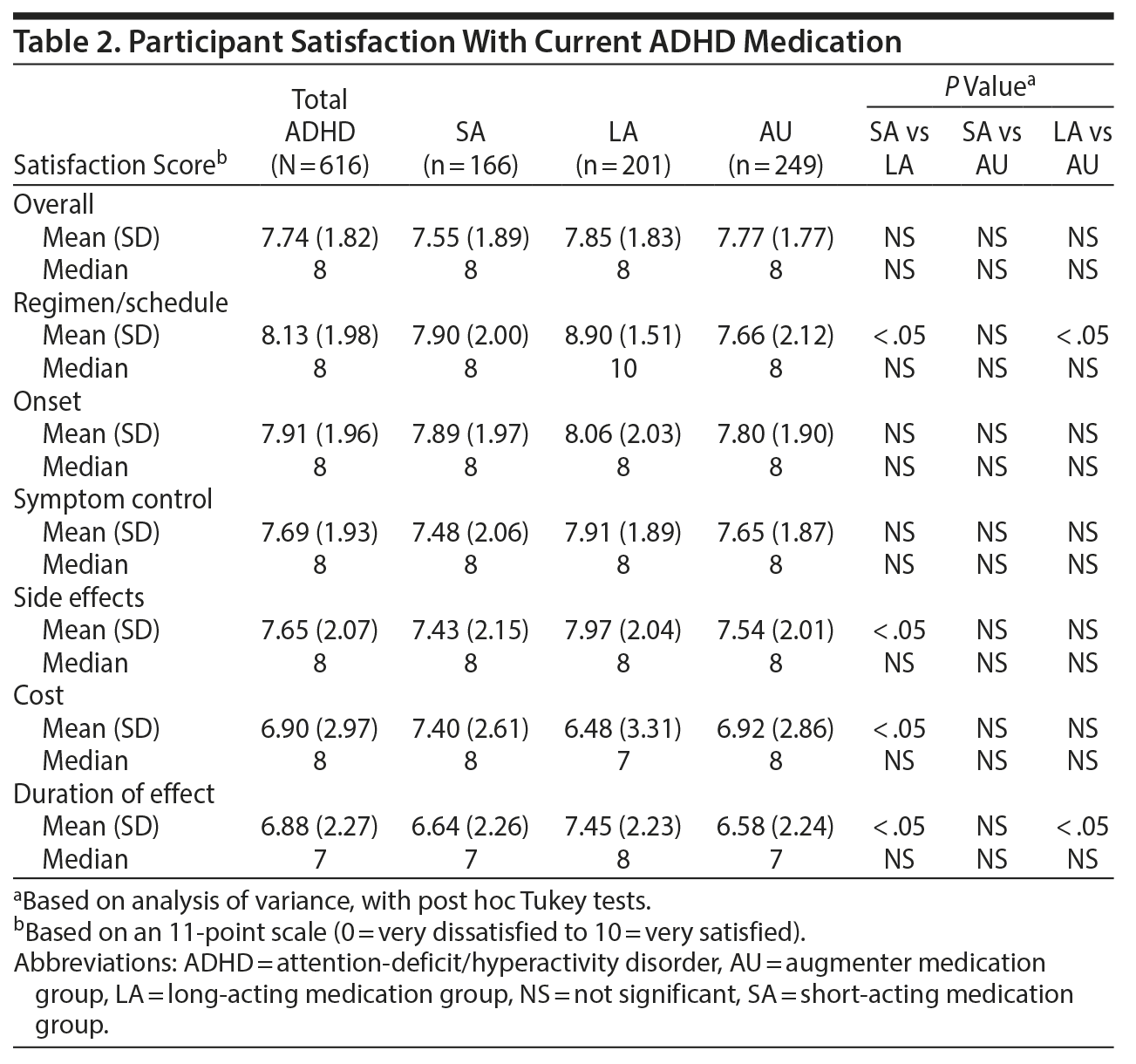
Satisfaction With Medication
Overall, ADHD participants were satisfied with their medication (Table 2). Mean satisfaction scores were lowest for duration of effect among all ADHD participants and significantly lower in the SA and AU subgroups versus the LA subgroup (both P < .05). There were no significant differences between ADHD subgroups for symptom control.
Medication Choice
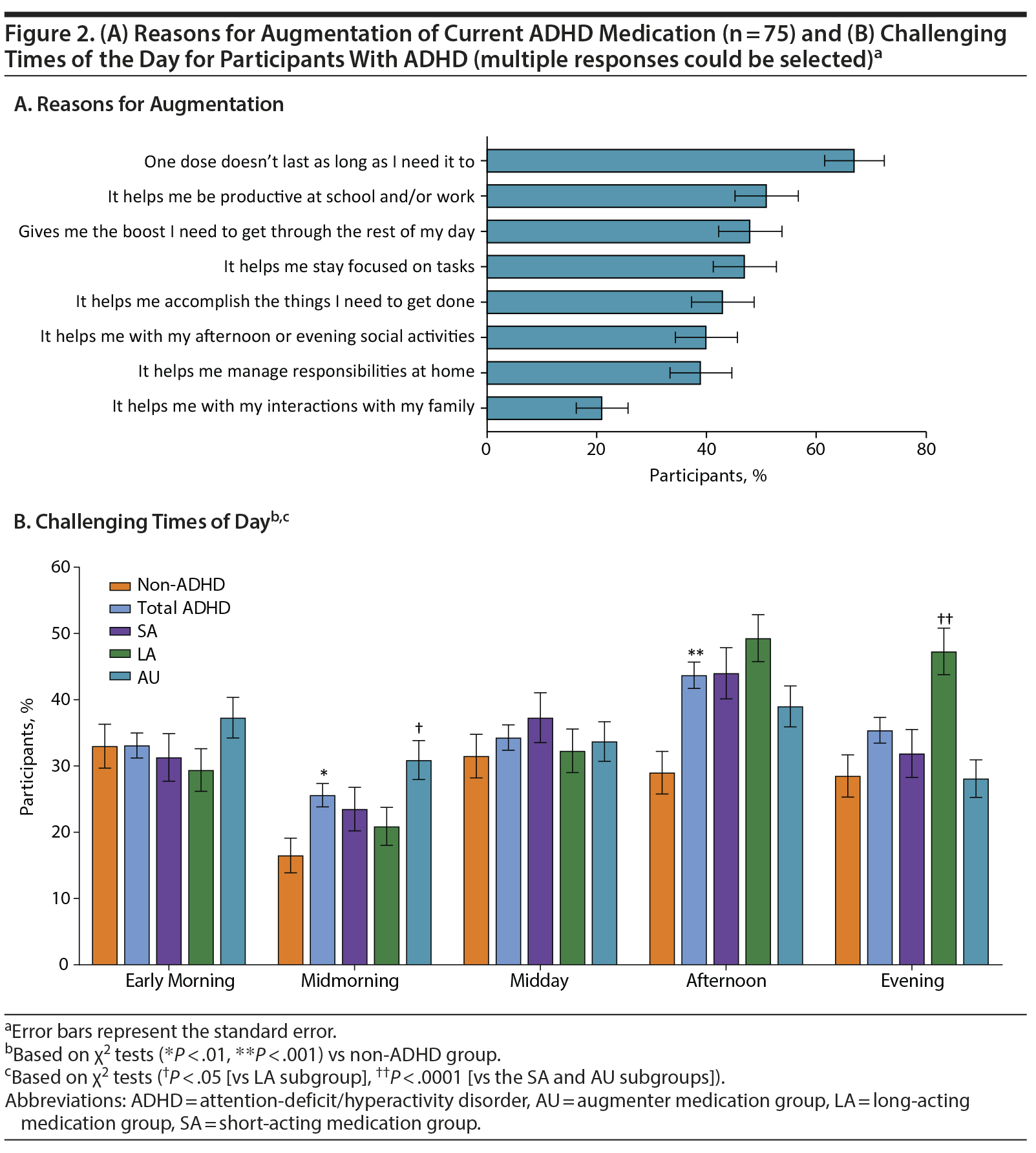
Duration of effect was an important factor for participants in choosing their current medication (43.5%), with doctor’s recommendation (59.6%) and insurance coverage (48.4%) also being important factors. The most frequently reported reason for augmentation in the AU subgroup was that 1 dose does not last long enough (Figure 2A). A significantly greater percentage of participants in the LA (67.7%) than AU subgroup (53.4%, P = .0022) chose their current medication based on the doctor’s recommendation, with the difference between the LA and SA subgroups approaching significance (67.7% vs 59.0%, P = .0871).
Forty-five percent of ADHD participants reported that they liked how long their current medication lasted, with the percentage being significantly greater in the LA (60.7%) than the SA (16.2%, P < .0001) and AU (51.4%, P = .0486) subgroups. Overall, 41.7% of ADHD participants reported that they had to plan activities around their medication wearing off, with the percentage being significantly greater in the AU (49.8%) than the SA (38.6%, P = .0242) and LA (34.3%, P = .0010) subgroups. A majority (55.8%) of ADHD participants indicated that the ideal medication would last all day, with this percentage being significantly lower in the SA (46.4%) than the LA (63.2%, P = .0013) and AU (56.2%, P = .0493) subgroups.
Challenging Times of the Day
The most challenging time of day was the afternoon for ADHD participants (43.7% vs 29.0% for non-ADHD participants, P = .0002) and early morning for non-ADHD participants (33.0% vs 33.1% for ADHD participants, P = .9757; Figure 2B). A significantly greater percentage of ADHD than non-ADHD participants reported midmorning to be the most challenging time of day (25.6% vs 16.5%, P = .0079). The evening was challenging for 35.4% of ADHD participants and 28.5% of non-ADHD participants (P = .0733). Among the ADHD subgroups, a significantly greater percentage of participants in the LA (47.3%) versus the SA (31.9%, P = .0029) and AU (28.1%, P < .0001) subgroups reported the evening to be a challenging time of day. A significantly greater percentage of the AU (30.9%) than the LA (20.9%, P = .0165) subgroups reported that midmorning was challenging (Figure 2B).
Medication Wearing Off
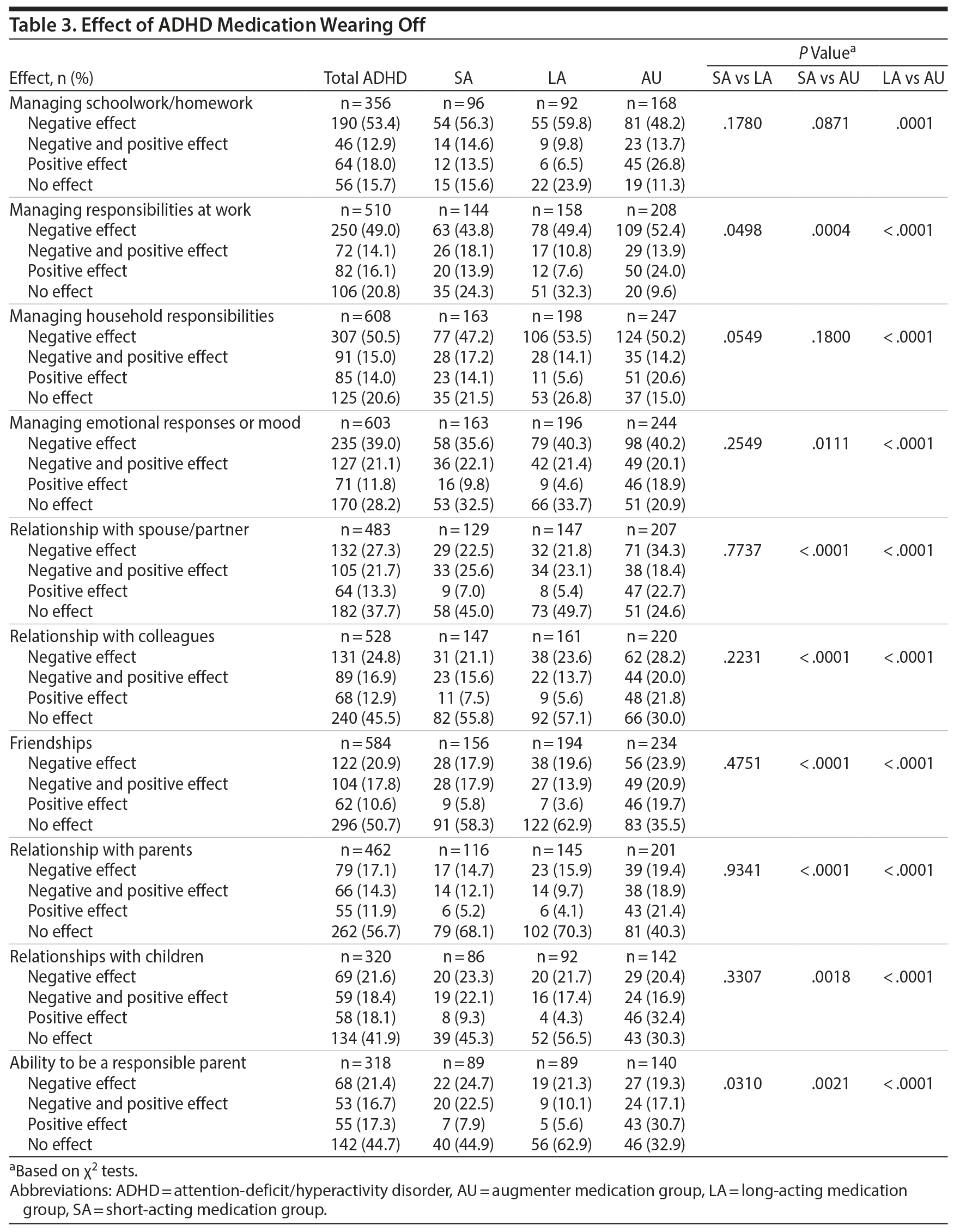
Participants with ADHD indicated experiencing effects of their medication wearing off, with > 90% of participants reporting 1 (11.7%) to ≥ 4 (45.1%) effects. Effects reported by > 10% of ADHD participants included having trouble focusing (62.5%), becoming scattered and disorganized (50.3%), feeling tired (42.9%), not performing tasks as well (42.4%), slowing down physically (39.3%), getting fidgety (29.7%), becoming moody and irritable (28.4%), and getting hungry (20.9%). Only 6.8% of ADHD participants could not tell when their medication was wearing off.
Participants frequently noted that their medication wearing off negatively affected their ability to manage schoolwork/homework, work and household responsibilities, emotional responses or mood, and relationships (Table 3). Significantly lower percentages of the AU subgroup reported no effect and higher percentages reported a positive effect regarding their medication wearing off on various relationships, including with their children and on their ability to be a responsible parent, than the SA and LA subgroups.
DISCUSSION
This survey demonstrates that adults being treated for ADHD continue to experience substantial impairment in multiple aspects of daily life. Greater percentages of ADHD than non-ADHD participants reported impairments and challenges at home, school, and work and in their relationships. ADHD participants reported experiencing difficulties at all times of the day, with the greatest percentages acknowledging challenges in the afternoon and evening when their medication was likely to have worn off. A significantly higher percentage of participants in the LA group than the SA or AU groups reported that the evening was challenging. Although seemingly counterintuitive, this could be the result of the participants’ current LA medication not having a sufficient duration of effect. This finding suggests that some participants in the LA group would have benefited from a longer-acting medication or from augmenting with another medication later in the day.
Duration of effect was an important factor for treatment choice and satisfaction, and a majority of ADHD participants reported that the ideal medication would last all day. In support of these findings, the highest level of satisfaction with duration of effect was reported by the LA group, with no difference observed between the SA and AU groups. However, across the ADHD groups, there were no significant differences in overall treatment satisfaction. This finding is not unexpected because many factors are likely to contribute to overall treatment satisfaction.
To fully understand adult ADHD, it is important to characterize the type and timing of impairment experienced.30 Previous studies5,9,31-35 in adults and adolescents indicate that ADHD is associated with greater impairment in various aspects of life compared with those without ADHD, resulting in a decreased quality of life. For example, the Lifetime Impairment Survey assessed the degree to which ADHD impairs everyday life and identified the areas of life most affected by ADHD.33,36 In adults with ADHD in the United Kingdom, greater impairments in social, family, emotional, academic, cognitive, and behavioral functioning were reported compared with those without ADHD.33
There is limited guidance available for treating adult ADHD in the United States. Pharmacotherapy is recommended for adults with ADHD, especially those with moderate or severe levels of impairment, as part of a comprehensive treatment program.37,38 Even with pharmacotherapy, some individuals with ADHD experience residual symptoms and functional impairments.19,39-41 A qualitative interview study35 conducted in the United Kingdom in adults with ADHD reported that individuals undergoing pharmacotherapy still experienced impairment in interpersonal relationships and work achievements. These findings are consistent with those of the current report and suggest clinicians should monitor their patients for ADHD-associated impairments across various times of the day, activities, and settings.
The preferences and needs of adults with ADHD relating to pharmacotherapy have received limited attention.9,32,42 In examining the current study, participants reported that duration of effect was an important factor for medication choice and satisfaction, along with physician recommendations and insurance coverage. This finding is supported by another study,33 which reported that, although the majority of adults with ADHD were satisfied with their treatment, a longer duration of effect was identified as an area requiring improvement. It is interesting that the number of participants reporting severe or very severe ADHD symptoms in the SA group was higher than expected. Furthermore, in the overall ADHD group and the AU group, use of SA medication was reported more frequently than LA medication. Although relationships between medication dose titration/optimization and ADHD severity were not examined in this study, the high utilization of SA medications could indicate that these individuals’ ADHD symptoms were not optimally managed.
It is important to note that study participants reported relatively high satisfaction scores for symptom control despite concurrently reporting considerable ADHD-associated impairments. This finding suggests that adults with ADHD may not always identify all of the aspects of impaired function associated with their ADHD symptoms and highlights that clinicians should ask their patients about symptom control and daily functioning. This approach would allow the clinician to discern where and how ADHD symptoms may continue to affect their patients’ lives. It is also worth noting that participants are likely to have experienced improvement in symptoms and daily life because of treatment, which could have contributed to the levels of treatment satisfaction observed in this study.
The impairments reported in this study suggest some individuals with ADHD may need a longer-acting medication to manage their symptoms throughout the day. Although the largest percentage of ADHD participants reported the afternoon hours as the most problematic time of day, a substantial minority reported impairment in interpersonal relationships, home life, and social life during the evening. Accordingly, physicians should consider tailoring treatment for adults with ADHD to meet the variety of individual patient needs throughout the day.
Previous studies43 indicate that the likelihood of medication compliance decreases as the number of daily doses increases. This finding suggests that missed doses could be an issue in individuals required to take ≥ 2 daily medication doses to manage their symptoms throughout the day. Consequently, LA formulations have largely replaced SA formulations as first-line ADHD treatments because a longer duration of effect is achieved with once-daily dosing.44,45 However, some patients may avoid LA formulations because of safety/tolerability concerns or personal beliefs that ADHD symptoms cause only workplace impairment.46-48 Alternatively, some patients may prefer augmentation of an LA formulation with an SA formulation because it allows them to experience less appetite suppression or feel more relaxed when the medication is not active, while allowing them to target specific times later in the day when they need to reintroduce the medication (eg, periods of homework or other activities in the evening).
This study has several limitations that could affect the generalizability of these data to a heterogeneous ADHD population. First, there is a potential for selection bias because participants were recruited through research participant panels, which may not be representative of the entire ADHD population. Second, the sex ratio of ADHD in adults is almost 1:1,49,50 but this study included a greater percentage of women. Third, non-ADHD participants were not matched with ADHD participants across all sociodemographic aspects. Nevertheless, many of the sociodemographic characteristics in the ADHD and non-ADHD groups were similar to those reported in another large survey9 conducted in the United States. Fourth, study participants were living in the United States, which may limit the relevance of these findings to other countries. Fifth, the AU subgroup was considered as a single group in this study. However, augmentation patterns in this group were likely heterogeneous and examining different patterns of augmentation (ie, use of a SA medication > 2 times a day versus use of a SA plus a LA medication) may have yielded different results. Sixth, the relationship between medication dose titration/optimization and ADHD symptoms could not be addressed because this information was not collected. Lastly, reports of the diagnosis and treatment of ADHD were self-reported and not physician confirmed. Thus, participants who did not meet the study entry criteria may have been included.
CONCLUSION
Currently available ADHD medications effectively reduce ADHD symptoms and improve many aspects of life in adults with ADHD. However, this study demonstrates that adults with ADHD being treated with pharmacotherapy continue to report impaired daily function. Overall, adults with ADHD reported impairment across multiple settings and, regardless of medication type, reported that there were some negative effects associated with their medication wearing off. Overall satisfaction with medication was high across all ADHD subgroups despite reports of impairment; however, participants in the LA subgroup had the highest satisfaction with their medication’s duration of effect and regimen/schedule. Although a proportion of participants from each ADHD subgroup reported challenges throughout the day, a significantly greater percentage of participants in the LA subgroup than the SA and AU subgroups reported that the evening was challenging. Furthermore, study participants described areas of unmet need, especially with respect to duration of treatment effect. The effects and challenges of ADHD symptoms across multiple dimensions of daily life emphasizes the need for clinicians to inquire about a patient’s daily functioning at specific times and for specific activities to help better tailor the patient’s treatment regimen so it optimizes coverage throughout the day.
Submitted: October 17, 2018; accepted February 15, 2019.
Published online: June 6, 2019.
Author contributions: All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Potential conflicts of interest: Dr Brown has received research support from Shire; served as a consultant for Ironshore, NLS, Sunovion, Supernus, and Shire; and has received publication royalties from American Psychiatric Publishing, Jossey-Bass/Wiley, Pearson, Routledge, and Yale University Press. Ms Romero, Mr Schwartz, and Dr Rhoten are employees of ICON, which was provided funding by Shire to perform the study. Mr Sarocco and Dr Atkins are former employees of Shire, a member of the Takeda group of companies, and Takeda stock holders.
Funding/support: This research was conducted by ICON (North Wales, Pennsylvania), with funding provided by the sponsor, Shire Development LLC, Lexington, Massachusetts, a member of the Takeda group of companies.
Role of the sponsor: The sponsor was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Shire Development LLC, a member of the Takeda group of companies, provided funding to Complete Healthcare Communications, LLC (CHC; North Wales, Pennsylvania), a CHC Group company, for support in writing and editing this manuscript.
Disclaimer: Shire, a member of the Takeda group of companies, develops and markets treatments for psychiatric disorders, including attention-deficit/hyperactivity disorder.
Previous presentations: The results of this study were previously presented at the Annual Meeting of the American Psychiatric Association; May 20-24, 2017; San Diego, California; and at the US Psychiatric and Mental Health Congress; September 16-19, 2017; New Orleans, Louisiana.
Acknowledgments: The authors thank Emuella Flood, BA (a former employee of ICON, which was funded by Shire Development LLC to conduct this study) for her contributions to the study design and analysis. The authors also thank Alexandra Khachatryan, MPH (an employee of Shire, a member of the Takeda group of companies) for her contributions to the development of this study. Editorial assistance, which was funded by Shire Development LLC, a member of the Takeda group of companies, was provided by Monique Curran, MSc (Oncue Writing, Auckland, New Zealand) and by Craig Slawecki, PhD (an employee of CHC). Editorial assistance in the form of proofreading and copyediting was also provided by CHC. There are no other conflicts of interest to disclose for these individuals.
Supplementary material: See accompanying pages.
REFERENCES
1. Ebejer JL, Medland SE, van der Werf J, et al. Attention deficit hyperactivity disorder in Australian adults: prevalence, persistence, conduct problems and disadvantage. PLoS One. 2012;7(10):e47404. PubMed CrossRef
2. Cheung CH, Rijdijk F, McLoughlin G, et al. Childhood predictors of adolescent and young adult outcome in ADHD. J Psychiatr Res. 2015;62:92-100. PubMed CrossRef
3. Langley K, Fowler T, Ford T, et al. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196(3):235-240. PubMed CrossRef
4. Erskine HE, Ferrari AJ, Polanczyk GV, et al. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry. 2014;55(4):328-336. PubMed CrossRef
5. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. PubMed CrossRef
6. Asherson P, Akehurst R, Kooij JJ, et al. Under diagnosis of adult ADHD: cultural influences and societal burden. J Atten Disord. 2012;16(suppl):20S-38S. PubMed CrossRef
7. Gjervan B, Torgersen T, Nordahl HM, et al. Functional impairment and occupational outcome in adults with ADHD. J Atten Disord. 2012;16(7):544-552. PubMed CrossRef
8. American Psychiatric Association. Attention-deficit/hyperactivity disorder. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013:59-61.
9. Bernardi S, Faraone SV, Cortese S, et al. The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychol Med. 2012;42(4):875-887. PubMed CrossRef
10. Biederman J, Faraone SV, Spencer TJ, et al. Functional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry. 2006;67(4):524-540. PubMed CrossRef
11. Barkley RA, Fischer M. Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: self-reported executive function (EF) deficits versus EF tests. Dev Neuropsychol. 2011;36(2):137-161. PubMed CrossRef
12. Barkley RA, Knouse LE, Murphy KR. Correspondence and disparity in the self- and other ratings of current and childhood ADHD symptoms and impairment in adults with ADHD. Psychol Assess. 2011;23(2):437-446. PubMed CrossRef
13. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41. PubMed CrossRef
14. Halm׸y A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187. PubMed CrossRef
15. Young JL, Goodman DW. Adult attention-deficit/hyperactivity disorder diagnosis, management, and treatment in the DSM-5 era. Prim Care Companion CNS Disord. 2016;18(6):e1-e11. PubMed CrossRef
16. Cortese S, Adamo N, Mohr-Jensen C, et al; European ADHD Guidelines Group (EAGG). Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open. 2017;7(1):e013967. PubMed CrossRef
17. Manos MJ. Psychosocial therapy in the treatment of adults with attention-deficit/hyperactivity disorder. Postgrad Med. 2013;125(2):51-64. PubMed CrossRef
18. Jain R, Katic A. Current and investigational medication delivery systems for treating attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2016;18(4). PubMed. 10.4088/PCC.4016r01979
19. Sikirica V, Flood E, Dietrich CN, et al. Unmet needs associated with attention-deficit/hyperactivity disorder in eight European countries as reported by caregivers and adolescents: results from qualitative research. Patient. 2015;8(3):269-281. PubMed CrossRef
20. Preuss U, Ralston SJ, Baldursson G, et al; ADORE Study Group. Study design, baseline patient characteristics and intervention in a cross-cultural framework: results from the ADORE study. Eur Child Adolesc Psychiatry. 2006;15(suppl 1):I4-I14. PubMed CrossRef
21. Lachaine J, Beauchemin C, Sasane R, et al. Treatment patterns, adherence, and persistence in ADHD: a Canadian perspective. Postgrad Med. 2012;124(3):139-148. PubMed CrossRef
22. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956.e4, e944. PubMed CrossRef
23. Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903-909. PubMed CrossRef
24. Bushmakin AG, Cappelleri JC, Taylor-Stokes G, et al. Relationship between patient-reported disease severity and other clinical outcomes in osteoarthritis: a European perspective. J Med Econ. 2011;14(4):381-389. PubMed CrossRef
25. Cleeland CS, Sloan JA; ASCPRO Organizing Group. Assessing the symptoms of cancer using patient-reported outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. 2010;39(6):1077-1085. PubMed CrossRef
26. Hewlett SA. Patients and clinicians have different perspectives on outcomes in arthritis. J Rheumatol. 2003;30(4):877-879. PubMed
27. Stacy M, Bowron A, Guttman M, et al. Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord. 2005;20(6):726-733. PubMed CrossRef
28. Pakhomov SV, Jacobsen SJ, Chute CG, et al. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14(8):530-539. PubMed
29. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. World Medical Association website. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed January 22, 2018.
30. Brown TE. Outside the Box: Rethinking ADD/ADHD in Children and Adults: A Practical Guide. Arlington, VA: American Psychiatric Association Publishing; 2017.
31. Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit hyperactivity disorder: a systematic review. Innov Clin Neurosci. 2012;9(5-6):10-21. PubMed
32. Able SL, Haynes V, Hong J. Diagnosis, treatment, and burden of illness among adults with attention-deficit/hyperactivity disorder in Europe. Pragmat Obs Res. 2014;5:21-33. PubMed
33. Pitts M, Mangle L, Asherson P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: results from the lifetime impairment survey. Arch Psychiatr Nurs. 2015;29(1):56-63. PubMed CrossRef
34. Molina BS, Hinshaw SP, Swanson JM, et al; MTA Cooperative Group. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500. PubMed CrossRef
35. Matheson L, Asherson P, Wong IC, et al. Adult ADHD patient experiences of impairment, service provision and clinical management in England: a qualitative study. BMC Health Serv Res. 2013;13(1):184. PubMed CrossRef
36. Caci H, Asherson P, Donfrancesco R, et al. Daily life impairments associated with childhood/adolescent attention-deficit/hyperactivity disorder as recalled by adults: results from the European Lifetime Impairment Survey. CNS Spectr. 2015;20(2):112-121. PubMed CrossRef
37. National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. NICE website. http://nice.org.uk/guidance/cg72. Accessed December 29, 2016.
38. Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA). Canadian ADHD Practice Guidelines. 3rd ed. Toronto, Canada: CADDRA; 2011.
39. Flood E, Gajria K, Sikirica V, et al. The Caregiver Perspective on Paediatric ADHD (CAPPA) survey: Understanding sociodemographic and clinical characteristics, treatment use and impact of ADHD in Europe. J Affect Disord. 2016;200:222-234. PubMed CrossRef
40. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763. PubMed CrossRef
41. Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10(1):99. PubMed CrossRef
42. Bushe C, Wilson B, Televantou F, et al. Understanding the treatment of attention deficit hyperactivity disorder in newly diagnosed adult patients in general practice: a UK database study. Pragmat Obs Res. 2015;6:1-12. PubMed
43. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296-1310. PubMed CrossRef
44. Hosenbocus S, Chahal R. A review of long-acting medications for ADHD in Canada. J Can Acad Child Adolesc Psychiatry. 2009;18(4):331-339. PubMed
45. Buitelaar J, Medori R. Treating attention-deficit/hyperactivity disorder beyond symptom control alone in children and adolescents: a review of the potential benefits of long-acting stimulants. Eur Child Adolesc Psychiatry. 2010;19(4):325-340. PubMed CrossRef
46. Christensen L, Sasané R, Hodgkins P, et al. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin. 2010;26(4):977-989. PubMed CrossRef
47. Setyawan J, Hodgkins P, Guérin A, et al. Comparison of therapy augmentation and deviation rates from the recommended once-daily dosing regimen between LDX and commonly prescribed long-acting stimulants for the treatment of ADHD in youth and adults. J Med Econ. 2013;16(10):1203-1215. PubMed CrossRef
48. Karlstad ט, Zo׫ga H, Furu K, et al. Use of drugs for ADHD among adults-a multinational study among 15.8 million adults in the Nordic countries. Eur J Clin Pharmacol. 2016;72(12):1507-1514. PubMed CrossRef
49. Cortese S, Faraone SV, Bernardi S, et al. Gender differences in adult attention-deficit/hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry. 2016;77(4):e421-e428. PubMed CrossRef
50. McCarthy S, Wilton L, Murray ML, et al. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr. 2012;12(1):78. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite