See letter by Modesto-Lowe and Aurilio
Benzodiazepines are classified as γ-aminobutyric acid–A (GABAA) receptor agonists. The greater affinity of the GABAA receptor for GABA increases the frequency of chloride-channel opening and potentiates the inhibitory effect of GABA in the central nervous system.1 In India, about 1.08% individuals (approximately 11.8 million people) currently take sedatives. Only a small percentage use sedatives in a harmful or dependent pattern. In the general population in India, about 0.11% (almost 1.18 million individuals) are using sedatives in a dependent pattern.2
Individuals with opioid use disorder who take benzodiazepines are at high risk for overdose. Interestingly, the use and misuse of benzodiazepines have contributed substantially to the current opioid overdose epidemic, with benzodiazepines involved in nearly 30% of opioid overdose deaths in 2015. 3
Abstinence or withdrawal of benzodiazepines results in withdrawal symptoms, which can be distressing and potentially fatal. Withdrawal syndrome is typically characterized by sleep disturbance, irritability, increased tension and anxiety, panic attacks, hand tremor, sweating, difficulty in concentration, nausea, some weight loss, palpitations, headache, muscular pain and stiffness, and multiple perceptual abnormalities.4 Gradual dose reduction is preferable to abrupt discontinuation of benzodiazepines. In primary care patients who had failed to stop benzodiazepine use with minimal intervention, gradual dose reduction was more effective than routine care in achieving cessation of use (51% vs 15%).5 At 15-month follow-up, 36% of those who received gradual dose reduction were abstinent based on benzodiazepine prescription data, compared with 15% of those who received routine care.6 Other medications, including carbamazepine, have been used to facilitate management of benzodiazepine dependence. Klein and colleagues7 reported on the use of carbamazepine to facilitate withdrawal from alprazolam in 3 patients with panic disorder who were unable to tolerate a gradual taper.
Here, we present the case of a patient with opioid and benzodiazepine dependence, wherein benzodiazepine detoxification was done by rapid taper with a long-acting benzodiazepine and the antiepileptic carbamazepine.
Case Report
A 36-year-old, married, employed man presented with a 12-year history of dependent opioid use of capsule Spasmo-Proxyvon (containing dicyclomine 10 mg, paracetamol 325 mg, and tramadol 50 mg). The patient exhibited craving, tolerance, withdrawal, preoccupation, and inability to control his use of Spasmo-Proxyvon, which had escalated to 30–40 capsules per day over time. He also had tobacco dependence characterized by craving, tolerance, and loss of control. The patient presented to our outpatient services in 2014 and was stabilized on tablet buprenorphine 14 mg/d. In subsequent years, the patient had multiple relapses and readmissions due to craving, and, subsequently, the dose of buprenorphine was optimized to 20 mg/d, with which he remained abstinent to opioids.
Around 2018, the patient was having adjustment issues due to an altercation with a romantic partner and had difficulty sleeping. He learned about tablet alprazolam from a nearby pharmacy. In the initial days, he used alprazolam to help with his sleep disturbances, but over the next 2 to 3 weeks he developed an intense urge to take it and had to increase the number of tablets. He would have headache, anxiety symptoms, difficulty sleeping, and a sense of uneasiness if he did not take the medication. This behavior continued, and he presented to our center in a withdrawal state due to loss of control from excessive use of tablet alprazolam (70–80 tablets of 0.5 mg) after using the drug for 7 months.
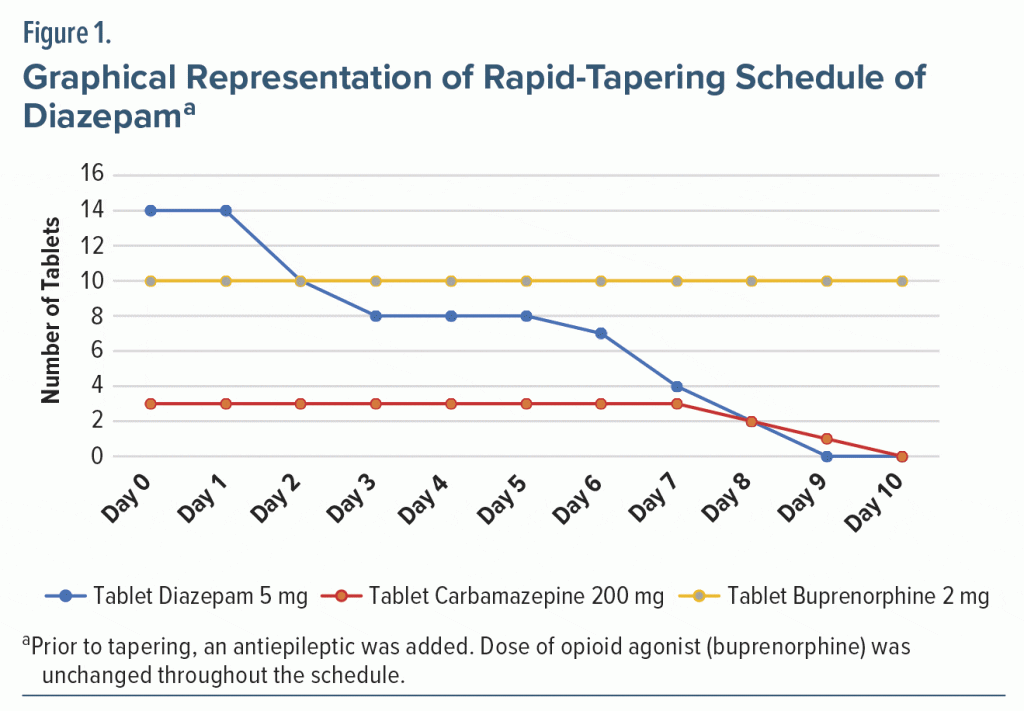
The patient had no history of premorbid anxiety or chronic sleep disorder prior to the adjustment reaction. He would take benzodiazepines to ameliorate the sleep disturbances and anxiousness due to ongoing psychosocial stressors. He was diagnosed with benzodiazepine dependence syndrome and was referred for inpatient detoxification. The patient was detoxified successfully with tablet diazepam 70 mg on the first day. He was started on tablet carbamazepine 600 mg as an antiepileptic cover at the start of the tapering process. The initial dose of tablet diazepam was tapered from 70 mg to 50 mg over the first 3 days. By the seventh day, the dose was reduced to 35 mg and stopped over the next 3 days (Figure 1). This tapering process was carried out over a period of 10 days, as the patient had expressed inability to stay in the hospital for a longer period. The doses of the opioid agonist (buprenorphine) were unchanged during tapering, while carbamazepine was stopped at day 10. Withdrawal symptoms were monitored twice daily and assessed with the Clinical Institute Withdrawal Assessment–Benzodiazepine scale.8 He had moderate withdrawal symptoms during the first 3 days of the tapering schedule, and mild-to-no withdrawal symptoms persisted afterward in the form of sleep difficulties, mild anxiousness, and heaviness of head, which were symptomatically managed. The patient was discharged after the 12th day of admission, although in the subsequent follow-ups for the next 7 months he complained of sleep difficulties and required medication. He was prescribed diazepam 10 mg and mirtazapine 7.5 mg at follow-up and remained abstinent to nonprescribed benzodiazepines. He regularly attended the follow-up appointments and was able to work and spend time with family members. He continues to be maintained well on tablet buprenorphine 20 mg.
Discussion
There are no definitive guidelines for benzodiazepine tapering in patients with benzodiazepine dependence; however, they are often categorized into rapid and slow tapers. In patients who receive benzodiazepines and opioids concurrently and need tapering for both medications, it is recommended to taper opioids first to avoid provoking anxiety. Abrupt cessation of benzodiazepines after a period of 1–6 months of use can cause life-threatening seizures and relapse to benzodiazepines, so the dose should be gradually reduced. The duration of weaning depends on the starting dose, duration of use, risk of relapse, and how well tapering is tolerated by the patient. Most studies in primary care have found that gradual withdrawal over at least 10 weeks is successful in achieving long-term abstinence.9 Few studies have commented on the optimal rate and duration of a benzodiazepine taper, which can range from weeks to months. Information in the literature is even more limited for rapid benzodiazepine tapers, although safe and successful rapid tapering of alprazolam using chlordiazepoxide has been done previously, wherein the short-acting benzodiazepine being abused by the individual is replaced by a long-acting benzodiazepine.10 For patients who have been on benzodiazepines for longer than 4 weeks or who have been on high doses, a slow taper might be better tolerated.
Rapid tapers are usually considered for patients who have taken benzodiazepines for less than 4 weeks. In this method, benzodiazepine discontinuation takes place by a relatively larger reduction in dose and more frequent dose reduction than in a slow taper. A rapid taper might involve an initial 25%–30% dose reduction weekly until 50% of the dose is reached, followed by 5%–10% dose reductions weekly.5 There are reports in the literature on the success of fast tapers. Fournier et al11 reported that successful rapid benzodiazepine tapering was conducted in an adolescent boy (aged 16 years) with severe benzodiazepine dependence (over 14 days) in an inpatient setting using an interdisciplinary approach.
In the present case, it was noted that the patient’s duration of use was more than 4 weeks (ie, 5 to 6 months), despite which a rapid taper was still possible. An advantage of rapid taper is a shorter stay in inpatient care, leading to less resource requirement. Possible explanations for successful rapid detoxification include our patient’s motivation for expedited treatment and use of an antiepileptic for potential seizures. An additional advantage of carbamazepine, apart from the antiepileptic effect, is its mild anxiolytic properties,7 which help in alleviating the withdrawal symptoms one endures during rapid benzodiazepine detoxification. Thus, rapid tapering of benzodiazepines with other benzodiazepines may be considered in some patients when required. However, further evidence is required to expand the literature on the efficacy and safety of rapid detoxification of benzodiazepines.
Article Information
Published Online: August 17, 2023. https://doi.org/10.4088/PCC.22cr03457
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(4):22cr03457
Submitted: November 30, 2022; accepted March 8, 2023.
To Cite: Munipati D, Mathur R, Sarkar S. Rapid detoxification in an adult with benzodiazepine dependence with the aid of an antiepileptic. Prim Care Companion CNS Disord. 2023;25(4):22cr03457.
Author Affiliations: All India Institute of Medical Sciences, New Delhi, India (all authors).
Corresponding Author: Rahul Mathur, MD, DNB, All India Institute of Medical Sciences, New Delhi, Delhi 110029, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report, and information, including dates, has been de-identified to protect anonymity.
References (11)

- Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147–1157. PubMed CrossRef
- Ministry of Social Justice and Empowerment. Magnitude of Substance Use in India. New Delhi: MSJE, Government of India. Muktangan.org website. 2019. https://www.muktangan.org/pdf/Magnitude_Substance_Use_India_REPORT.pdf
- Votaw VR, Geyer R, Rieselbach MM, et al. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol Depend. 2019;200:95–114. PubMed CrossRef
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455–1459. PubMed CrossRef
- Closser MH, Brower KJ. Treatment of alprazolam withdrawal with chlordiazepoxide substitution and taper. J Subst Abuse Treat. 1994;11(4):319–323. PubMed CrossRef
- Voshaar RC, Couvée JE, van Balkom AJ, et al. Strategies for discontinuing long-term benzodiazepine use: meta-analysis. Br J Psychiatry. 2006;189(3):213–220. PubMed CrossRef
- Klein E, Uhde TW, Post RM. Preliminary evidence for the utility of carbamazepine in alprazolam withdrawal. Am J Psychiatry. 1986;143(2):235–236. PubMed CrossRef
- SA Health. Government of South Australia. Benzodiazepine withdrawal scale (CIWA-B). SA Health website. Accessed July 20, 2023. https://www.sahealth.sa.gov.au/wps/wcm/connect/0f6337804077201f9318bb222b2948cf/benzodiazepine_withdrawal_scale_ciwab_dassa%5B1%5D.pdf?MOD=AJPERES
- Denis C, Fatséas M, Lavie E, et al. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst Rev. 2006;3(3):CD005194. PubMed CrossRef
- Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr. 2015;38(5):152–155. PubMed CrossRef
- Fournier C, Jamoulle O, Chadi A, et al. Severe benzodiazepine use disorder in a 16-year-old adolescent: a rapid and safe inpatient taper. Pediatrics. 2021;147(1):e20201085. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite