Prim Care Companion CNS Disord 2021;23(5):20l02862
To cite: Mahgoub Y, Jolly T, Singh J, et al. Prophylactic antidepressants in a patient with multiple sclerosis receiving interferon-β. Prim Care Companion CNS Disord. 2021;23(5):20l02862.
To share: https://doi.org/10.4088/PCC.20l02862
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Health, Penn State College of Medicine, Hershey, Pennsylvania
*Corresponding author: Yassir Mahgoub, MD, Penn State Hershey Medical Center, 500 University Dr, Hershey, PA 17033 ([email protected]).
Multiple sclerosis (MS) is a complex neurologic disease that results in demyelination plaques with inflammation, causing axonal injury and loss. Approximately, 85% of patients present with a relapsing-remitting course, which is characterized by gradual progression of disability between either acute relapses or in the absence of relapses.
Multiple psychiatric disorders are prevalent among patients with MS, with some studies1 estimating the lifetime prevalence of depression to be near 54%. Interferon-β (INF-β) was approved in 1993 for the treatment of MS, and the US Food and Drug Administration (FDA) provides instructions to educate patients to report any depressive and suicidal symptoms that develop during treatment.2 The relation between INF-β and depression and suicidality has been investigated. Unlike INF-α, which is used for hepatitis B, C, and some malignancies, the relationship between INF-β and depression and suicidality has not been strongly established.
Case Report
A 60-year-old White woman, with a history of MS diagnosed at the age of 28 years and no other medical comorbidities, presented with new onset of depression and suicidal ideations. She had no prior psychiatric history; however, she was prescribed fluoxetine 30 mg/d, prophylactically as per her understanding, when she was started on INF-β for the treatment of MS 10 years prior to the current presentation. She reported that the dose was titrated up to 30 mg/d, on which she had been stable. Since then, she had slow and gradual progression of her neurologic symptoms, with no symptoms of depression until 3 weeks prior to her presentation. She reported feeling disappointed for not being able to refinance her house at a lower rate, although this did not result in financial hardship. She subsequently discontinued fluoxetine but continued INF-β for MS for unclear reasons. She was on a maintenance subcutaneous dose of INF-β 44 mcg 3 times/week. Within 2 weeks after stopping fluoxetine, she developed an episode of severe depression with significant pervasive depressed mood, anhedonia, lack of motivation, poor appetite, hopelessness, and suicidal ideation. Subsequently, she overdosed on several baclofen tablets with an intention to end her life and was admitted to the medical floor. She was intubated and was also treated with a course of steroids for concerns of acute MS exacerbation, during which period her INF-β was suspended. Following medical stabilization, her fluoxetine was restarted back at her previous dose of 30 mg/d, and within 1 week her depressive symptoms and suicidal ideations resolved. She was transferred to a psychiatric facility for further management. She continued to have a sustained good mood with no further symptoms of depression. Following recovery, she was not able to explain the rapid deterioration of her mood that occurred following fluoxetine discontinuation. She further added that her mood improved within days after resuming fluoxetine. She was restarted on INF-β, was maintained on fluoxetine 30 mg/d, and was observed for potential recurrence of depressive symptoms, which were not noted, and she was subsequently discharged for outpatient follow-up.
Discussion
Interferons are glycoproteins secreted in response to viral infections to protect other cells against infection. In addition, interferons have complex immunomodulatory and antiproliferative effects. Interferons are divided into 3 types: INF-1, INF-2 or INF-γ, and INF-3. Many medications focus on INF-1, and 2 types of INF-1 have been identified by functional and molecular characteristics, which are INF-α and INF-β.
INF-α
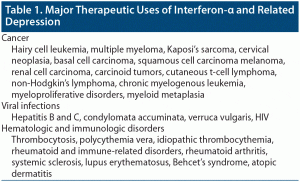
INF-α was approved by the FDA for hairy cell leukemia in 1984.3 Since then, there has been an expansion of its use for different malignancies, viral infections, and hematologic disorders4 (Table 1).
Several studies found that depression is common among patients receiving INF-α. In hepatitis C, it is estimated that mild to moderate depression develops in 45%–60% of patients treated with INF-α, moderate to severe depression in 15%–40%, and major depression in 15%–45%.4,5 In hairy cell leukemia, it was found that 3.3% developed depression while on interferon treatment.6 In malignant melanoma, it was found that 45% developed depression while on interferon treatment.7
Some risk factors for INF-α−induced depression or suicide were found including depression during previous treatment with INF-α; depressive symptoms before treatment is started, although this is not associated with outcome; sleep disturbances before treatment; early vegetative symptoms (such as sleep, loss of appetite) during treatment, baseline stress, and lack of social support; and biomarkers of inflammation before starting treatment.5
The use of prophylactic antidepressants has become a widely accepted practice. The precedent of prophylactic use of antidepressants occurred after a study7 of patients with malignant melanoma who received INF-α. Forty patients without prior depression were studied: 1 group with placebo and the second group with paroxetine. The rate of depression was 11% among the paroxetine group in comparison to 45% in the placebo group.7 This study7 propelled further exploration of antidepressant prophylaxis among patients receiving INF-α for hepatitis B and C. Two interventional approaches were studied. The first involved prophylactic treatment with the goal of preventing or attenuating depressive symptoms. The second was the traditional approach of rescue that involves the quick initiation of treatment of depression following the emergence of symptoms. Most of these studies were done in patients with hepatitis C, in which patients also received ribavirin.8 Schaefer et al5 used escitalopram prophylactically among patients with hepatitis C and found that 32% of patients developed depression in the escitalopram group in comparison to 59% in the placebo group. However, Diez-Quevedo et al9 also used escitalopram for prophylaxis but was unable to find differences between the treatment group and placebo group. Gleason et al10 concluded that pretreatment of patients with history of MDD using escitalopram prevented recurrence of MDD during the course of treatment with INF-α.
Raison et al4 used paroxetine for prophylaxis and Schaefer et al11 used citalopram and both found that pretreatment increased treatment adherence to INF-α. Schaefer et al11 suggested considering citalopram as a first-line agent for treatment, considering underlying hepatic function, drug-induced hepatoxicity, and other side effects. They recommended the use of escitalopram, paroxetine, mirtazapine, or sertraline as a second-line agent.11 Other studies8 and clinicians report that rescue treatment is a reasonable strategy when depression evolves during the course of treatment with INF-α.
INF-β
INF-β was approved by the FDA in 1996 for the treatment of MS.12 Two forms are available: INF-β1a, which is a glycosylated recombinant mammalian product with an amino acid sequence identical to that of natural INF-β, and INF-β1b, which is a non-glycosylated recombinant bacterial product in which the serine is substituted with cysteine at position 17. Both forms are used for the treatment of relapsing-remitting MS.
There is a high prevalence of depression in MS patients, with some studies estimating the lifetime prevalence to be around 54%. However, it does not appear to be related to the severity of neurologic features or duration of the symptoms.1 With the introduction of INF-β and the concerns about its association with depression and suicidality, similar to INF-α, several studies and reports12–17 investigated such associations, but results were inconsistent. The initial clinical trials done to obtain FDA approval for the treatment of MS in 1993 found that fatigue/depression accounted for almost 60% of medication discontinuation, and depression was observed at increased frequency among subjects; however, the association with depression was not statistically significant in patients treated with INF-β. The FDA still provided guidelines for providers to educate MS patients about the potential risk for developing depression and suicidal ideations.12 Jacobs et al13 studied the introduction of INF-β during the first demyelination event. They found that patients treated with INF-β were more likely to develop depression than placebo.13 However, large studies such as the SPECTRIMS study,14 which followed 365 subjects for 3 years to evaluate the association of depression with subjects treated with INF-β; the PRISMS trials,15 which had 560 subjects; and the European study groups on secondary progressive MS all failed to establish any association between INF-β with increased risk of depression and suicidality. Mohr et al16 followed a group of depressed patients before and after treatment with INF-β and compared them with a group of nondepressed subjects, and there was an initial improvement of depression in the first 2 weeks of treatment for patients on INF-β and return back to baseline level of depression in 8 weeks, while the nondepressed subject group had no significant change in mood after treatment. Mohr et al17 also studied the association between reported depression among patients with MS treated with INF-β. However, they used a questionnaire (follow-up Betaseron questionnaire) rather than a standard self-report scale or validated tool to assess depression. The authors17 concluded that 41% of the study subjects (85 subjects) reported new or increased depression.
While the data on the relationship between INF-β and development of depression and suicidality in patients with MS are not solid in comparison to INF-α, it is important to educate patients receiving INF-β about the possibility, especially with the high rate of depression among patients with MS, as treatment can result in improvement of symptoms of depression as well as compliance with INF-β. In our patient, the justification for initiating prophylactic antidepressants when INF-β was introduced was not clear and is not noted to be a common practice for the lack of supportive evidence justifying it. An argument can be made that poorly recalled or subsyndromal symptoms were among the reasons for initiating and titrating fluoxetine during the introduction of INF-β. She reported being disappointed and stressed out prior to stopping fluoxetine. She stated that her INF-β doses cost thousands of dollars per month and were provided to her through charity. However, she was not able to explain the reason behind continuing INF-β while stopping fluoxetine alone. It is possible that the financial stress contributed to a depressive episode. However, she was not in a state of financial hardship at that time, and she also had the support of her family. She continued to take INF-β following the discontinuation of fluoxetine, and she subsequently developed severe depression and attempted suicide within 1 month. INF-β was stopped on the medical floor due to the concerns of active MS exacerbation. Her depressive symptoms resolved within 1 week after she resumed fluoxetine. This case continues to raise the question about the potential association of INF-β use in MS patients, with development or worsening of depression and suicidal ideations. Despite not having enough support in the practice of using prophylactic antidepressants in patients with MS receiving INF-β, appropriate and aggressive treatment should be implemented if depression emerges during the course of treatment while still continuing treatment with INF-β. Routine administration of depression screening scales should be considered for patients who are being started on this class of medicine. While there are robust studies4,5,7,8,10,11 on the prophylactic use of antidepressants in patients treated with INF-α, there is no general consensus on the same when it comes to INF-β. Our report as well as previous research that indicates a high prevalence of depression among patients with MS pave the way for more robust and well-designed studies to look at the prophylactic use of antidepressants for INF-β.
Published online: September 30, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: This case was accepted for a poster presentation at the Annual Meeting of the American Psychiatric Association in 2020 but was not presented due to cancellation of the annual meeting.
Patient consent: Verbal consent was obtained from the patient to publish this case report, and personal details were de-identified to protect anonymity.
References (17)

- Minden SL, Orav J, Reich P. Depression in multiple sclerosis. Gen Hosp Psychiatry. 1987;9(6):426–434. PubMed CrossRef
- Betaseron (interferon beta-1b) [package insert]. Whippany, NJ: Bayer HeathCare Pharmaceuticals Inc; 2016.
- Quesada JR, Reuben J, Manning JT, et al. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984;310(1):15–18. PubMed CrossRef
- Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. PubMed CrossRef
- Schaefer M, Capuron L, Friebe A, et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol. 2012;57(6):1379–1390. PubMed CrossRef
- Martin A, Nerenstone S, Urba WJ, et al. Treatment of hairy cell leukemia with alternating cycles of pentostatin and recombinant leukocyte A interferon: results of a phase II study. J Clin Oncol. 1990;8(4):721–730. PubMed CrossRef
- Navinés R, Gómez-Gil E, Puig S, et al. Depression in hospitalized patients with malignant melanoma treated with interferon-alpha-2b: primary to induced disorders. Eur J Dermatol. 2009;19(6):611–615. PubMed CrossRef
- Kraus MR, Schäfer A, Faller H, et al. Paroxetine for the treatment of interferon-α-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16(6):1091–1099. PubMed CrossRef
- Diez-Quevedo C, Masnou H, Planas R, et al. Prophylactic treatment with escitalopram of pegylated interferon alfa-2a-induced depression in hepatitis C: a 12-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(4):522–528. PubMed CrossRef
- Gleason OC, Fucci JC, Yates WR, et al. Preventing relapse of major depression during interferon-alpha therapy for hepatitis C—a pilot study. Dig Dis Sci. 2007;52(10):2557–2563. PubMed CrossRef
- Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42(6):793–798. PubMed CrossRef
- Jacobs LD, Cookfair DL, Rudick RA, et al; The Multiple Sclerosis Collaborative Research Group (MSCRG). Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39(3):285–294. PubMed CrossRef
- Jacobs LD, Beck RW, Simon JH, et al; CHAMPS Study Group. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343(13):898–904. PubMed CrossRef
- Patten SB, Metz LM; SPECTRIMS Study Group. Interferon beta1a and depression in secondary progressive MS: data from the SPECTRIMS Trial. Neurology. 2002;59(5):744–746. PubMed CrossRef
- PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352(9139):1498–1504. PubMed CrossRef
- Mohr DC, Dick LP, Russo D, et al. The psychosocial impact of multiple sclerosis: exploring the patient’s perspective. Health Psychol. 1999;18(4):376–382. PubMed CrossRef
- Mohr DC, Goodkin DE, Likosky W, et al. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997;54(5):531–533. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite