Sturge-Weber syndrome (SWS) is a rare embryological neurocutaneous malformation involving the excessive uncontrolled proliferation of blood vessels, with subsequent calcifications over time.1 Blood vessel proliferation is predominant in 3 areas of the head—the brain (leptomeningeal angiomas), the eyes (glaucoma), and the face (facial capillary hemangioma). SWS has a prevalence of occurrence among 1 in 20,000–50,000 live births with more predominance in White individuals and those of female biological sex.1,2 Thus far, very little is known about the extensive manifestation of SWS, especially in adulthood. In terms of etiology, the GNAQ gene mutation overexpression in genome-wide association studies has been attributed to the pathology.2,3 Though few disorders such as those that lead to neurologic, behavioral, and mood symptoms have been described in adulthood,4,5 there remains a gap in the literature regarding psychiatric manifestations. Psychosis has been described in other neurocutaneous malformations like tuberous sclerosis6 and neurocutaneous melanosis,7 but there is no concrete relationship between SWS and psychosis. Since research on psychiatric manifestation among SWS patients is still ongoing, there remains an unanswered question regarding psychosis. Hence, we present a case of an SWS patient with progressive psychosis over time and subsequent diagnosis of schizophrenia. We highlight the possible association between SWS and schizophrenia pathology.
Case Report
The emergency medical service brought in a 30-year-old African American man in the context of aggressive behavior toward his family. The patient had a medical history of morbid obesity, bronchial asthma, and SWS with an associated seizure disorder. On evaluation in the emergency department (ED), the patient was agitated, internally preoccupied, talking to self, and exhibiting paranoid and persecutory delusions. He continued to express concerns that his family had been stealing his personal items and cash. He remained very talkative and refused to follow safety instructions, and he exhibited poor frustration tolerance, poor impulse control, and poor reality testing. The patient was disorganized in behavior, as he kept pacing the unit and attempting to elope. He was subsequently admitted to the inpatient psychiatric unit and started on oral olanzapine 10 mg once daily and phenytoin sodium 200 mg twice daily for seizures.
On inpatient evaluation, the patient was hyper-talkative and difficult to interrupt. Regarding the circumstance leading to his admission, he reported that his aunt does not want him to live with her anymore and activated 911. He denied any disorganized or aggressive behavior toward the family before the activation of 911. He reported that his aunt hides his stuff from him all the time, including his samurai sword. He stated that his sword was like a toy to him and that he had no intent to hurt anyone with it. He reported keeping the samurai sword as a collectable. He further stated that he felt people were always following him and trying to rob him of his sword. The patient also admitted to over a year’s history of non-distressing auditory hallucination that occurred daily and was last experienced during his psychiatric ED evaluation. He said the voice told him “You have to get out of this place. The security guys are trying to jump on you.” The patient denied commanding hallucinations. The patient denied any family history of mental disorders and denied any history of head trauma.
Per collateral reports, the patient was increasingly agitated during the week prior to presentation to the ED. Due to worsening agitation and aggression toward family members, 911 was finally activated. The patient’s intermittent agitation reportedly started 8 years before presentation and was associated with periods of disorganized behavior and paranoia. He was treated for delusional disorder and never had a deteriorating clinical manifestation that required inpatient psychiatric admission. The patient was also diagnosed with intellectual disability and required special education services.
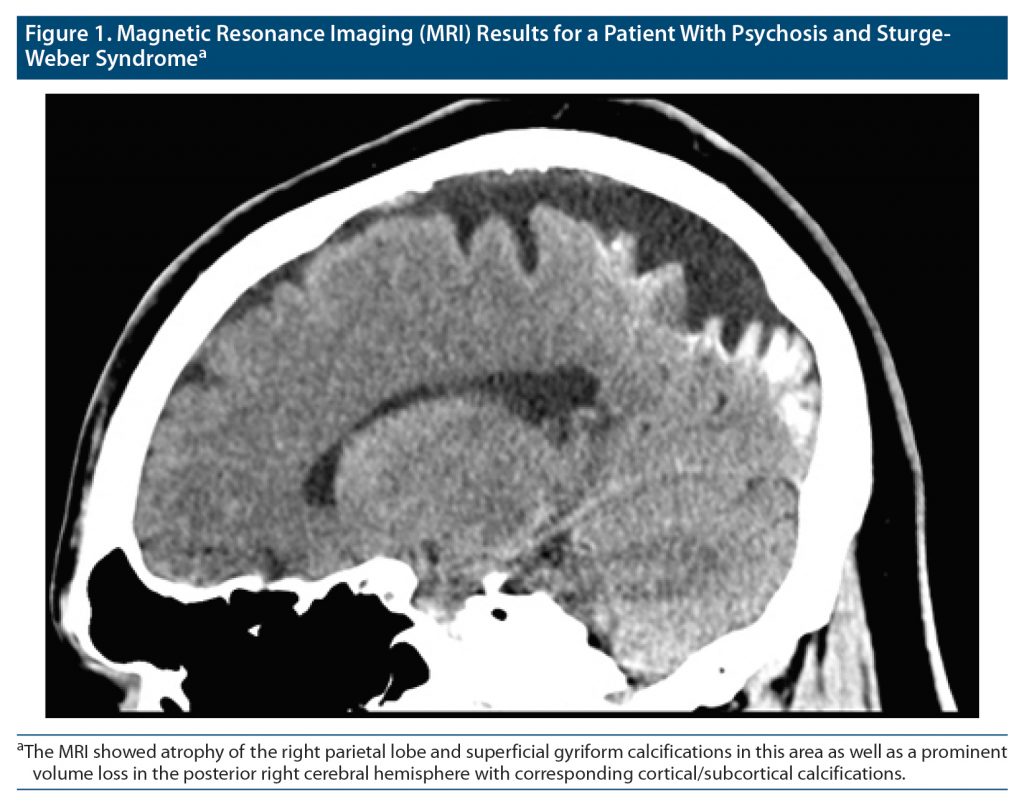
On further evaluation on the unit, the patient had a Mini-Mental Status Examination (MMSE)8 score of 24, and the Wechsler Adult Intelligence Scale (WAIS)9 showed a total IQ of 73 (verbal IQ, 75; non-verbal IQ, 71). The patient’s laboratory results were unremarkable. Brain imaging was also performed (see Figure 1 for the results of magnetic resonance imaging [MRI]), which showed there was atrophy of the right parietal lobe and superficial gyriform calcifications in this area. There was a prominent volume loss in the posterior right cerebral hemisphere with corresponding cortical/subcortical calcifications. Given the patient’s psychiatric presentation, he was diagnosed with schizophrenia (DSM-5-TR criteria). The treatment team adopted an integrated care approach to effectively manage his psychiatric and medical comorbidity.
The patient tolerated all his medications and began to show clinical improvement and good behavioral control by day 6 of his hospital stay. He was subsequently discharged back home following psychiatric stability.
Discussion
It is noteworthy that SWS is associated with a myriad of neurologic abnormalities, and the severity of its involvement varies among those affected.5 Seizures are prevalent from infancy and childhood in most patients. Other notable neurologic sequelae include developmental disabilities, intellectual disabilities in the form of mild/moderate partial muscle weakness, or one-sided paralysis (hemiparesis).5 Additionally, hemodynamic complications of SWS in the form of transient ischemic attacks (TIAs) and strokes have been described in the literature.10 These may lead to permanent visual defects (hemianopsia). Behavioral issues have been described in individuals with SWS including attention-deficit/hyperactive disorder (ADHD), mood disorders, and substance use disorders. However, the association with psychosis remains understudied to date. Specifically, no publication on SWS and schizophrenia comorbidity was found in our review of the extant literature found.
After a search and review of literature in the PsycINFO, EMBASE, and PubMed databases, results suggested a gap with regard to SWS-related psychosis. We identified only 1 case report on associated psychosis in a female Asian patient with SWS.11 Though SWS is non-hereditary, it has been attributed to a mutation in the GNAQ gene,3 a gene responsible for producing proteins that regulate signaling pathways for the development of blood vessels. Enhanced signaling results from mutation in the gene, which leads to abnormal and excessive proliferation of blood vessels.
In terms of pathophysiology, there is scientific evidence in the literature that suggests the schizophrenia comorbidity in individuals with SWS might be attributable to the GNAQ mutation. Laboratory studies conducted among monkeys, for example, showed increased expression of the altered GNAQ gene in schizophrenia.3 Since our patient had no positive family history of mental disorder, the gene mutation appears to be a plausible linkage between the SWS and schizophrenia comorbidity. Similarly, the 2015 study by Rukova et al12 on genome-wide methylation profiling among people with schizophrenia showed that the GNAQ gene was one of the differentially methylated regions among female schizophrenic patients. Alternatively, the psychosis could be attributable to genetic activation from interaction with environmental factors (eg, cannabis use) in genetically susceptible individuals. While the patient in this case report used cannabis—although sparingly—he did not have a family history of a psychotic disorder. Additionally, unlike in most males with schizophrenia, the first episode of psychosis in this patient occurred at a later age. Hence, we believe a diagnosis of schizophrenia in this patient with SWS, without the well-known risk factors for psychosis, is unique.
Conclusion
This case report presents an unusual relationship between psychosis and SWS in a patient with no family history of mental illness (including schizophrenia). While no absolute relationship between SWS and schizophrenia is purported by this case, we hope it stimulates studies into the role of the GNAQ gene in the development of schizophrenia. Finally, health care personnel are encouraged to have a high index of suspicion for the emergence of schizophrenia spectrum disorder among patients with SWS.
Article Information
Published Online: August 8, 2023. https://doi.org/10.4088/PCC.22cr03459
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(4):22cr03459
Submitted: December 1, 2022; accepted March 15, 2023.
To Cite: Nkemjika S, Jordan E, Fouron P, et al. Psychosis in a patient with Sturge-Weber syndrome: a case report and literature review. Prim Care Companion CNS Disord. 2023;25(4):22cr03459.
Author Affiliations: Department of Psychiatry and Behavioral Sciences, Interfaith Medical Center, Brooklyn, New York (Nkemjika, Fouron, Saeed, Olupona); Medical University of the Americas, Saint Kitts and Nevis (Jordan); Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at Houston, Houston, Texas (Olayinka).
Corresponding Author: Stanley Nkemjika, MD, MPH, Interfaith Medical Center, 1545 Atlantic Ave, Brooklyn, NY 11233 ([email protected]).
Relevant Financial Relationships: The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this article.
Funding/Support: None.
Patient Consent: Permission was obtained from the patient to publish this case report, and information was de-identified to protect anonymity.
References (12)

- Singh AK, Keenaghan M. Sturge-Weber syndrome. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- Sloneem J, Moss J, Powell S, et al. The prevalence and profile of autism in Sturge-Weber syndrome. J Autism Dev Disord. 2022;52(5):1942–1955. PubMed CrossRef
- MacDonald ML, Ding Y, Newman J, et al. Altered glutamate protein co-expression network topology linked to spine loss in the auditory cortex of schizophrenia. Biol Psychiatry. 2015;77(11):959–968. PubMed CrossRef
- Turin E, Grados MA, Tierney E, et al. Behavioral and psychiatric features of Sturge-Weber syndrome. J Nerv Ment Dis. 2010;198(12):905–913. PubMed CrossRef
- Luat AF, Juhász C, Loeb JA, et al. Neurological complications of Sturge-Weber syndrome: current status and unmet needs. Pediatr Neurol. 2019;98:31–38. PubMed CrossRef
- Pokharel S, Jyotsana P, Maharjan RS, et al. Tuberous sclerosis complex-associated neuropsychiatric disorder (TAND) in a low-resource setting—from seizure to psychosis: a case report. Ann Med Surg (Lond). 2012;2020(60):734–736. PubMed
- Azzoni A, Argentieri R, Raja M. Neurocutaneous melanosis and psychosis: a case report. Psychiatry Clin Neurosci. 2001;55(2):93–95. PubMed CrossRef
- Creavin ST, Wisniewski S, Noel-Storr AH, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. 2016;2016(1):CD011145. PubMed CrossRef
- Merz ZC, Van Patten R, Hurless N, et al. Furthering the understanding of Wechsler Adult Intelligence Scale-fourth edition factor structure in a clinical sample. Appl Neuropsychol Adult. 2021;28(1):12–23. PubMed CrossRef
- Jansen FE, van der Worp HB, van Huffelen A, et al. Sturge-Weber syndrome and paroxysmal hemiparesis: epilepsy or ischaemia? Dev Med Child Neurol. 2004;46(11):783–786. PubMed CrossRef
- Chen S-W, Sun T-F, Lui C-C, et al. Sturge-Weber syndrome associated with auditory hallucination. Taiwanese J Psychiatry. 2006;20(4):321–327.
- Rukova B, Staneva R, Hadjidekova S, et al. Genome-wide methylation profiling of schizophrenia. Balkan J Med Genet. 2015;17(2):15–23. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite