Prim Care Companion CNS Disord 2021;23(4):21br02930
To cite: Xu KY, Mintz CM, Borodovsky JT, et al. Prevalence of kratom use and co-occurring substance use disorders in the United States. Prim Care Companion CNS Disord. 2021;23(4):21br02930.
To share: https://doi.org/10.4088/PCC.21br02930
© Copyright 2021 Physicians Postgraduate Press, Inc.
aHealth and Behavior Research Center, Department of Psychiatry, Washington University School of Medicine, St Louis, Missouri
bCenter for Technology and Behavioral Health, Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire
cWilliam Greenleaf Eliot Division of Child Psychiatry, Washington University School of Medicine, St Louis, Missouri
dAlvin J Siteman Cancer Center, Barnes Jewish Hospital and Washington University School of Medicine, St Louis, Missouri
eDepartments of Family and Community Medicine and Health and Outcomes Research, St Louis University, St Louis, Missouri
*Corresponding author: Kevin Y. Xu, MD, MPH, Department of Psychiatry, Washington University School of Medicine, 420 South Euclid Ave, Campus Box 8134, St Louis, MO 63110 ([email protected]).
Kratom (Mitragyna speciosa) is a novel psychoactive drug that is often used to self-manage pain and opioid withdrawal.1–3 While kratom, in itself, has been found to lack the addictive potential of classic opioids and may have promising therapeutic properties,4 there are concerns about the safety of unregulated kratom products in the United States.5 As kratom has increasingly been found in association with overdoses, usually in the presence of other substances,1 it is important to understand the co-occurring substance use disorders (SUDs) associated with kratom use. The aim of the present study was to describe the prevalence of kratom use and co-occurring substance use using the National Survey on Drug Use and Health (NSDUH).
Methods
We used data from the 2019 NSDUH (n = 56,136, weighted interview response rate 72.1% for adolescents, 64.2% for adults), the first year for which kratom data were available. NSDUH is conducted annually among the civilian, noninstitutionalized US population, aged 12 years and older. We calculated prevalence estimates of self-reported history of kratom use, as well as co-occurring past-year opioid, stimulant, alcohol, marijuana, sedative, and other SUDs. Prevalence ratios were calculated via log-binomial regression. We tabulated the number of SUDs per individual and examined if whether kratom use was associated with increased risk of multiple SUDs. Analyses were done with STATA 16; all estimates accounted for complex survey design. This study was exempted from review by the Washington University Institutional Review Board.
Results
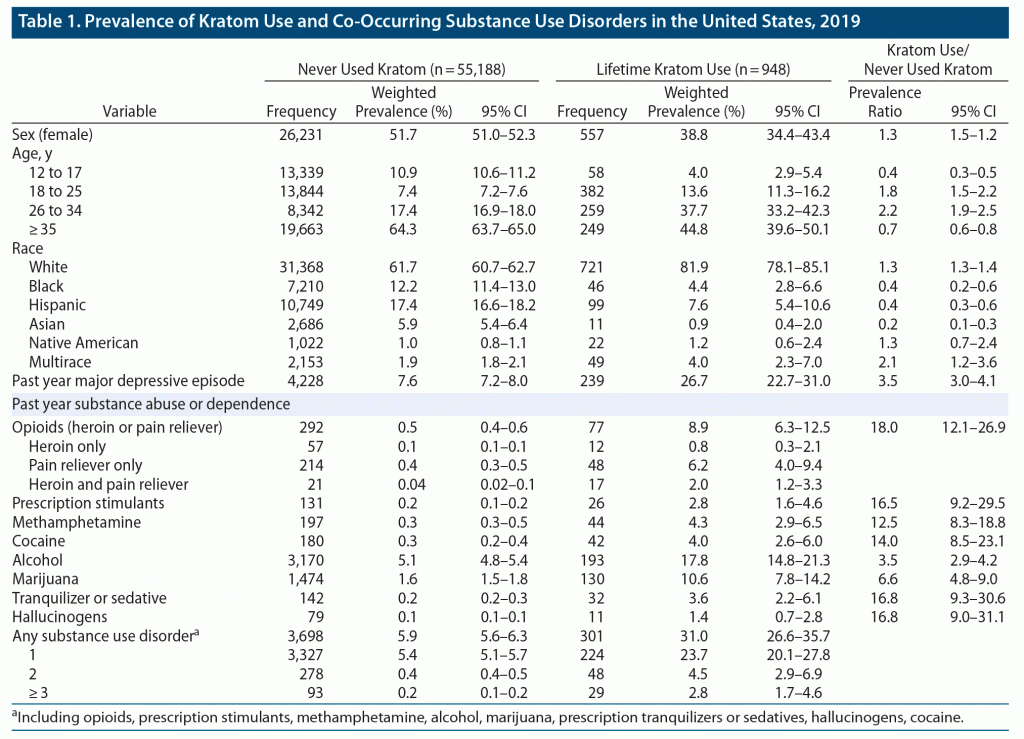
As shown in Table 1, prevalence of lifetime kratom use in the United States was 1.5% (95% CI, 1.4%–1.6%). Among those who used kratom, 50.9% (46.8%–54.9%) used more than 1 year ago, 28.4% (24.4%–32.8%) used within the past year, and 20.7% (17.2%–24.6%) used within the past month. Most lifetime kratom users were male (61.2%, 56.6%–65.6%), white (81.9%, 78.1%–85.1%), and between the ages of 18 and 34 (55.2%, 49.9%–60.4%). Few users were under the age of 18.
Almost one-third (31%, 26.6%–35.7%) of kratom users had at least 1 SUD. Whereas the prevalence of opioid use disorder was 0.5% (0.4%–0.6%) among never users, this increased 18-fold to 8.9% (6.3%–12.5%) among lifetime kratom users. Kratom use was also associated with increased prevalence of prescription stimulant (16.5-fold, 9.2–29.5), methamphetamine (12.5-fold, 8.3–18.8), cocaine (14-fold, 8.5–23.1), and tranquilizer or sedative use disorders (16.8-fold, 9.3–31.1).
Lifetime kratom use was also associated with a higher burden of multiple SUDs. Among never users, the past-year prevalence of experiencing 3 or more co-occurring SUDs was 0.16%. However, this prevalence increased almost 20-fold to 2.81% among kratom users. In addition, whereas only 7.6% (7.2%–8.0%) of never users reported a major depressive episode in the last year, this increased to 26.7% (22.7%–31.0%) for kratom users.
Discussion
Nearly one-third of lifetime kratom users in the United States have at least 1 SUD; over one-fourth experienced past–year major depression. Lifetime kratom use is associated with a higher risk of other SUDs, especially opioids, stimulants, and sedatives. Importantly, the high burden of co-occurring SUDs among kratom users should not be interpreted to suggest that kratom causes SUDs; existing studies6–8 show that kratom itself does not typically produce a “high” in comparison to classic opioids, with the vast majority of users not meeting DSM criteria for kratom-related SUDs. Multiple studies have found that kratom alkaloids have unique binding properties that show potential for management of pain,9 as well as ameliorate opioid withdrawal symptoms with minimal central nervous system depression.10 More research using nationally representative data is needed to elucidate the interplay between kratom’s therapeutic promise and safety concerns, especially given a current lack of quality control surrounding kratom products.5
Published online: August 5, 2021.
Potential conflicts of interest: Dr Bierut is listed as an inventor on US Patent 8080371 “Markers for Addiction,” covering use of SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr Borodovsky serves on the board of directors and is treasurer of the nonprofit MySafeRx Inc but does not receive any financial compensation for this work. Drs Xu, Mintz, Glaser, and Grucza declare no financial interests. None of the authors have financial relationships with organizations that may have an interest in this work.
Funding/support: This work was supported by National Institutes of Health (NIH R25 MH112473-01, Dr Xu; R21 DA044744, Dr Grucza; U10 AA008401, R01 DA036583, Dr Bierut; K12 DA041449, Dr Mintz; R21 AA02568901 and F32 AA027941, Dr Borodovsky).
Role of the sponsor: The funding sources had no role in the study design, implementation, or interpretation of results.
Acknowledgments: The authors acknowledge the support of Nuri B. Farber, MD, and the Psychiatry Residency Research Education Program (PRREP) of Washington University (R25 MH112473-01) as well as the National Center for Advancing Translational Sciences of the National Institutes of Health under UL1 TR002345. Dr Farber has no conflicts of interest related to the subject of this report.
References (10)

- Anwar M, Law R, Schier J. Notes from the field: kratom (Mitragyna speciosa) exposures reported to poison centers—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(29):748–749. PubMed CrossRef
- Schimmel J, Amioka E, Rockhill K, et al. Prevalence and description of kratom (Mitragyna speciosa) use in the United States: a cross-sectional study. Addiction. 2021;116(1):176–181. PubMed CrossRef
- Grundmann O. Patterns of Kratom use and health impact in the US—results from an online survey. Drug Alcohol Depend. 2017;176:63–70. PubMed CrossRef
- Veltri C, Grundmann O. Current perspectives on the impact of kratom use. Subst Abuse Rehabil. 2019;10:23–31. PubMed CrossRef
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70–77. PubMed CrossRef
- Grundmann O, Babin JK, Henningfield JE, et al. Kratom use in the United States: a diverse and complex profile. Addiction. 2021;116(1):202–203. PubMed CrossRef
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. PubMed CrossRef
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340–348. PubMed CrossRef
- Todd DA, Kellogg JJ, Wallace ED, et al. Chemical composition and biological effects of kratom (Mitragyna speciosa): in vitro studies with implications for efficacy and drug interactions. Sci Rep. 2020;10(1):19158. PubMed CrossRef
- Wilson LL, Harris HM, Eans SO, et al. Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend. 2020;216:108310. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite