Tardive dyskinesia is persistent and repetitive involuntary movements caused by prolonged exposure to antipsychotics or other dopamine receptor antagonists.1 It most commonly involves the face, tongue, and lips and can be associated with choreatic movements of the trunk and extremities.2
A few known risk factors associated with tardive dyskinesia include the following:
- Advanced age: >60 years
- Sex: females > males
- Prolonged duration of exposure to antipsychotics
- Presence of previous movement disorder
- Organic brain dysfunction and brain atrophy
- Alcohol abuse/dependence
- Genetic: cytochrome P450 2D6 and both the dopamine D2 (DRD2) and D3 (DRD3) receptor genes.3
This case report describes a patient prescribed phentermine for low energy and weight control, who subsequently developed tardive dyskinesia a month after starting the medication. The case underscores the importance of routine monitoring for tardive dyskinesia in patients taking phentermine.
Case Report
A 79-year-old woman with a past medical history of major depressive disorder, generalized anxiety disorder, and attention and concentration deficit presented to the clinic with a chief complaint of involuntary facial movements. Since August 2022, she has been treated with desvenlafaxine (100 mg/d), buspirone (20 mg/d), hydroxyzine (25 mg/d), and lorazepam (0.5 mg/d). Atomoxetine (25–60 mg/d) was added to her treatment regimen in April 2023, but while taking it, she noted having fluctuating mood and energy. In August 2023, she switched to phentermine (Adipex) 37.5-mg tablet once daily for low energy and weight control. At her 4-week follow-up in September 2023, her mood and energy had improved, but she began experiencing involuntary perioral and periorbital movements, noticing them 20 days after starting phentermine. No hand tremor, limb rigidity, bradykinesia, or other signs of parkinsonism were observed. Due to the presumptive diagnosis of tardive dyskinesia (the Abnormal Involuntary Movement Scale score was 64), phentermine was discontinued, and atomoxetine 40 mg was restarted. At her follow-up in October 2023, she reported relief from the involuntary facial movements, and she remains stable and compliant with her medications and is regularly following up at the clinic.
Discussion
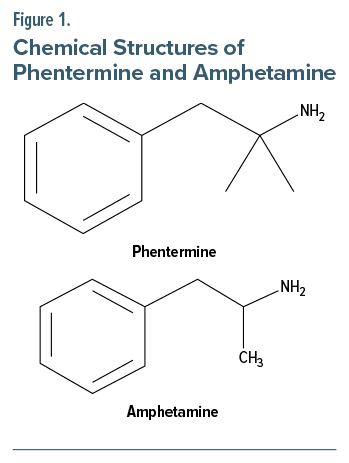
Phentermine is a short-term appetite suppressant that works by increasing the secretion of norepinephrine and epinephrine, acting as an agonist at the trace amine associated (TAAR1) receptor site in the hypothalamus.5 The spectrum of side effects of phentermine ranges from dry mouth to tachycardia, hypertension, visual symptoms, nausea, hyperhidrosis, insomnia, anxiety, and manic-like episodes.6 Phentermine-induced dyskinesia has not been well studied or reported. However, from the literature, there is an observed association between tardive dyskinesia with amphetamines and other central stimulants, which are structurally similar (Figure 1).7 Amphetamine is a nonselective stimulator of synaptic noradrenaline, dopamine, and serotonin release.8 However, unlike amphetamine, phentermine has very little effect on the release of dopamine at the neuronal synapse.9 It is known that phentermine does not release dopamine at a clinically significant quantity compared with amphetamine; however, we cannot conclude whether phentermine has some or no effect on dopamine.10
A phase IV clinical study of US Food and Drug Administration data done on August 9, 2023, reported that 14,395 people had side effects when taking phentermine. Among them, 18 people (0.13%) had tardive dyskinesia.11 This case illustrates the potential for tardive syndromes associated with stimulant use. While atypical, it is important to recognize the potential occurrence of this side effect. To the best of our knowledge, this is the third reported case showing an association between phentermine intake and tardive dyskinesia.12 No studies have yet investigated the development of movement disorders related to phentermine, although these symptoms can appear repeatedly and cause social impairment. Therefore, further research about phentermine-induced tardive dyskinesia is needed.
AnchorArticle Information
Published Online: June 27, 2024.
https://doi.org/10.4088/PCC.24cr03710
© 2024 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2024;26(3):24cr03710
Submitted: January 23, 2024; accepted March 27, 2024.
To Cite: Prasad P, Corsten MN, Shebak SS, et al. A rare case of phentermine-related tardive dyskinesia. Prim Care Companion CNS Disord. 2024;26(3):24cr03710.
Author Affiliations: Dayanand Medical College, Ludhiana, India (Prasad); Wayne State University Physician Assistant Program, Detroit, Michigan (Corsten); Department of Psychiatry, Michigan State University College of Human Medicine, East Lansing, Michigan (Shebak); Northern Kentucky University, Highland Heights, Kentucky (Makki).
Corresponding Author: Pooja Prasad, MBBS, Dayanand Medical College, 70A/207 Shivam Apt. Patel marg, Mansarovar, Jaipur, Rajasthan, India 302020 (prasad.pooja09@gmail.com).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report, and information (including dates) has been de-identified to protect anonymity.
ORCID: Pooja Prasad: https://orcid.org/0009-0007-0155-0644
References (12)

- Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N.Y.). 2013;3:tre-03-161-4138-1. PubMed CrossRef
- Cornett EM, Novitch M, Kaye AD, et al. Medication induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162–174. PubMed
- Balasubramaniam R, Ram S. Orofacial movement disorders. Oral Maxillofac Surg Clin North Am. 2008;20(2):273–285, vii. PubMed CrossRef
- Identifying Tardive Dyskinesia: Risk Factors, Functional Impact, and Diagnostic Tools. Psychiatrist.com. Accessed November 17, 2023. https://www.psychiatrist.com/jcp/evaluatingpatients-for-tardive-dyskinesia/
- Son JW, Kim S. Comprehensive review of current and upcoming anti-obesity drugs. Diabetes Metab J. 2020;44(6):802–818. PubMed CrossRef
- Jo HS, Wang SM, Kim JJ. Recurrent psychosis after phentermine administration in a young female: a case report. Clin Psychopharmacol Neurosci. 2019;17(1):130–133. PubMed CrossRef
- Thiel A, Dressler D. Dyskinesias possibly induced by norpseudoephedrine. J Neurol. 1994;241(3):167–169. PubMed CrossRef
- Frei K. Tardive dyskinesia: who gets it and why. Parkinsonism Relat Disord. 2019;59:151–154. PubMed CrossRef
- Hong SI, Kim MJ, You IJ, et al. Phentermine induces conditioned rewarding effects via activation of the PI3K/Akt signaling pathway in the nucleus accumbens. Psychopharmacology (Berl). 2016;233(8):1405–1413. PubMed CrossRef
- Devan GS. Phentermine and psychosis. Br J Psychiatry. 1990;156:442–443. PubMed CrossRef
- Phentermine and Tardive Dyskinesia, a Phase IV Clinical Study of FDA data. eHealthMe. Accessed November 17, 2023. https://www.ehealthme.com/ds/phentermine/tardive-dyskinesia/#google_vignette%20(1)
- A Case of Tardive Dyskinesia and Parkinsonism Following Use of Phentermine for Weight Loss. Department of Neurology, College of Medicine, University of Florida. Accessed November 17, 2023. https://neurology.ufl.edu/2018/04/18/a-case-oftardive-dyskinesia-and-parkinsonism-following-useof-phentermine-for-weight-loss/
Please sign in or purchase this PDF for $40.
Save
Cite