aMaltepe University, Faculty of Medicine, Department of Neurology, Istanbul, Turkey
*Corresponding author: Miruna Florentina Ates, MD, Maltepe University, Faculty of Medicine, Department of Neurology, Feyzullah St No: 39, 34844 Maltepe, Istanbul, Turkey ([email protected]).
Prim Care Companion CNS Disord 2021;23(2):21cr02931
To cite: Ates MF, Karsidag S. Ptosis and COVID-19: an unusual initial finding. Prim Care Companion CNS Disord. 2021;23(2):21cr02931.
To share: https://doi.org/10.4088/PCC.21cr02931
© Copyright 2021 Physicians Postgraduate Press, Inc.
The severe acute respiratory syndrome coronavirus 2 or coronavirus disease 2019 (COVID-19) caused by novel coronavirus was reported as a neurotropic virus.1 The literature suggests that Guillain Barre syndrome, isolated cranial neuropathies, encephalopathy, encephalitis, myopathy, myositis, myasthenia gravis, and stroke were reported related with COVID-19.2 In this report, a COVID-19 case with hemiptosis is presented.
Case Report
A 61-year-old woman presented to the neurology outpatient clinic with drooping of the left eyelid and vertigo. Neurologic examination revealed partial unilateral ptosis on the left side. Ocular movements, diameter of the pupils, and light reflex were normal. Ptosis did not show fluctuation, and a forced eyelid closure test was negative for fatigue. Muscle strength, deep tendon reflexes, and cerebellar tests were within normal limits.
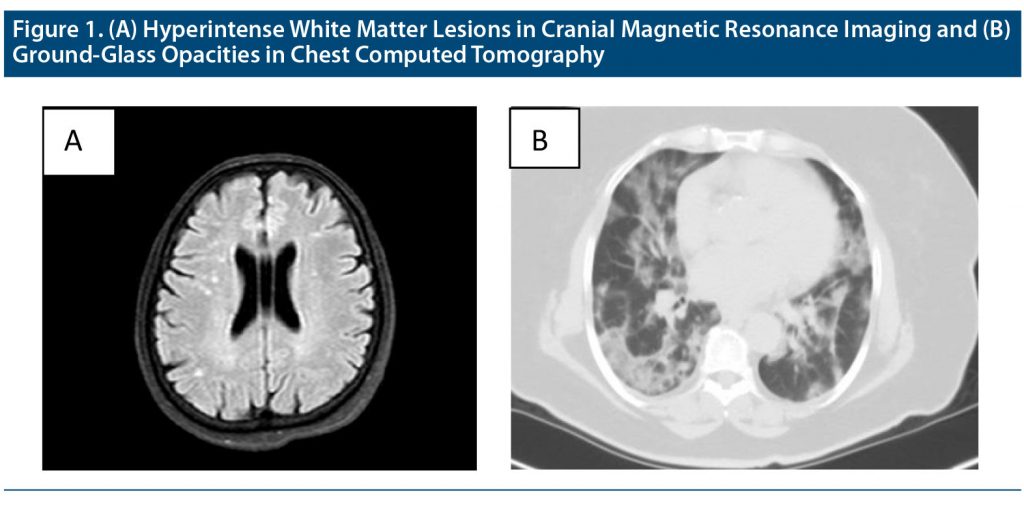
The routine biochemical analysis results were normal except for slightly increased erythrocyte sedimentation rate (39 mm/h). Some white matter hyperintensities in centrum semiovale were detected on brain magnetic resonance imaging (MRI), which does not show diffusion restriction or contrast enhancement (Figure 1A). The fluorescent antinuclear antibody test and rheumatoid factor were investigated for suspicion of vasculitis and were negative.
Methylprednisolone 64 mg/d was initiated. After 6 days, the patient was hospitalized with high fever, myalgia, and cough. A COVID-19 nasopharyngeal swab polymerase chain reaction test was positive, and ground-glass opacities were found in her lung computed tomography scan (Figure 1B).
Favipiravir was started (1,600 mg twice daily followed by 600 mg twice daily for 5 days). The laboratory tests were notable in terms of elevated inflammatory markers including C-reactive protein: 226 mg/L (range, 0–5 mg/L), fibrinogene: 625 mg/dL (range, 200–400 mg/dL), creatine kinase: 255 U/L (range, 26–192 U/L), D-dimer: 1.2 mg/L (range, 0–0.5 mg/L), procalcitonin: 1.14 ng/mL (< 0.5 ng/mL), eosinophile count: 0.02/mm3 (range, 0.05–0.5/mm3), and lymphocytes count: 1.1/mm3 (range, 1–4.8/mm3).
Hemiptosis was completely resolved after a week. After 15 days, control MRI was performed, and it showed similar white matter lesions that were found previously. However, thickened sinus mucosa was seen in maxillary and ethmoid sinuses on the left side.
Discussion
In this case, hemiptosis was observed as an early symptom at day 6 before COVID-19 symptoms started. Dinkin et al3 reported 2 cases with cranial neuropathy containing the third and sixth nerves. MRI showed hyperintensity on T2-weighted sequence and gadolinium enhancement on the third cranial nerve and optic nerve sheaths in these cases.3 Oliveira et al4 reported a case with trochlear nerve palsy secondary to vertebrobasilar vasculitis in duration of COVID-19 infection. Facial, glossopharyngeal, vagal, and trigeminal neuropathy is described in COVID-19 infection.5–8
Huber et al9 reported a patient who developed postinfectious myasthenia gravis during COVID-19 infection. Ptosis did not fluctuate in our patient. Pathophysiologic mechanisms such as ACE-2 expression in nervous tissue cells, access to central nervous system through olfactor bulbus gate, the retrograde dissemination of the virus, and the neuronal changes in the hypothalamus and cortex might play a role in neurologic involvement of COVID-19.10
In this case, ptosis may be a result of myopathic or neuromuscular junction dysfunction that develops secondary to retrograde invasion from the nasal and ethmoid cavity. Old multiple small possible ischemic lesions or changes of blood-brain barrier permeability in the eye area in the motor cortex may be responsible for reversible hemiptosis. The aim of our presented case is to underscore that neurologic symptoms can be seen before viremia of COVID-19 starts.
Published online: April 8, 2021.
Author contributions: Concept: Dr Karsidag, design: Dr Ates, supervision: Dr Karsidag, literature search: Dr Ates, manuscript writing: Dr Ates, critical review: Dr Karsidag.
Potential conflicts of interest: None.
Funding/support: None.
Patients consent: Consent was obtained from the patient to publish the case report, and information was de-identified to protect anonymity.
References (10)

- Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020;77(6):679–680. PubMed CrossRef
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. PubMed CrossRef
- Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95(5):221–223. PubMed CrossRef
- Oliveira RMC, Santos DH, Olivetti BC, et al. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arq Neuropsiquiatr. 2020;78(6):385–386. PubMed CrossRef
- Wan Y, Cao S, Fang Q, et al. Coronavirus disease 19 complicated with Bell’s palsy: a case report. Research Square; 2020. Accessed March 17, 2021. https://www.researchsquare.com/article/rs-23216/v1
- Aoyagi Y, Ohashi M, Funahashi R, et al. Oropharyngeal dysphagia and aspiration pneumonia following coronavirus disease 2019: a case report. Dysphagia. 2020;35(4):545–548. PubMed CrossRef
- Ferreira ACAF, Romão TT, Macedo YS, et al; Fellow of the American Academy of Neurology (FAAN). COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27(9):1748–1750. PubMed CrossRef
- Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. PubMed CrossRef
- Huber M, Rogozinski S, Puppe W, et al. Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front Neurol. 2020;11:576153. PubMed CrossRef
- Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite