Prim Care Companion CNS Disord 2022;24(5):21cr03191
To cite: Gomaa H, Joshi A, Baweja R. Rapid-onset nonallergic peripheral edema with sertraline and fluoxetine in the postpartum period. Prim Care Companion CNS Disord. 2022;24(5):21cr03191.
To share: https://doi.org/10.4088/PCC.21cr03191
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Health, Penn State College of Medicine, Hershey, Pennsylvania
*Corresponding author: Hassaan Gomaa, MD, Department of Psychiatry and Behavioral Health, Penn State College of Medicine, 500 University Dr, Hershey, PA 17033 ([email protected]).
Selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat depressive disorders.1 Common side effects associated with SSRI use include headache, nausea, vomiting, diarrhea, constipation, dry mouth, insomnia, restlessness, and sexual side effects.2 A few cases of nonallergic edema have been reported with some SSRIs such as escitalopram3,4 and paroxetine,5 as well as antidepressants such as venlafaxine,5 mirtazapine,6,7 and trazodone.6 Despite the low potential risk of nonallergic edema in patients using sertraline (< 2%)8 and fluoxetine (< 1%),9 there are no previously reported nonallergic edema cases associated with the use of either medication. We report a unique case of a patient who developed peripheral edema shortly after starting both sertraline and fluoxetine in the postpartum period.
Case Report
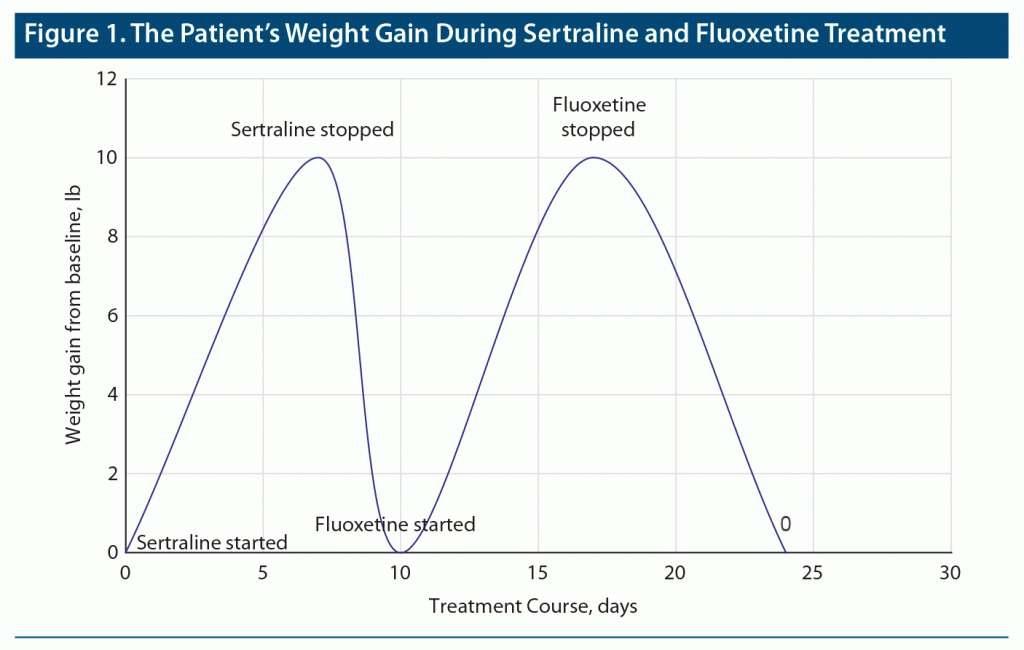
A married multiparous woman in her mid-30s with a medical history of polycystic ovary syndrome and bariatric surgery for obesity and no prior psychiatric history presented to an obstetrics clinic for a 4- to 6-week follow-up after vaginal delivery. She presented with depressed mood, anhedonia, worthlessness, fatigue, insomnia, irritability, decreased appetite, and lack of concentration that affected her ability to take care of her newborn and was diagnosed with major depressive disorder with peripartum onset. During that time, she was taking perinatal vitamins, thiamine, and acetaminophen as needed for headaches before starting sertraline 50 mg/d during the same visit. She noticed swelling in her lower limbs and hands with noticeable weight gain on the second day. Within a week, she gained 9 to 10 lb. Sertraline was discontinued, and her symptoms resolved completely within 2 days. She was then started on fluoxetine 10 mg/d and had similar symptoms of peripheral edema with a gradual increase in weight of about 9 to 10 lb within a week. Fluoxetine was stopped after 1 week with subsequent resolution of peripheral edema and weight gain (Figure 1). The patient had no known history of renal, cardiovascular, liver, immune, or thyroid diseases. Her blood pressure was within the normal range during and after pregnancy. She did not complain of any symptoms suggestive of deep vein thrombosis, preeclampsia, peripartum cardiomyopathy, or other medical problems. Her body mass index at the time of the assessment was 31 kg/m2, and there were no reported symptoms suggestive of anorexia nervosa. Her most recent laboratory studies at time of childbirth, about 4 weeks prior to starting sertraline, showed a white blood cell count of 13.84/µL. Her hemoglobin was 7.9 g/dL, and ferritin was 4.2 µg/L (low) for which she was receiving ferrous sulfate 325 mg/d. She also had a history of diabetes mellitus that was significantly improved with bariatric surgery 2 years prior to the adverse events from SSRIs. Her hemoglobin A1C was 5.8 about 9 months prior to developing edema from sertraline and fluoxetine.
Discussion
Sertraline and fluoxetine rarely cause peripheral edema in patients. We searched PubMed for “edema and sertraline” as well as “edema and fluoxetine,” and both searches revealed no cases of nonallergic edema as a side effect of either sertraline or fluoxetine. In this case, the edema was severe and rapid, which is an uncommon presentation. Edema occurrence with SSRIs may be related to the decrease in pressure-induced myogenic constriction with SSRIs,10 which increases vascular permeability. It is also proposed that peripheral edema may be due to the direct effect of blocking 5-HT2 receptors, leading to relaxation of smooth muscles and thus increasing permeability and edema.11 An in vitro animal study12 showed that estrogen and progesterone can affect epithelial sodium channels in renal collecting tubules and cause premenstrual edema. Another study13 assessed the effect of serotonin on both rat and human epithelial sodium channels in the alveolar epithelium and showed it has an inhibitory effect that can cause edema. We assume that serotonin effect on epithelial sodium channels in the collecting tubules in the kidneys would be similar. Although there is a paucity of data derived from human studies, based on these animal studies,12,13 it could be hypothesized that a combination of a serotonergic agent and sudden drop in estrogen and progesterone in the postpartum period may have caused the peripheral edema. In this instance, the possibility of hormonal changes playing a role in the development of edema appears to be minimal and facilitative of the serotonergic side effect due to the close temporal relationship of the development and resolution of edema to the start and discontinuation of both sertraline and fluoxetine.
An allergic reaction to both medications was less likely due to absence of other signs and symptoms typically associated with such a reaction. The absence of other changes in medication regimen around the time of manifestation and resolution of edema also suggests correlation with sertraline and fluoxetine. The patient’s sodium level was within the normal range, which excludes peripheral edema due to hyponatremia.
Peripheral edema may occur with sertraline and fluoxetine. The mechanism for development of this adverse effect is unclear but may be related to the serotonergic action on blood vessel smooth muscles as well as estrogen and progesterone effect on renal collecting tubules. We recommend assessing patients for adverse events like peripheral edema after commencement of SSRIs including sertraline and fluoxetine, especially in postpartum women.
Published online: October 6, 2022.
Relevant financial relationships: Dr Joshi’s spouse is a principal investigator on a research grant made available by AstraZeneca through the BIG 10 cancer consortium and participated in an advisory board for Seagen. Drs Gomaa and Baweja report no relevant financial relationships.
Funding/support: None.
Previous presentation: This case was presented as a poster at the online American Psychiatric Association Annual Meeting; May 1–3, 2021.
Additional information: Information was de-identified to protect patient anonymity.
References (13)

- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15(7):220–226. PubMed CrossRef
- Bet PM, Hugtenburg JG, Penninx BW, et al. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol. 2013;23(11):1443–1451. PubMed CrossRef
- Ravi PB, Ravishankar GM, Andrade C. Bilateral peripheral edema as a rare adverse effect of escitalopram. Indian J Psychiatry. 2014;56(1):97. PubMed CrossRef
- Masdrakis VG, Oulis P, Kouzoupis AV, et al. Bilateral ankle oedema in a patient taking escitalopram. World J Biol Psychiatry. 2009;10(4 Pt 3):939–941. PubMed CrossRef
- Uguz F. Rapid weight gain associated with edema after use of paroxetine and venlafaxine: 2 case reports. Clin Neuropharmacol. 2014;37(1):34–35. PubMed CrossRef
- Yang HT, Liu YW, Kuo SC, et al. Repeated leg edema induced by trazodone and mirtazapine in a patient with depression with successful agomelatine substitution: a case report. (published online ahead of print October 1, 2020) Am J Ther. 2020. PubMed CrossRef
- Lai FY, Shankar K, Ritz S. Mirtazapine-associated peripheral oedema. Aust N Z J Psychiatry. 2016;50(11):1108. PubMed CrossRef
- Zoloft (sertraline hydrochloride) Label. (N.d.). Fda.Gov website. Accessed September 25, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839S74S86S87_20990S35S44S45lbl.pdf
- Fluoxetine hydrochloride - Drug Summary. Prescribers’ digital reference (PDR). PDR website. Accessed September 25, 2021. https://www.pdr.net/drug-summary/Fluoxetine-Tablets-fluoxetine-hydrochloride-1416
- Ungvari Z, Tarantini S, Yabluchanskiy A, et al. Potential adverse cardiovascular effects of treatment with fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) in patients with geriatric depression: implications for atherogenesis and cerebromicrovascular dysregulation. Front Genet. 2019;10:898. PubMed CrossRef
- Ganong WF. Excitable Tissue: Muscle. Review of Medical Physiology. 17th ed. East Norwalk, CT: Appleton and Lange; 1995:56–74.
- Chang CT, Sun CY, Pong CY, et al. Interaction of estrogen and progesterone in the regulation of sodium channels in collecting tubular cells. Chang Gung Med J. 2007;30(4):305–312. PubMed
- Goolaerts A, Roux J, Ganter MT, et al. Serotonin decreases alveolar epithelial fluid transport via a direct inhibition of the epithelial sodium channel. Am J Respir Cell Mol Biol. 2010;43(1):99–108. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite