ABSTRACT
Objective: To determine the prevalence of tardive dyskinesia (TD) identified by clinicians in naturalistic data in a real-world treatment setting.
Methods: Electronic medical record data were analyzed from a single large community mental health treatment center for all psychiatric provider encounters of 120,431 unique adult and child patients during a 5-year period from January 2013 through December 2017, focusing on clinician-identified TD in patients prescribed antipsychotic medication.
Results: Only half of the antipsychotic-prescribed patients had Abnormal Involuntary Movement Scale (AIMS) information recorded in their medical records, and only 1% of those with AIMS data had a positive AIMS identifying TD. AIMS testing represented the largest source of all identified TD in these patients, but only one-third of the patients with a positive AIMS in the record had a clinical diagnosis of TD recorded in the prescriber’s diagnostic impression list from billing code data. The clinical identification of only 1% of antipsychotic-prescribed patients with TD in this study is far below generally established TD prevalence estimates of previous research. An important methodological contributor to this discrepancy is generation of the data by treating clinicians in this study who greatly under identified TD relative to systematic research methodology.
Conclusions: Given the recent availability of US Food and Drug Administration–approved pharmaceutical agents for treatment of TD, it is now more important than ever to identify and intervene in TD. Agency-wide policies and procedures can be established to ensure that TD assessments are systematically conducted with regularity and accuracy among all antipsychotic-prescribed patients.
Prim Care Companion CNS Disord 2022;24(4):21m03069
To cite: North CS, McDonald K, Hunter J, et al. Prevalence of tardive dyskinesia in an electronic medical record study at a large community mental health treatment center. Prim Care Companion CNS Disord. 2022;24(4):21m03069.
To share: https://doi.org/10.4088/PCC.21m03069
© 2022 Physicians Postgraduate Press, Inc.
aMetrocare Services, Dallas, Texas
bDepartment of Psychiatry, The University of Texas Southwestern Medical Center, Dallas, Texas
*Corresponding author: Carol S. North, MD, MPE, DLFAPA, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Ste NE5.102, Dallas, TX 75390-9070 ([email protected]).
Tardive dyskinesia (TD) is an involuntary, generally irreversible movement disorder associated with long-term antipsychotic medication treatment. TD was first described in 1957, not long after the introduction of antipsychotic agents into general use in psychiatric practice.1,2 The subsequent introduction and widespread use of second-generation antipsychotic agents generated expectations that their potential for TD would be much lower than with the first-generation antipsychotic agents, but research findings have been disappointingly inconsistent.1,3–5
The occurrence of TD is of major concern because the condition is associated with poorer quality of life, embarrassment and stigma, greater social isolation, functional impairment, poorer ratings of health, increased mortality, and medication nonadherence.1,6–8 Established risk factors for TD include female sex, advancing age, Black race, diabetes, HIV-positive status, history of brain injury, cognitive impairment, alcohol and drug abuse, primary mood disorder, chronicity of psychotic illness, duration and dosages of antipsychotic medication use, treatment with first-generation antipsychotic agents, early emergence of acute extrapyramidal symptoms, and treatment with antiparkinsonian agents.1,3,6,7,9
TD is of further concern for patients treated with antipsychotic medications because it has been found to be present in substantial proportions of these patients.8 The potential for TD is especially important for patients with chronic schizophrenia for whom these medications represent the primary treatment. Numerous studies have examined TD prevalence in antipsychotic-treated patient populations; a comprehensive review and meta-analysis6 of 41 studies collectively representing 11,493 antipsychotic-prescribed patients found an overall mean TD prevalence of 25%. The individual study estimates of antipsychotic-induced TD in this review6 varied widely, however, were attributed to methodological and research design differences and inclusion of assorted patient populations in a variety of prescriber practices.7,8 Different patient populations and practice settings may vary considerably in TD prevalence, as illustrated by studies3,10,11 finding a considerably higher prevalence of TD in state psychiatric hospital inpatients than in other patient populations.
The majority of patients receiving psychiatric treatment in the public sector obtain most of their care in outpatient settings, but the outpatient public sector psychiatric treatment population has not been well represented in TD prevalence studies. Research is needed not only to determine the relative prevalence of TD in these patients but also to document the identification of this condition by their treating clinicians in naturalistic data from real-world treatment settings. The current study addresses this gap by examining clinician documentation of TD in a large electronic medical record database from a single large community mental health treatment center comprising > 120,000 patients, including nearly 30,000 children and almost 45,000 antipsychotic-treated patients.
METHODS
This study of TD in clinical care was conducted at Metrocare Services, a large nonprofit community mental health treatment center in Dallas County in Texas that provides outpatient care for serious mental illness and developmental disabilities for 62,000 unique adults and children annually. Because the analysis of the database used for this study was determined by the institutional review boards (IRBs) of Metrocare Services and The University of Texas Southwestern Medical Center to not represent human subject research, it did not require IRB approval or oversight or documentation of informed consent. The study’s database contained information from all psychiatric provider encounters of 120,431 unique patients during a 5-year period (January 2013–December 2017). De-identified data on selected study variables from these encounters were downloaded from electronic medical records into Microsoft Excel spreadsheets by the agency’s information resources personnel. Unique research participant identifiers were generated to allow grouping of all encounters within unique patients.
Demographic and clinical variables included in the study database were patient age group (child: <18 years of age, adult: ≥18 years of age), temporal order of patient encounters within the study timeframe, and an active prescription for antipsychotic medication (of drug classes including phenothiazines, butyrophenones, thioxanthenes, dibenzodiazepines, diphenylbutylpiperidines, dihydroindolones, and dibenzoxazepines used for the treatment of psychotic illness). Psychotic disorder diagnoses were identified using corresponding DSM billing codes. DSM billing codes for TD diagnoses (tardive dyskinesia, tardive dystonia, tardive akathisia, and neuroleptic-induced tardive disorder) entered by the provider in the diagnostic impression list of the medical record for each encounter were included in the database. Nursing notes identifying TD through 3 fields for recording information about psychomotor abnormalities, reported side effects, and provision of patient education about any identified “tardive” or “tardive dyskinesia” findings were included as well.
Abnormal Involuntary Movement Scale (AIMS)4 ratings were collected from patient medical records. The AIMS is a widely used tool for rating abnormal movements, and it is embedded in the agency’s medical record software. This software prompts, but does not require, providers to assess patients for abnormal movements and record the findings in the medical record at least annually for all patients prescribed antipsychotic medication. The classic Kane-Schooler algorithm12 was used for scoring of the AIMS by 2 methods, with a positive clinician rating satisfied either by scoring ≥ 2 in ≥ 2 anatomic areas or scoring ≥ 3 in ≥ 1 areas from a total of 7 anatomic locations each scored from 0 (none) to 4 (severe). The AIMS also provided global overall severity and incapacity clinician ratings. For patients with AIMS data recorded at any encounter, findings from the most recent previous encounter were carried forward to subsequent encounters in the database if new data were not entered by the provider. Analysis of TD-related data in this study examined AIMS scores, diagnostic billing codes for TD, and nursing notes identifying TD in the most recent encounter for each patient.
Data Analysis
The study database was imported into SAS version 9.4 (SAS Institute, Cary, North Carolina) for analysis. Descriptive data are presented as counts and proportions of unique patients represented in dichotomous variables, and continuous data for AIMS scores are presented as mean and standard deviation (SD). Comparisons of 2 dichotomous variables were conducted using χ2 tests with the odds ratio option, substituting Fisher exact tests for any instances of expected cell sizes < 5. Comparisons of 2 continuous variables were compared using Pearson correlations. The level of statistical significance was set as α ≤ .05.
RESULTS
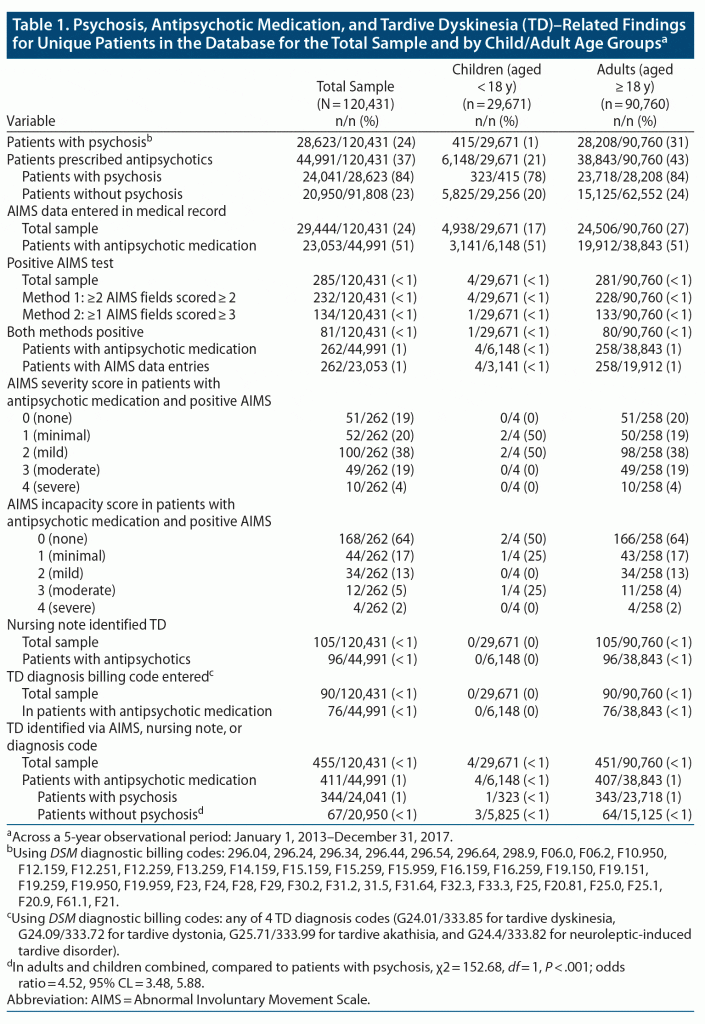
The patient population of Metrocare Services, from which this sample was drawn, is 54% male, and its racial distribution is 48% Black, 46% White, and 6% other race, with Hispanic ethnicity represented in 26%. Over the 5-year observation period of this study, the patients in the database with psychosis had a mean of 2.4 visits per unique patient among adults and 1.5 for children, and the mean number of visits among patients with AIMS data was 2.4. Table 1 presents data for unique patients including psychosis diagnosis, antipsychotic medication prescribed, and TD-related variables. Children < 18 years of age represented 25% of the patients. About one-fourth of the patients had a psychotic disorder diagnosis in the database, including nearly one-third of adults aged ≥ 18 years, but very few children. About one-third (n = 44,991) of all patients in the sample were prescribed antipsychotic medication, including the majority of both children and adults with a psychotic disorder diagnosis and almost one-fourth of patients without psychosis.
Only about one-half of the patients prescribed antipsychotic medication had AIMS data entered in the medical record. Of patients with AIMS data, only about 1% had a positive AIMS (half that amount among all adult patients prescribed antipsychotic medication; see Table 1). A positive AIMS was rarely (in about 1 of every 12,554) identified in children with AIMS data who were prescribed antipsychotic medication. For patients prescribed antipsychotic medication who had a positive AIMS, the largest numbers of patients had global AIMS severity scores in the mild category, with about one-fourth in the moderate or severe categories. Nearly two-thirds had AIMS global incapacity scores in the no incapacity category, and few scored in the moderate or severe range. The AIMS severity and incapacity scores were highly correlated (r = 0.51, P < .001).
In the entire sample of patients prescribed antipsychotic medication and AIMS data entered (not shown in the tables), the mean (SD) AIMS anatomic location category score for facial and oral movements overall was 0.9 (0.8), with specific movements rated as 0.9 (1.1) for muscles of facial expression, 1.1 (1.2) for lips and perioral area, 0.8 (1.1) for jaw, and 0.8 (1.0) for tongue. The mean (SD) AIMS anatomic location category score for extremity movements overall was 1.1 (0.9), with specific movements rated as 1.2 (1.2) for upper extremities and 1.0 (1.2) for lower extremities. The mean (SD) overall score for trunk movements was 0.6 (1.0), reflecting movements specific to neck, shoulders, and hips. The trunk category was scored significantly higher for children than for adults (child mean = 1.8, SD = 1.3 vs adult mean = 0.6, SD = 1.0, t = 2.20, df = 260, P = .028). The score for lips and perioral area was scored significantly higher for adults than for children (adult mean = 1.2, SD = 1.2 vs child mean = 0.0, SD = 0.0, t = 15.94, df = 257, P < .001). No other anatomic location category or specific movements were rated differently for adults versus children.
As shown in Table 1, <1% of patients prescribed antipsychotic medication had TD identified in a nursing note (all adults, no children). Fewer than 1% of patients prescribed antipsychotic medication had a TD diagnostic billing code entered by a psychiatric provider (again, all adults and no children). Altogether, 407 adults and 4 children prescribed antipsychotic medication in the database were identified with TD by any of the 3 methods: AIMS test, nursing note, or provider diagnostic billing code, representing about 1% of patients prescribed antipsychotic medications. The proportion of patients prescribed antipsychotic medication who were identified with TD by any means was higher for adults than for children (1.05% vs 0.07%, χ2 = 56.63, df = 1, P < .001) and also in patients with than without psychosis (about 4.5 times the odds). There were 44 adult patients (30 with psychosis, 14 without psychosis) but no children without antipsychotic medications prescribed who were identified with any TD (not shown in the tables).
The AIMS test alone identified 58% (n = 265 patients including 261 adults and 4 children) of a total of 455 patients with TD in the sample, nursing notes alone identified 21% (n = 95, all adults), and physician’s diagnosis code alone identified 16% (n = 71, all adults). Only 5% of TD cases were identified by > 1 of these 3 sources. Only 32% (90 of 285) of patients with a positive AIMS result had a TD diagnosis billing code entered.
DISCUSSION
This study examined TD identified by treating clinicians at a large public outpatient mental health center. Data were examined from electronic medical records of > 120,000 patients in this real-world treatment setting, including nearly 45,000 antipsychotic-prescribed patients. Only half of the antipsychotic-prescribed patients had AIMS information recorded in their medical records, and only 1% of this subset with AIMS data had a positive AIMS. AIMS testing represented the largest source of all identified TD in these patients, but only one-third of the patients with a positive AIMS in the record had a clinical diagnosis of TD recorded in the prescriber’s diagnostic impression list from billing code data.
The identification of only 1% of antipsychotic-prescribed patients with TD in this study is far below generally established prevalence estimates of 20% (for second-generation antipsychotic agents) to 30% (for first-generation agents), as summarized in a review and meta-analysis6 and reported in a more recent study.7 The current study’s far lower 1% estimate is, however, consistent with 1%–2% reported by another recent study13 also using clinical practice data. Important methodological differences may have contributed to this wide disparity of estimates: both the Loughlin et al13 study and the current study used retrospective medical record data entered by treating clinicians. In contrast, the Carbon et al6 meta-analysis and the Caroff et al7 study used TD data collected by carefully trained researchers dedicated to systematic assessment and documentation of data, rather than relying on TD findings that busy clinicians managed to document in the patients’ medical records.
Methodological advantages of studies with systematic collection of research data over studies using retrospective medical record data have been major contributors to inconsistent findings on the prevalence of TD among antipsychotic-prescribed patient populations. Realities of clinical practice, including lack of training and insufficient time and staffing to conduct systematic and regular formal assessments of abnormal movements, are known to limit the identification of TD.4,7 Additional methodological differences that might have contributed substantially to disparities in prevalence estimates are differences in study populations, sampling methods, and TD measures used. Variable proportions prescribed first- and second-generation antipsychotic agents in research populations could have further contributed to inconsistent findings, but not enough for this factor alone to generate such wide disparity of estimates.
In this study, a substantial proportion (nearly one-fourth) of patients who did not have psychotic illness were prescribed antipsychotic medication. The use of antipsychotic agents in patients without psychosis may be for other US Food and Drug Administration (FDA)–approved indications or for off-label purposes. The latter has become a common prescribing approach even though empirical evidence for this practice is uncertain and it exposes increasing numbers of patients to adverse effects, including TD.4,13–16 Prior studies3,6,7 have identified primary mood disorders as risk factors for TD, but in this study, TD was found to be more prevalent in the patients with psychotic disorders. This inconsistency may be at least partly explained by known confounding of lower doses and shorter treatment durations and also with predilection for use of second-generation antipsychotic agents in patients with primary mood disorders and other nonpsychotic psychiatric indications.6,7
Among children prescribed antipsychotic medication, 95% did not have psychotic illness, and very few were identified with TD. The anatomic distribution of TD identified in children was predominantly truncal, in contrast to the predominantly upper extremity and orofacial distributions observed in adult patients, suggesting that either abnormal upper extremity and orofacial movements were not well recognized in children or that more focus on abnormal truncal movements may help direct clinicians’ attention to the most likely evidence of potential TD in children.
An important strength of this study was the collection of clinical record data on a large antipsychotic-treated patient population, including > 6,000 children, in a real-world clinical care setting at a public sector mental health treatment center. The largest study of antipsychotic-treated children of all ages represented that was found in the published literature to date examined 118 children.17 The standardized agency-wide use of the AIMS for patients prescribed antipsychotic medication allowed comparison of the findings with AIMS data from many other studies.4,6 The 1% TD prevalence identified in this study’s patient population likely represents a vast underestimate based on medical record data entered by treating clinicians not generating TD data as systematically and accurately as in research dedicated to this mission. These findings, however, provide a realistic view of how clinicians actually assess, identify, and document the information about TD in their patients’ medical records. The scope of this study was limited because it did not include information about the specific antipsychotic medications prescribed, the dose, the duration of medication use, or patient medication adherence to examine the role of these variables in risk for TD. The database also did not include information regarding the severity of psychosis in the patients in the study and did not differentiate new from existing diagnoses. Additionally, carrying AIMS results forward to subsequent encounters could have contributed to inconsistency with provider diagnoses and nursing notations of TD in the last encounters specifically examined in this study.
This study’s findings have some logical implications for clinical practice in public sector psychiatry settings wherein antipsychotic medications are widely used and many patients are at risk for developing TD. Given the recent availability of FDA-approved pharmaceutical agents for treatment of TD, it is now more important than ever to identify TD so that treatment may be provided and antipsychotic medications adjusted or discontinued to reduce further liability for TD.4 Recognition of TD by clinicians may be augmented by adoption of some of the strategies applied by researchers to achieve systematic and accurate TD assessment in populations. Agency-wide policies and procedures can be established to ensure that TD assessments are conducted with regularity and accuracy on all antipsychotic-prescribed patients. This can be operationalized and implemented through electronic medical record systems requiring periodic TD assessment with formal tools such as the AIMS. Additionally, improved recognition of TD by clinicians may be enhanced by specific training on TD assessment, provision of resources including time and staff support, and removal of disincentives for identification of TD imposed by additional work burdens needed to address it.4,7 Additionally, attention to TD by clinicians can be incentivized by building AIMS assessment and intervention documentation into quality improvement expectation tools such as in HEDIS (Healthcare Effectiveness Data and Information Set). The findings of this study suggest the need for research demonstrating ways to improve clinicians’ recognition of TD in their patients, with the ultimate goals of identifying and successfully treating TD and minimizing the likelihood of TD in the treatment of psychiatric illness with antipsychotic medication.
Submitted: July 8, 2021; accepted October 5, 2021.
Published online: June 28, 2022.
Relevant financial relationships: None.
Funding/support: None.
Additional information: The dataset used for this study is housed and controlled at Metrocare Services. Information about access to the data can be obtained from the corresponding author, and restrictions apply to the availability of these data.
Clinical Points
- Clinicians in a real-world community care setting identified tardive dyskinesia in only 1% of antipsychotic-prescribed patients, far below established 20%–30% prevalence estimates.
- Given recently available US Food and Drug Administration–approved treatments for tardive dyskinesia, it is now more important than ever to identify and treat the disorder.
- Recognition of tardive dyskinesia by clinicians can be improved through agency-wide policies facilitating systematic and accurate assessment strategies.
References (17)

- Correll CU, Kane JM, Citrome LL. Epidemiology, prevention, and assessment of tardive dyskinesia and advances in treatment. J Clin Psychiatry. 2017;78(8):1136–1147. PubMed CrossRef
- Citrome L, Dufresne R, Dyrud JM. Tardive dyskinesia: minimizing risk and improving outcomes in schizophrenia and other disorders. Am J Manag Care. 2007;13:1–12.
- D’Abreu A, Akbar U, Friedman JH. Tardive dyskinesia: epidemiology. J Neurol Sci. 2018;389:17–20. PubMed CrossRef
- Kane JM, Correll CU, Nierenberg AA, et al; Tardive Dyskinesia Assessment Working Group. Revisiting the Abnormal Involuntary Movement Scale: proceedings from the Tardive Dyskinesia Assessment Workshop. J Clin Psychiatry. 2018;79(3):17cs11959. PubMed CrossRef
- Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): current concept and modern approaches to its management. Psychiatry Clin Neurosci. 2015;69(6):321–334. PubMed CrossRef
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264–e278. PubMed CrossRef
- Caroff SN, Yeomans K, Lenderking WR, et al. RE-KINECT: a prospective study of the presence and healthcare burden of tardive dyskinesia in clinical practice settings. J Clin Psychopharmacol. 2020;40(3):259–268. PubMed CrossRef
- Widschwendter CG, Hofer A. Antipsychotic-induced tardive dyskinesia: update on epidemiology and management. Curr Opin Psychiatry. 2019;32(3):179–184. PubMed CrossRef
- Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162–174. PubMed
- Brar JS, Parepally H, Chalasani L, et al. The impact of olanzapine on tardive dyskinetic symptoms in a state hospital population. Ann Clin Psychiatry. 2008;20(3):139–144. PubMed CrossRef
- Woerner MG, Kane JM, Lieberman JA, et al. The prevalence of tardive dyskinesia. J Clin Psychopharmacol. 1991;11(1):34–42. PubMed CrossRef
- Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486–487. PubMed CrossRef
- Loughlin AM, Lin N, Abler V, et al. Tardive dyskinesia among patients using antipsychotic medications in customary clinical care in the United States. PLoS One. 2019;14(6):e0216044. PubMed CrossRef
- Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–184. PubMed CrossRef
- Carton L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280–3297. PubMed CrossRef
- Trémeau F, Citrome L. Antipsychotics for patients without psychosis? what clinical trials support. Curr Psychiatr. 2006;5:33–44.
- Wonodi I, Reeves G, Carmichael D, et al. Tardive dyskinesia in children treated with atypical antipsychotic medications. Mov Disord. 2007;22(12):1777–1782. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite