Objective: To identify the changes in QT dispersion (QTd), corrected QTd (QTcd), and P-wave dispersion (Pd) values with long-term alcohol abuse that could lead to severe ventricular arrhythmia, atrial fibrillation, and sudden death in alcohol use disorder (AUD) patients with excessive alcohol use.
Methods: This cross-sectional study included 48 individuals diagnosed with AUD based on DSM-5 criteria. Patients with a history of psychiatric diseases were not included. The control group comprised 48 individuals with no psychiatric diagnosis who did not abuse alcohol or other substances. Participants with body mass index > 24.9 kg/m2 were excluded. Twelve-derivation electrocardiograms (ECG) were obtained from all participants.
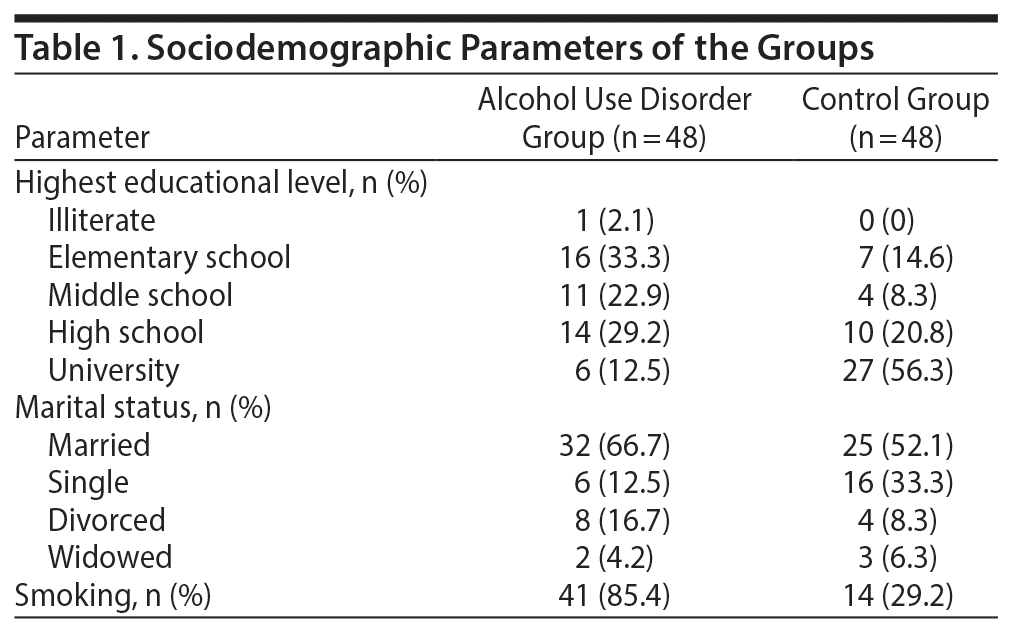
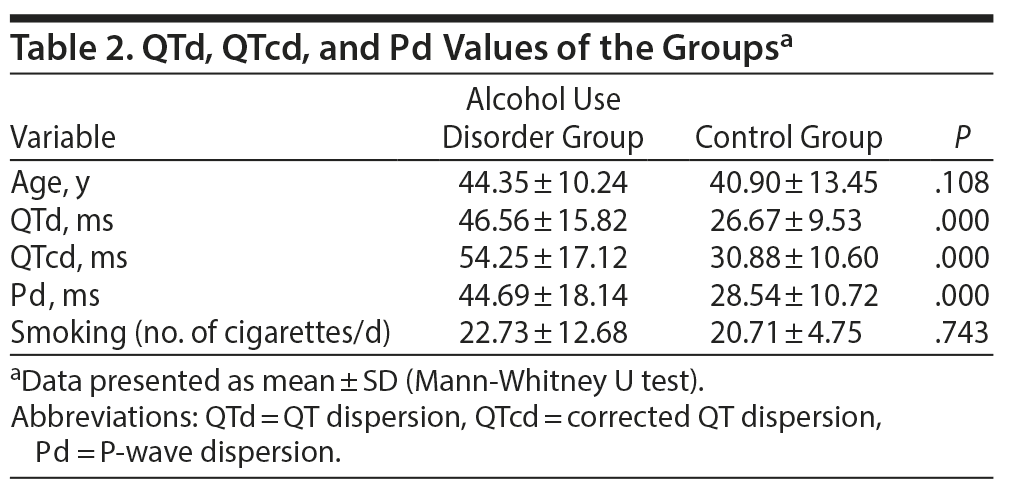
Results: The mean ± SD age was 44.35 ± 10.24 years in the AUD group and 40.90 ± 13.45 years in the control group. There was no significant difference between the groups based on age (P = .108). There was a significant difference between the groups based on smoking status (P = .000). The mean ± SD period of alcohol use was 20.71 ± 12.04 years, and the alcohol intake was 5.88 ± 1.65 units/d. The AUD group demonstrated elevations in all ECG measures (QTd: 46.56 vs 26.67 ms, QTcd: 54.25 vs 30.88 ms, Pd: 44.69 vs 28.54 ms, all P = .000).
Conclusions: AUD patients with excessive alcohol use had a higher risk of arrhythmia and sudden death compared to the control group. Consideration of ECG and referral to cardiologic examinations would contribute to the follow-up and health of patients with AUD.
ABSTRACT
Objective: To identify the changes in QT dispersion (QTd), corrected QTd (QTcd), and P-wave dispersion (Pd) values with long-term alcohol abuse that could lead to severe ventricular arrhythmia, atrial fibrillation, and sudden death in alcohol use disorder (AUD) patients with excessive alcohol use.
Methods: This cross-sectional study included 48 individuals diagnosed with AUD based on DSM-5 criteria. Patients with a history of psychiatric diseases were not included. The control group comprised 48 individuals with no psychiatric diagnosis who did not abuse alcohol or other substances. Participants with body mass index > 24.9 kg/m2 were excluded. Twelve-derivation electrocardiograms (ECG) were obtained from all participants.
Results: The mean ± SD age was 44.35 ± 10.24 years in the AUD group and 40.90 ± 13.45 years in the control group. There was no significant difference between the groups based on age (P = .108). There was a significant difference between the groups based on smoking status (P = .000). The mean ± SD period of alcohol use was 20.71 ± 12.04 years, and the alcohol intake was 5.88 ± 1.65 units/d. The AUD group demonstrated elevations in all ECG measures (QTd: 46.56 vs 26.67 ms, QTcd: 54.25 vs 30.88 ms, Pd: 44.69 vs 28.54 ms, all P = .000).
Conclusions: AUD patients with excessive alcohol use had a higher risk of arrhythmia and sudden death compared to the control group. Consideration of ECG and referral to cardiologic examinations would contribute to the follow-up and health of patients with AUD.
Prim Care Companion CNS Disord 2020;22(1):19m02541
To cite: Baykara S, Ocak D, Berk SS, et al. Analysis of QT dispersion, corrected QT dispersion, and P-wave dispersion values in alcohol use disorder patients with excessive alcohol use. Prim Care Companion CNS Disord. 2020;22(1):19m02541.
To share: https://doi.org/10.4088/PCC.19m02541
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, School of Medicine, Fırat University, Elazig, Turkey
bNecip Fazıl City Hospital, Kahramanmaraş, Turkey
*Corresponding author: Sema Baykara, MD, Department of Psychiatry, Firat University Hospital, 23119 Elazig, Turkey ([email protected]).
Alcohol use disorder (AUD) is a psychoactive substance dependency that is highly prevalent worldwide. According to World Health Organization estimates, 6% of global deaths are caused by alcohol use, and the mortality rate due to alcohol-induced cardiovascular disease is 12%.1 QT dispersion (QTd) is the difference between the maximum QT value and the minimum QT value measured with 12-derivation surface electrocardiogram (ECG). Corrected QT (QTc) refers to the QT interval adjusted for the heart rate. QTc dispersion (QTcd) is obtained by measuring the difference between the maximum QTc and the minimum QTc in milliseconds by any ECG derivation.2 Prolongation in QT indicates cardiac autonomy imbalance and increases susceptibility to malignant ventricular arrhythmias.3 QT prolongation was reportedly observed in 22%–46.9% of patients with AUD.4 Age, female sex, left ventricular hypertrophy, ischemia, slow heart rate, and electrolyte imbalance could lead to QT prolongation.5 Prolonged QT, a rare condition in healthy individuals (0.0017%–0.31%), could be observed as a result of alcohol use or hypomagnesemia caused by alcohol use and may lead to tachyarrhythmia and sudden death.6 P-wave dispersion (Pd) indicates the difference between the maximum P (Pmax) and the minimum P (Pmin) value. Pd prolongation is a risk factor for atrial fibrillation.7 Although there are a few studies8–12 that investigated QTd changes in patients diagnosed with AUD in the literature, no studies were found on Pd. The present study aimed to identify the changes in QTd, QTcd, and Pd values with long-term alcohol abuse that could lead to severe ventricular arrhythmia, atrial fibrillation, and sudden death in AUD patients with excessive alcohol use.
METHODS
This cross-sectional study compared the ECG QTd, QTcd, and Pd values of patients with long-standing AUD with that of controls with no history of alcohol abuse from a hospital alcohol and substance addiction therapy and research center outpatient clinic in Turkey. All participants signed an informed consent form. The study was approved by the Ethics Committee of Fırat University. Procedures were strictly in accordance with the ethical standards of the Institutional and National Committee on Human Experimentation and with the Declaration of Helsinki.13
Subjects
Both the AUD and control groups were composed of men. The AUD group was aged between 21 and 66 years and the control group between 20 and 65 years. Exclusion criteria for both groups included history of any cardiologic (cardiac arrhythmias, unstable coronary heart disease, atrioventricular blocks or bundle branch blocks, heart failure), neurologic, endocrinologic, metabolic, or psychiatric disorders; any electrolyte imbalance; systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg; and body mass index > 24.9 kg/m2. All participants included in the study were normotensive.
AUD group. Sixty-five consecutive patients diagnosed with AUD and history of abuse for at least 10 years (per DSM-5 criteria14) who applied to the outpatient clinic for treatment were invited to participate in the study. Seven patients declined to participate, 4 did not attend the appointment, and 6 did not meet further study criteria. A total of 48 individuals participated in the study.
Control group. The control group included 48 healthy individuals with no history of alcohol or substance abuse for the past year. The control group comprised men who were referred to the outpatient clinic by any official institutions to prove that they were free of substances of abuse based on urine analyses.
All participants completed a sociodemographic form, which included alcohol history. One unit of liquor was accepted to contain 8–10 g of alcohol (1 unit of liquor: 33 cl beer, 1 glass of wine, single raki, 1 shot of liquor).15 Alcohol consumption parameters were as follows: 0.1–9.9 g ethanol/d was defined as low, 10–30 g ethanol/d as moderate, and > 30 g ethanol/d as heavy.16
Height and weight of all participants were measured, and BMI was calculated. Blood pressure of the participants was measured with an automatic sphygmomanometer (Omron HEM-7113, Omron Healthcare, Lake Forest, Illinois) in secant after 10 minutes of rest. The participants with systolic blood pressure ≥ 140 mm Hg and diastolic blood pressure ≥ 90 mm Hg were excluded in both groups. After 10 minutes of resting, 12-derivation ECG (Cardiofax S, Nihon Kohden, Japan) with 3 standard (I–III), 3 unipolar (aVR, aVL, aVF), and 6 precordial (V1–V6) at a 25 mm/s paper speed and 1.0 mV/cm amplitude standardization was applied. The results were evaluated manually by the cardiologist (S.K.) who was blind to the groups. The distance from the beginning of the Q wave to the end point where the T wave returned to the isoelectric line was measured in milliseconds as the QT interval. The lowest point of the combined section of the T wave and the U wave was accepted as the finishing point of the T wave if there was a U wave.17 The mean of 3 consecutive QT intervals was accepted as the final value. QTd values were calculated by subtracting the minimum value from the maximum value after measurement of the maximum and minimum values within the 12 derivation of each interval. The QTc durations were calculated according to the Bazett formula: QTc = QT/√(R-R).18 The QTc was considered long (long QT) at ≥ 450 ms in men and ≥ 460 ms in women according to the guidelines.19 The duration of the P-wave was found by measuring the line between the intersection of the starting point of the P-wave and the isoelectric line and the end point of the P-wave and the isoelectric line. Pd was calculated as the difference between the maximum P-wave duration and the minimum P-wave duration.20
Analyses
The χ2 test was used for categorical variables. Since the groups did not exhibit normal distribution, Mann-Whitney U test was used for comparisons.
RESULTS
The mean ± SD age of the AUD group was 44.35 ± 10.24 years and of the control group was 40.90 ± 13.45 years. There was no significant difference between the 2 groups based on age (P = .108). There was a significant difference between the groups based on smoking status (P = .000); however, there was no significant difference between the groups based on the number of cigarettes smoked daily (P = .743). The sociodemographic characteristics of both groups are presented in Table 1. The mean ± SD period of alcohol use was 20.71 ± 12.04 years, and the alcohol consumption was 5.88 ± 1.65 units/d. Mean ± SD alcohol consumption in the AUD group was 5.88 ± 1.65/d, which indicates an excessive consumption rate of about 58 g of alcohol/d.
Patients in the AUD group had significantly higher QTd, QTcd, and Pd values compared to the control group (all P = .000), indicating that patients with a long history of AUD with heavy alcohol consumption have a high risk of arrhythmia and resulting sudden death risk. The QTd, QTcd, and Pd values of the groups are presented in Table 2.
DISCUSSION
In the present study, the AUD group’s (including male, normotensive, excessive alcohol users) QTd, QTcd, and Pd values were statistically higher compared to those of the control group. Chronic alcohol use may lead to autonomous nervous system dysfunction and QT prolongation,4 alcoholic cardiomyopathy, myocarditis, myocyte degeneration, and immune system damage.9 Chronic alcohol use could cause arrhythmia and contractile dysfunction, leading to heart failure, myocardial infarction, and sudden death.21 The most common cause of death due to cardiovascular disease in patients with AUD is arrhythmia.22 QTd reflects regional heterogeneity of ventricular repolarization and is an important predictor of arrhythmia and sudden death risk.23 There are studies3,24–27 that investigated the effects of acute alcohol intake on QT dispersion in the literature. However, to our knowledge, only 1 study9 has investigated QTd values in patients with a long AUD and excessive alcohol use history. The Pd indicates the difference between the Pmax and Pmin values. Pd prolongation is a risk factor for atrial fibrillation.7 Our literature review revealed no study on the effects of alcohol on Pd.
A correlation between high blood alcohol concentration and QTd has been reported.25 It was suggested that acute ethanol intoxication affects several physiologic mechanisms in the heart such as the autonomous nervous system, ion channels that play a role in action potential, and modulation of receptor proteins, and changes in these mechanisms lead to changes in QTd and Pd.25 Murata et al28 and Yokoyama12 demonstrated that acute alcohol intake affected sympathetic activity, decreased parasympathetic activity, and prolonged QTc in their respective studies.
The results of studies that investigated chronic alcohol use toxicity in the heart were contradictory. It is possible that hypomagnesemia, hypopotassemia, and hypocalcemia,29 which are common in chronic alcohol use, could play a role in ECG differences. In a study30 that investigated the etiology of QT prolongation and increase in T-wave voltage in chronic alcoholic patients, it was reported that prolongation incidence, T-wave high voltage levels in V2, sinus tachycardia, and high QRS voltage continued after the 35-day abstinence period. However, QT prolongation did not correlate with any electrolyte level including calcium but did correlate with alcohol use period, consumption volume, and the abstinence period length. Thus, contrary to Härtel et al,8 the authors30 concluded that it was difficult to explain the cardiologic abnormalities observed in chronic alcohol use with electrolyte imbalance, and alcoholic myocardial damage could be a cause of QT prolongation.30 However, in more recent years, it was reported that laboratory findings demonstrated that ethanol inhibited Kv1.5 channel currents and ventricular repolarization.31,32 Thus, since QT interval and P-wave were determined by repolarization and depolarization mediated by the ion channels, it was concluded that alcohol use could lead to changes in QT and P-waves by causing electrolyte abnormalities.11 It has also been shown that chronic alcohol use leads to prolongation in the QRS complex, and this finding demonstrates that left ventricular hypertrophy could indirectly lead to changes in QT interval and ST-T configuration and P-wave.30 In another study,10 no evidence of cardiac arrhythmia, especially atrial fibrillation, was determined in alcoholic patients; however, an increase in β-receptor density was reported. QTd > 50 ms in healthy individuals indicates high risk for arrhythmia.33 In the present study, the mean AUD group QTd value was measured as 66.06 ms. The findings of a study by Li et al11 were consistent with those of the present study. In the study by Li et al11 conducted with 11,269 people, QTc was more prolonged in individuals with heavy alcohol consumption compared to those who did not consume alcohol, which is consistent with the findings of the present study. The authors11 also noted a difference between the sexes, as QTc prolongation was 1.4 times higher in males and 2.3 times higher in females compared to healthy controls. In the present study, the AUD group included only male patients due to hospital conditions.
It is known that the alcohol consumption volume is significant in the development of cardiologic effects of alcohol. It was reported that moderate alcohol consumption is associated with increased QTd,3 and high alcohol concentration leads to prolongation of the P-wave, PR interval, QRS complex, and QTc interval.26 The present study group was considered to consume excessive alcohol with 58 g of alcohol consumption per day (5.88 ± 1.65/d) and included patients with at least 10 years of alcohol consumption history (mean ± SD of 20.71 ± 12.04 years). In a study27 that investigated the effects of alcohol on ECG, alcohol injection prolonged the QRS complex period in all subjects and prolonged the P-wave period in some. Uyarel et al3 reported that alcohol intoxication increased the P-wave period by 9.1%. Similarly, Aasebø et al26 reported that P-wave and QTc were prolonged in patients with alcohol intoxication compared to the control group. In the present study, the Pd period was significantly longer in patients with long-term and excessive alcohol consumption compared to healthy controls (P = .000).
In a study conducted by Corovic et al9 in patients with a history of excessive alcohol use for more than a decade, it was reported that the frequency of prolongation in QTc was more than 2-fold compared to healthy controls. It was demonstrated that 24.5% of patients had a QTc value of 480 ms, which is known as the threshold value for the development of malignant ventricular arrhythmias; however, the same increase was not observed in healthy controls. In the same study,9 the QTcd was 50.0 ms in 34.7% of the AUD group, while the same rate was found in only 2.0% of controls. This finding was statistically significant. Furthermore, QT interval, QTd, QT interval index, QTcd, and QTc interval index were significantly higher in the AUD group compared to healthy controls.9 Smoking is another factor that affects the electrical stimulation of the heart. It was reported that heart rate, QTc maximum, and QTd were higher in smokers compared to nonsmokers, and adrenergic effects of smoking contributed to this prolongation.34,35 In the present study, there was a significant difference between the groups based on smoking favoring the AUD group.
The present study has some limitations. The sample included only male subjects due to hospital conditions, which might prevent generalization of the findings to both sexes. Another limitation is that the mean age of the AUD group was higher than the mean age of the control group. Factors such as nutrition, regular exercise, and smoking were confounding variables that are difficult to exclude. The present study was conducted with a cross-sectional design. Prospective studies including both sexes and a larger patient sample and minimization of confounding factors may contribute further to the literature.
CONCLUSION
In the present study, significant differences were found between the groups based on QTd, QTcd, and Pd values favoring the AUD group. Patients with a long history of AUD with excessive alcohol consumption had a high risk of arrhythmia and resulting sudden death risk compared to the control group. ECG, which can be easily obtained during the follow-up period, and subsequent referral for cardiologic examination would contribute to the follow-up and health of patients with AUD.
Clinical Points
- Patients with alcohol use disorder had significantly higher QT dispersion, corrected QT dispersion, and P-wave dispersion values compared to the control group.
- Patients with a long history of alcohol use disorder with excessive alcohol use had a higher risk of arrhythmia and sudden death compared to the control group.
- Electrocardiogram, which could be easily obtained, and subsequent referral to cardiologic examination would contribute to the follow-up and health of patients with alcohol use disorder.
Submitted: September 6, 2019; accepted November 15, 2019.
Published online: February 13, 2020.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1. World Health Organization. Global Status Report on Alcohol and Health, 2014. Geneva, Switzerland: World Health Organization; 2014.
2. Day CP, James OF, Butler TJ, et al. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet. 1993;341(8858):1423–1428. PubMed CrossRef
3. Uyarel H, Ozdol C, Gencer AM, et al. Acute alcohol intake and QT dispersion in healthy subjects. J Stud Alcohol. 2005;66(4):555–558. PubMed CrossRef
4. Yokoyama A, Ishii H, Takagi T, et al. Prolonged QT interval in alcoholic autonomic nervous dysfunction. Alcohol Clin Exp Res. 1992;16(6):1090–1092. PubMed CrossRef
5. Ambhore A, Teo SG, Bin Omar AR, et al. Importance of QT interval in clinical practice. Singapore Med J. 2014;55(12):607–611, quiz 612. PubMed CrossRef
6. Moulin SR, Mill JG, Rosa WC, et al. QT interval prolongation associated with low magnesium in chronic alcoholics. Drug Alcohol Depend. 2015;155:195–201. PubMed CrossRef
7. Aytemir K, Ozer N, Atalar E, et al. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23(7):1109–1112. PubMed CrossRef
8. Härtel G, Louhija A, Konttinen A. Cardiovascular study of 100 chronic alcoholics. Acta Med Scand. 1969;185(6):507–513. PubMed
9. Corović N, Duraković Z, Misigoj-Duraković M. Dispersion of the corrected QT and JT interval in the electrocardiogram of alcoholic patients. Alcohol Clin Exp Res. 2006;30(1):150–154. PubMed CrossRef
10. Mäki T, Toivonen L, Koskinen P, et al. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol. 1998;82(3):317–322. PubMed CrossRef
11. Li Z, Guo X, Liu Y, et al. Relation of heavy alcohol consumption to QTc interval prolongation. Am J Cardiol. 2016;118(8):1201–1206. PubMed CrossRef
12. Yokoyama A. Prognostic significance of QT prolongation and autonomic nervous dysfunction in alcoholics with diabetes mellitus. Keio J Med. 1993;42(4):141–148. PubMed
13. Riis P. Perspectives on the Fifth Revision of the Declaration of Helsinki. JAMA. 2000;284(23):3045–3046. PubMed CrossRef
14. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
15. Parry H, Cohen S, Schlarb JE, et al. Smoking, alcohol consumption, and leukocyte counts. Am J Clin Pathol. 1997;107(1):64–67. PubMed CrossRef
16. Nogueira LC, do Rio RF, Lollo PCB, et al. moderate alcoholic beer consumption: the effects on the lipid profile and insulin sensitivity of adult men. J Food Sci. 2017;82(7):1720–1725. PubMed CrossRef
17. Camm AJ, Janse MJ, Roden DM, et al. Congenital and acquired long QT syndrome. Eur Heart J. 2000;21(15):1232–1237. PubMed CrossRef
18. Robyns T, Willems R, Vandenberk B, et al. Individualized corrected QT interval is superior to QT interval corrected using the Bazett formula in predicting mutation carriage in families with long QT syndrome. Heart Rhythm. 2017;14(3):376–382. PubMed CrossRef
19. Rautaharju PM, Surawicz B, Gettes LS, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e241–e250. PubMed CrossRef
20. Szabó Z, Kakuk G, Fülöp T, et al. Effects of haemodialysis on maximum P wave duration and P wave dispersion. Nephrol Dial Transplant. 2002;17(9):1634–1638. PubMed CrossRef
21. Whitman IR, Agarwal V, Nah G, et al. Alcohol abuse and cardiac disease. J Am Coll Cardiol. 2017;69(1):13–24. PubMed CrossRef
22. Mustroph J, Lebek S, Maier LS, et al. Mechanisms of cardiac ethanol toxicity and novel treatment options. Pharmacol Ther. 2019;197:1–10. PubMed CrossRef
23. Cuddy TE, Halli PS, Tate RB. QT dispersion and heart rate predict the risk of sudden unexpected cardiac death in men: the Manitoba Follow-Up Study. Prev Cardiol. 2009;12(1):27–33. PubMed CrossRef
24. Uyarel H, Ozdöl C, Karabulut A, et al. Acute alcohol intake and P-wave dispersion in healthy men. Anadolu Kardiyol Derg. 2005;5(4):289–293. PubMed
25. Aasebø W. QT interval dispersion in acute alcohol intoxication. International Journal of Advance in Medical Science. 2016;4:1.
26. Aasebø W. ECG-voltage in alcoholics and non-alcoholics with acute alcohol intoxication. J Forensic Leg Med. 2009;16(7):381–384. PubMed CrossRef
27. Cardy MA, Donnerstein RL, Kelly LF, et al. Acute effects of ethanol ingestion on signal-averaged electrocardiograms. Am J Cardiol. 1996;77(15):1356–1357. PubMed CrossRef
28. Murata K, Araki S, Yokoyama K, et al. Autonomic neurotoxicity of alcohol assessed by heart rate variability. J Auton Nerv Syst. 1994;48(2):105–111. PubMed CrossRef
29. Kuchly B, Tiksrail A, Baglioni P. Electrolyte disturbances in chronic alcohol-use disorder. N Engl J Med. 2018;378(2):203. PubMed
30. Koide T, Ozeki K, Kaihara S, et al. Etiology of QT prolongation and T wave changes in chronic alcoholism. Jpn Heart J. 1981;22(2):151–166. PubMed CrossRef
31. Hu H, Zhou J, Sun Q, et al. Effects of ethanol on action potential of rat myocardium and human Kv1.5 channel [article in Chinese]. Sheng Li Xue Bao. 2011;63(3):219–224. PubMed
32. Keiver K, Weinberg J. Effect of duration of maternal alcohol consumption on calcium metabolism and bone in the fetal rat. Alcohol Clin Exp Res. 2004;28(3):456–467. PubMed CrossRef
33. Zaidi M, Robert A, Fesler R, et al. Dispersion of ventricular repolarisation: a marker of ventricular arrhythmias in patients with previous myocardial infarction. Heart. 1997;78(4):371–375. PubMed CrossRef
34. Ileri M, Yetkin E, Tandoğan I, et al. Effect of habitual smoking on QT interval duration and dispersion. Am J Cardiol. 2001;88(3):322–325. PubMed CrossRef
35. Dilaveris P, Pantazis A, Gialafos E, et al. The effects of cigarette smoking on the heterogeneity of ventricular repolarization. Am Heart J. 2001;142(5):833–837. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite