Background: We studied the patterns and predictors of long-acting injectable (LAI) antipsychotic (AP) use in the treatment of schizophrenia and the effect of introducing a new LAI (paliperidone palmitate [paliperidone-LAI]) in the Spanish province of Tarragona.
Methods: This noninterventional, naturalistic, retrospective study included electronic medical record data from a large population-based database of 1,646 patients who were diagnosed with schizophrenia according to ICD-10 criteria and treated between January 2011 and December 2013.
Results: During the study period, 42.0% of patients were treated with an LAI AP. The most frequently prescribed initial LAI was risperidone (52.0% of patients). A total of 23% of patients initially treated with an oral AP were switched to an LAI AP, a change that was associated with younger age (P = .001), undifferentiated schizophrenia (P = .015), substance abuse (P < .001), and neuropsychiatric comedication with the following agents: anticonvulsants (P = .004), anticholinergics (P < .001), and hypnotics/sedatives (P = .03). The change from an oral AP to paliperidone-LAI was predicted by younger age (P < .001). Overall, 27.5% of patients switched to another LAI AP, and paliperidone-LAI was the preferred option in 64.7% of cases. The most frequent change involved patients taking risperidone-LAI, many of whom transitioned to paliperidone-LAI (85.0% of cases), particularly patients with a disease duration > 5 years (P = .019).
Conclusions: There was a progressive increase in the use of LAI formulations in our catchment area. These agents were preferentially prescribed to patients with chronic disease and a history of substance abuse, as well as patients receiving neuropsychiatric comedication. One-month LAI formulations were commonly used in young patients.
Background: We studied the patterns and predictors of long-acting injectable (LAI) antipsychotic (AP) use in the treatment of schizophrenia and the effect of introducing a new LAI (paliperidone palmitate [paliperidone-LAI]) in the Spanish province of Tarragona.
Methods: This noninterventional, naturalistic, retrospective study included electronic medical record data from a large population-based database of 1,646 patients who were diagnosed with schizophrenia according to ICD-10 criteria and treated between January 2011 and December 2013.
Results: During the study period, 42.0% of patients were treated with an LAI AP. The most frequently prescribed initial LAI was risperidone (52.0% of patients). A total of 23% of patients initially treated with an oral AP were switched to an LAI AP, a change that was associated with younger age (P = .001), undifferentiated schizophrenia (P = .015), substance abuse (P < .001), and neuropsychiatric comedication with the following agents: anticonvulsants (P = .004), anticholinergics (P < .001), and hypnotics/sedatives (P = .03). The change from an oral AP to paliperidone-LAI was predicted by younger age (P < .001). Overall, 27.5% of patients switched to another LAI AP, and paliperidone-LAI was the preferred option in 64.7% of cases. The most frequent change involved patients taking risperidone-LAI, many of whom transitioned to paliperidone-LAI (85.0% of cases), particularly patients with a disease duration > 5 years (P = .019).
Conclusions: There was a progressive increase in the use of LAI formulations in our catchment area. These agents were preferentially prescribed to patients with chronic disease and a history of substance abuse, as well as patients receiving neuropsychiatric comedication. One-month LAI formulations were commonly used in young patients.
Prim Care Companion CNS Disord 2017;19(2):16m02044
https://doi.org/10.4088/PCC.16m02044
© Copyright 2017 Physicians Postgraduate Press, Inc.
aHospital Universitari Institut Pere Mata, IISPV, Universitat Rovira i Virgili, CIBERSAM, Reus, Spain
bDepartament de Ciרncies Experimentals i la Salut, Institute of Evolutionary Biology (UPF-CSIC), Universitat Pompeu Fabra, PRBB, Barcelona, Spain
*Corresponding author: Elisabet Vilella, PhD, Hospital Universitari Institut Pere Mata Ctra de l’ Institut Pere Mata, s/n 43206, Reus, Spain ([email protected]).
Schizophrenia is characterized by a chronic course that in most cases requires continued, long-term treatment.1 Core pharmacologic treatment involves the use of antipsychotics (APs),2 although medication responsiveness varies widely among affected patients. Approximately half of these patients require a change in their AP medication due to limited effectiveness, intolerance of side effects, lack of adherence, or psychiatric comorbidities.3-5
Typical long-acting injectable (LAI) APs, which are administered every 2 to 4 weeks, were developed in the 1960s to improve partial or covert nonadherence rates and to simplify complex medication schedules in the long-term maintenance treatment of psychiatric diseases.6 More recently, with the introduction of atypical APs, which are characterized by improved efficacy and tolerability profiles,7 several LAI formulations have also become available and increased the number of pharmacotherapeutic options for schizophrenia.8
Most clinical guidelines1,9,10 recommend using LAI APs for maintenance treatment in patients who prefer them to oral APs and in patients with a history of multiple relapses and nonadherence. In addition, it has been increasingly recognized that LAI APs can be effective in all phases of schizophrenia,11 particularly during the early stages of the illness.12 However, their use in routine clinical practice is still restricted and in most cases is limited to chronic patients with severe symptoms, patients with a history of multiple psychotic episodes, and patients who are likely to have a poor outcome.13,14 Moreover, there are no formal recommendations regarding the use of specific LAIs when initiating treatment because, as shown in a recent review,15 there are only a few available head-to-head studies and even fewer studies assessing which medications are more effective when transitioning from an oral AP or another LAI formulation. Prescription of LAIs compared to oral APs for outpatient maintenance varies considerably across countries from 10% to 80%.16,17
At the time of this study, 2 main typical LAI APs were prescribed in our catchment area, namely, fluphenazine decanoate (fluphenazine-LAI) and zuclopenthixol decanoate (zuclopenthixol-LAI), which are both administered as an intramuscular (IM) injection every 2 weeks. In addition, 2 atypical LAI APs were prescribed, risperidone microspheres (risperidone-LAI), which are also administered as an IM injection every 2 weeks, and paliperidone palmitate (paliperidone-LAI), which is the primary active metabolite of risperidone,18 is administered as a monthly IM injection, and was authorized in Spain for maintenance treatment of schizophrenia in March 2011.
A systematic review19 on the prescription patterns of oral and LAI APs in real-life settings in nonrandomized studies has shown that data are lacking regarding the patterns and predictors of use of different LAI formulations, and the effect of introducing new LAI drugs has drawn even less attention.20 The aim of this study was to analyze LAI AP prescription patterns during a 3-year follow-up period in the Spanish province of Tarragona and to identify predictors of medication changes, specifically when a new LAI is introduced.
METHODS
Design
This noninterventional, naturalistic, retrospective study included all patients who were diagnosed with schizophrenia and treated at any community mental health care center in the province of Tarragona in southern Catalonia, Spain.

- Real-world data confirm that use of long-acting injectable (LAI) formulation antipsychotics is increasing in our catchment area.
- LAI antipsychotic formulations are mainly prescribed to chronic complex patients.
- Prescription of 1-month LAI antipsychotic formulation in young patients is increasing.
Data Extraction
Data pertaining to patients older than 18 years with a diagnosis of schizophrenia (ICD-10 F20.xx) who visited at least 1 mental health center between January 1, 2011, and December 31, 2013, were extracted from electronic medical records as previously described.21 The variables analyzed included age, sex, marital status, schizophrenia subtype, duration of disease, psychiatric comorbidities (affective disorders, personality disorders, intellectual disabilities, and substance abuse disorders), and substance use (alcohol, tobacco, cannabis, and cocaine). Principal APs, which were defined as the drugs that were prescribed continuously for the longest amount of time, and additional psychiatric drugs were categorized according to their anatomic therapeutic chemical classifications as antiepileptics, anticholinergics, anxiolytics, hypnotics and sedatives, or antidepressants. Moreover, monotherapy was defined as treatment with a single AP (oral or LAI) throughout the entire study period, and polytherapy was defined as simultaneous treatment with 2 or more APs for more than 3 consecutive months. The routes and doses of APs marketed in Spain during the study period were grouped as oral formulations and LAIs, namely, fluphenazine-LAI, zuclopenthixol-LAI, risperidone-LAI, and paliperidone-LAI.
The study received ethical approval from the Clinical Research Ethics Committee of the Hospital Universitari de Sant Joan and the Clinical Research Committee of the Hospital Universitari Institut Pere Mata, Reus, Spain.
Statistical Analysis
All statistical analyses were performed using R Statistical Software (version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria). Descriptive analysis results are expressed as means and standard deviations for continuous variables and as percentages for categorical variables. The Kruskal-Wallis test was used to compare quantitative variables, and a χ2 test was used to compare proportions. Multivariate linear regression analyses and binary multiple logistic regression models were used to analyze predictive factors pertaining to the continuous and categorical variables, respectively. Moreover, time series analysis was used to identify trends in incident LAI AP use during the study period. Finally, we used Kaplan-Meier survival curves and a log rank test to determine the cumulative probability of switching to another LAI and Cox proportional hazards regression (stratified by LAI treatment) to simultaneously adjust for potential confounders, namely, sex, age, marital status, and psychiatric comorbidities. The level of significance was set at α < .05, and Bonferroni correction was used when necessary.
RESULTS
Demographic and Clinical Characteristics Associated With Long-Acting Injectable Prescription
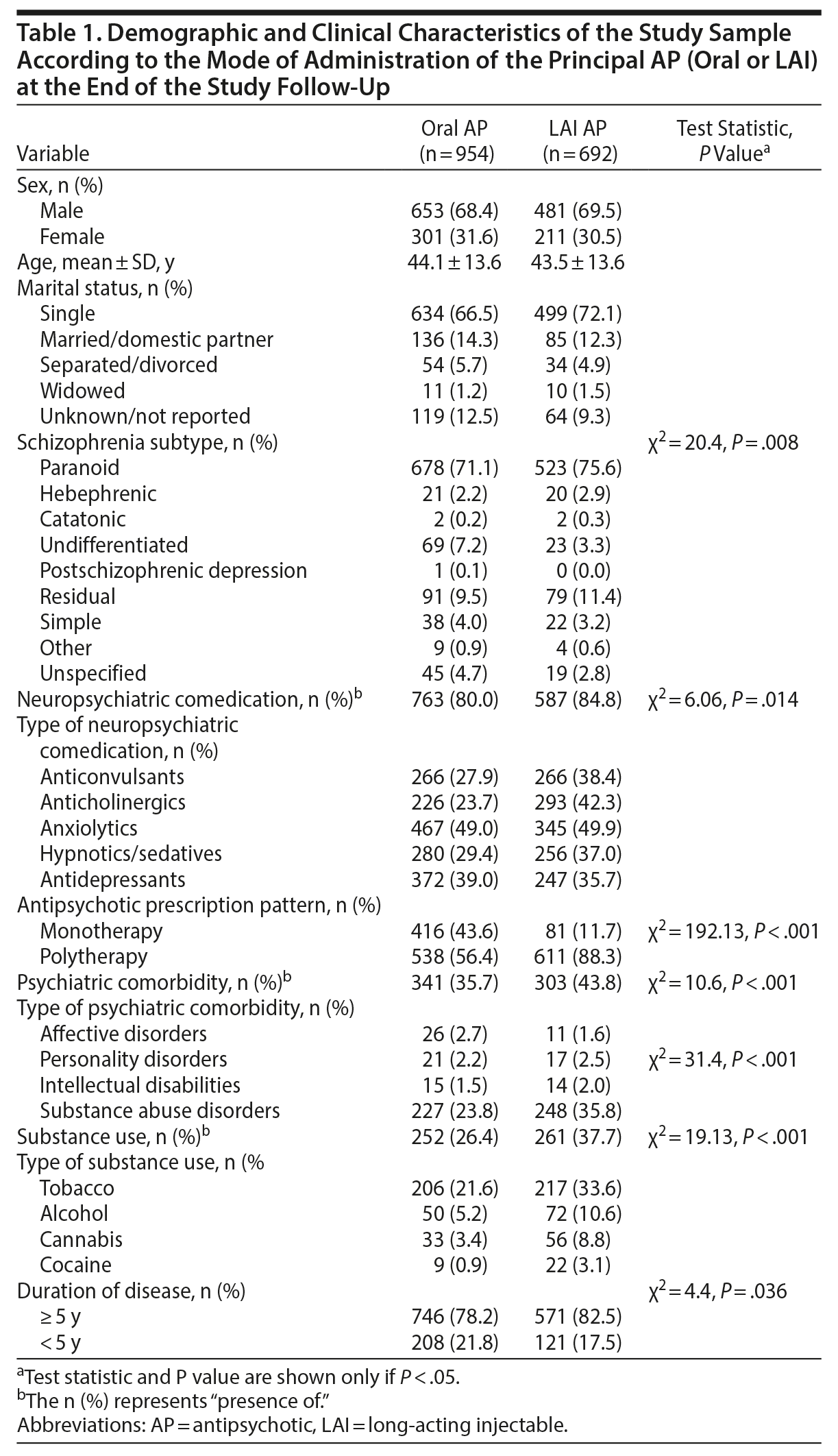
This study included a total of 1,646 patients who were diagnosed with schizophrenia and prescribed at least 1 AP, which during the follow-up period was an oral medication in 954 patients (57.9%) and an LAI medication in 692 patients (42.0%) (Table 1). At the end of the follow-up period, 509 (74%) patients continued receiving an LAI. Overall, the majority of patients were male (68.4% and 69.5% in the oral and LAI AP groups, respectively), and the mean age was similar between the groups (44.1 ± 13.6 years in the oral AP group and 43.5 ± 13.6 years in the LAI AP group). Patients treated with an LAI AP were more likely to have been diagnosed with paranoid, hebephrenic, catatonic, or residual schizophrenia than patients treated with an oral AP (P = .008). Furthermore, patients treated with an LAI AP exhibited more psychiatric comorbidities (P < .001), were more likely to be treated with neuropsychiatric comedication (P = .014), were more likely to be substance users (P < .001), and were more likely to have lived with their disease for longer than 5 years (P = .036) (Table 1) compared to the oral AP group. Only 12% of patients who were treated with an LAI as their principal AP received monotherapy; 43.6% of patients in the oral AP group received monotherapy (P < .001). The mean doses (mg/injection) of fluphenazine-LAI, zuclopenthixol-LAI, risperidone-LAI, and paliperidone-LAI were 16.5 ± 9.6, 167.3 ± 82.0, 45.9 ± 17.4, and 122.2 ± 34.1, respectively.
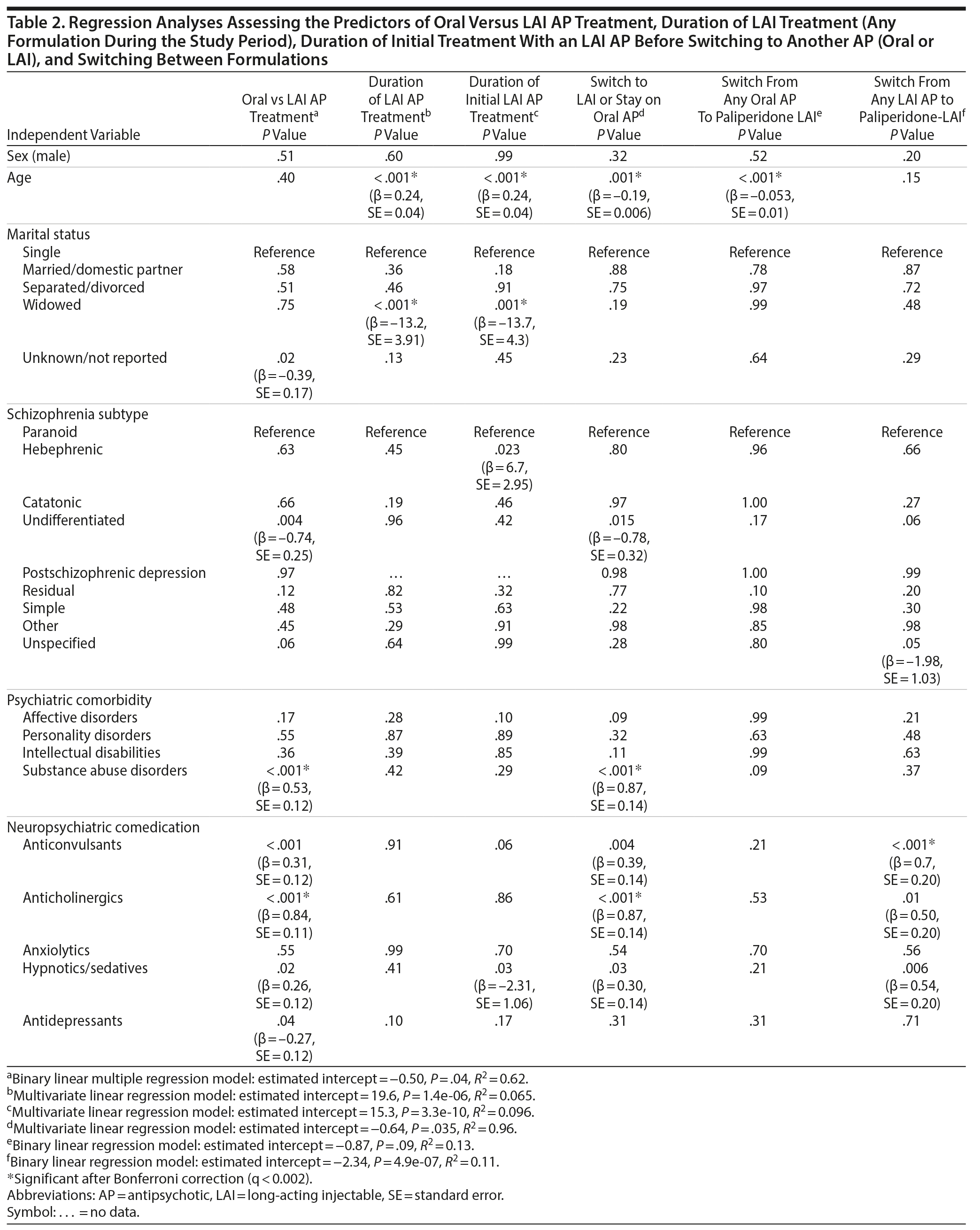
Multiple binary logistic regression analysis showed that the variables associated with prescription of an LAI AP versus an oral AP were undifferentiated schizophrenia (β = −0.74, P = .004), substance abuse (β = 0.53, P < .001), and neuropsychiatric comedication with the following agents: anticonvulsants (β = 0.31, P < .001), anticholinergics (β = 0.84, P < .001), hypnotics/sedatives (β = 0.26, P = .02), and antidepressants (β = −0.27, P = .04) (Table 2).
Patterns of Long-Acting Injectable Prescription According to Duration of Treatment
The most frequently prescribed initial LAI formulation during the study period was risperidone-LAI (in 52.0% of patients), followed by zuclopenthixol-LAI (in 20.1%), paliperidone-LAI (in 14.3%), and fluphenazine-LAI (in 12.9%). Multivariate linear regression analysis showed that older age (β = 0.24, P < .001) and widowhood (β = −13.2, P < .001) were associated with the duration of treatment with any LAI during the study period (Table 2).
Patterns of Long-Acting Injectable Prescription in Patients Who Switched Antipsychotic Medications
Overall, 391 (23%) patients who were initially treated with an oral AP were switched to an LAI AP during the study period. Ninety-two (23.5%) were switched to a typical LAI AP (16.4% to zuclopenthixol-LAI and 7.2% to fluphenazine-LAI), 200 (51.2%) were switched to risperidone-LAI, and 99 (25.3%) were switched to the newly available paliperidone-LAI. Bivariate logistic regression showed that the switch from an oral AP to an LAI AP among LAI-naive patients was associated with younger age (β = −0.19, P = .001), undifferentiated schizophrenia (β = −0.78, P = .015), substance abuse disorders (β = 0.87, P < .001), and comedication with anticonvulsants (β = 0.39, P = .004), anticholinergics (β = 0.87, P < .001), or hypnotics/sedatives (β = 0.30, P = .03) (Table 2). Moreover, the only demographic or clinical characteristic that predicted the change from an oral AP to paliperidone-LAI was younger age (β = −0.053, P < .001) (Table 2).
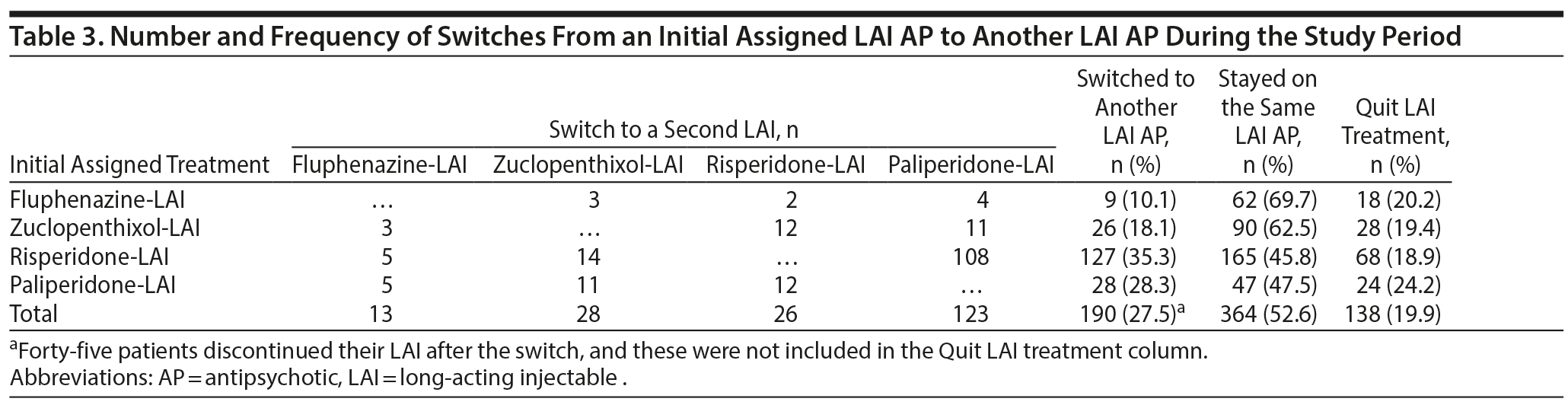
A total of 190 (27.5%) of 692 patients who were treated with an LAI AP switched from their initial LAI formulation to an oral AP or another LAI AP for any reason during the study period, and 138 patients (19.9%) discontinued LAI treatment, which was slightly more frequent among patients who were treated with paliperidone-LAI (Table 3). A multiple linear regression model showed that the variables associated with the duration of treatment with the initial prescribed LAI were age (β = 0.24, P < .001), widowhood (β = −13.7, P = .001), hebephrenic schizophrenia (β = 6.7, P = .023), and comedication with hypnotics/sedatives (β = −2.31, P = .03) (Table 2). Paliperidone-LAI was the preferred second option in 64.7% of patients (n = 123) who switched between LAI formulations (Table 3), and the only demographic and clinical predictors of this particular change were unspecified schizophrenia (β = −1.98, P = .05) and comedication with anticonvulsants (β = 0.70, P = .001), anticholinergics (β = 0.50, P < .01), and hypnotics/sedatives (β = 0.54, P = .006) (Table 2). Among all patients treated with LAI APs, the most frequent switch involved patients who were treated with risperidone-LAI, as 127 (35.3%) transitioned to another LAI formulation, mainly paliperidone-LAI (n = 108, 85.0%) (Table 3). The only difference between patients who stayed on risperidone-LAI (n = 165) and those who switched to paliperidone-LAI (n = 108) was that patients in the latter group were more likely to have lived with their disease > 5 years (χ2 = 5.4, P = .019).
Patterns of Long-Acting Injectable Prescription According to the Time and Impact of Paliperidone Palmitate Availability
There was a substantial increase in the total number of patients treated with 1 of the available LAI formulations during the months preceding paliperidone-LAI use, launched on March 31, 2011, in Spain and November 30, 2011, in our catchment area, a trend that was most marked for risperidone-LAI but least noticeable for typical LAI APs (fluphenazine-LAI and zuclopenthixol-LAI) (Figure 1A). After the introduction of paliperidone-LAI, there was a substantial decrease in the number of patients treated with risperidone-LAI, which coincided with a sharp increase in the number of patients treated with paliperidone-LAI, although the prescription rate of both formulations continued to increase until the end of the study follow-up. Conversely, paliperidone-LAI availability did not seem to significantly impact the number of patients who were treated with typical LAI APs. Finally, Kaplan-Meier estimates of the time to switch medications showed that patients taking fluphenazine-LAI and zuclopenthixol-LAI were more likely to stay on their assigned medication for a longer period than patients taking risperidone-LAI (χ2 = 51.2, P < .001) or paliperidone-LAI (χ2 = 51.9, P < .001) (Figure 1B). Of note, the time to switch in patients treated with risperidone-LAI decreased abruptly, coinciding with the time point at which the introduction of paliperidone-LAI took place in our area (Figure 1B).
Patterns of Long-Acting Injectable Prescription According to Duration of Disease
Overall, patients with a disease duration longer than 5 years were more likely to be older (mean ± SD age of 45.5 ± 13.8 vs 36.9 ± 10.3 years, P < .001) and have a diagnosis of hebephrenic or residual schizophrenia (P < .001) and were less likely to be treated with atypical APs (P = .0017) (Supplementary Table 1) than patients in the early phase of their disease (illness duration < 5 years). Regarding differences in the initial LAI AP prescribed, we observed that risperidone-LAI was the most frequently used formulation regardless of the duration of disease (55.3% when < 5 years and 51.3% when ≥ 5 years), but patients with a disease duration < 5 years took paliperidone-LAI more frequently than patients with a disease duration ≥ 5 years (χ2 = 12.9, P = .05, Supplementary Figure 1).
DISCUSSION
Patterns of Long-Acting Injectable Prescription
In the present study, we assessed LAI AP prescription practices during a 3-year follow-up period that began shortly before the marketing authorization and use of a new LAI formulation in the Tarragona area of Spain in late 2011. We observed that the prescription of all LAI formulations increased gradually with time and that at the end of the study, around one-third of patients were receiving an LAI. This finding falls within the mean of the wide range of LAI AP prescription rates reported, ranging from 10% to 80%.16,17
We observed that patients treated with an LAI AP had longer disease durations and were more likely to suffer from other psychiatric comorbidities including substance abuse than patients treated with an oral AP; however, only undifferentiated schizophrenia, substance abuse disorder, and neuropsychiatric comedication predicted the use of an LAI over an oral AP. These findings confirm previous observations that LAI formulations are preferred in challenging chronic disease patients13,14 and are also in agreement with the finding that LAI formulations are generally recommended to improve nonadherence in patients with comorbid substance abuse disorders.22,23 Moreover, our results confirm previous observations that a high proportion of patients taking LAIs use adjunctive psychotropic medications in real-world settings.24
Our data also show that hebephrenic schizophrenia and the need of hypnotic/sedative prescription predicted the treatment duration with the initially assigned LAI AP. Hebephrenic schizophrenia is linked to AP nonresponsiveness, use of comedications, and poor long-term prognoses.25
When we assessed the profiles of LAI-naive patients who switched from their initial assigned oral AP medication to an LAI formulation, we observed that younger age, substance abuse disorders, and concomitant use of anticholinergics strongly predicted a switch. Although we did not assess specific reasons for switching, our findings are consistent with previous studies reporting that LAI formulations are appropriate in younger patients because these patients are at higher risk for noncompliance.22,26 In addition, the higher frequency of anticholinergic treatment among subjects who changed from oral to LAI formulations can be considered an indirect indicator of the presence of extrapyramidal effects, which are one of the main reasons why AP treatments need to be changed.1
The transition from any LAI formulation to paliperidone-LAI (which in 65% of cases was the preferred second LAI option) was strongly predicted by a higher probability of neuropsychiatric comedication. This finding is in agreement with a previous report27 showing a high rate of concomitant prescription of mood stabilizers in patients treated with paliperidone-LAI compared with risperidone-LAI and zuclopenthixol-LAI, respectively. Because comorbid psychiatric disorders were not predictors of this particular switch, it is possible that this subgroup of patients required intensive treatment, leading to more adverse effects and a chronic disease course, which suggests that paliperidone-LAI is being prescribed for more severely affected patients (channeling effect).20
Impact of Paliperidone Palmitate Availability
Risperidone-LAI was the most frequently prescribed LAI AP regardless of the duration of illness. Switching from risperidone-LAI to paliperidone-LAI was the most frequent transition between LAI formulations, particularly in patients with illness duration > 5 years. However, as an initial LAI prescription, paliperidone-LAI was more frequently used among patients in the early phase of their disease (duration < 5 years). This pattern seems to explain the rapid acceptance and widespread use of paliperidone-LAI in our health care area, as we observed that while its introduction did not significantly impact the frequency of typical LAI AP prescription, the use of risperidone-LAI decreased in parallel with an initial sharp increase in its use. Similarly, the time to switch to another LAI formulation was longer among patients treated with a typical LAI but was much shorter among patients treated with risperidone-LAI immediately after the market authorization of paliperidone-LAI. This pattern is consistent with the findings of previous reports20 showing that the introduction of a new AP to the market is followed by very quick adoption by physicians and patients because of the convenience of its monthly administration. The increase in the number of overall LAI prescriptions throughout the study period may indicate a change in psychiatrist prescribing practices because of the availability of new second-generation LAI APs, which have increased the number of therapeutic options for patients with schizophrenia8 and also attenuated the negative attitudes of patients and psychiatrists, especially in the early phases of the illness, toward LAI APs compared with oral APs.13,28
Strengths and Limitations
The present study was based on a large sample of the overall population of schizophrenic patients in Tarragona who were taking APs, which is representative of southern Europe in terms of sociodemographic characteristics. Most importantly, the study was conducted in a real-life clinical setting, thus allowing the inclusion of patients who were otherwise excluded from randomized clinical trials. One of the limitations of this study was its retrospective design, which by nature means that some of the variables were recorded poorly and may have biased our results. Moreover, medication regimens were extracted from patient medical records, and we did not have access to information regarding patients’ reasons for medication discontinuation and nonadherence, which prevented us from analyzing a potential association between efficacy and tolerability and the switch from an oral or another LAI AP to paliperidone-LAI.
CONCLUSIONS
Our results provide further evidence that LAI formulations are generally prescribed to patients with longer disease durations, a greater number of associated psychiatric comorbidities, and more frequent substance use as well as to patients receiving concomitant neuropsychiatric medication compared with patients taking oral APs. The number of patients treated with at least 1 LAI formulation increased from 18.0% to 30.9% during the study period, and although risperidone-LAI was the most frequently prescribed LAI, we observed a decrease in the use of risperidone-LAI in parallel with an initial sharp increase in the use of paliperidone-LAI, particularly in young patients. Additional extensive long-term observational studies are needed to address whether the selective prescription of new LAI formulations is a channeling effect or the result of its favorable efficacy and tolerability profile and its advantageous mode of delivery.
Submitted: September 13, 2016; accepted November 28, 2016.
Published online: March 23, 2017.
Drug names: paliperidone (Invega), risperidone (Risperdal and others).
Potential conflicts of interest: None.
Funding/support: This work was supported by grants from Hospital Universitari Institut Pere Mata, Lundbeck Espa×±a SA, and Otsuka Pharmaceutical, SA to Drs Gaviria and Vilella.
Role of the sponsor: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments: Mײnica Gratacײs, PhD, who provides freelance service, and American Journal Experts, Durham, North Carolina, provided support in the manuscript preparation and editing and received payment from Hospital Universitari Institut Pere Mata and report no other conflicts of interest related to the subject of this article.
Supplementary material: See accompanying pages.
REFERENCES
1. Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients With Schizophrenia, 2nd ed. Am J Psychiatry. 2004;161(2 suppl):1-56. PubMed
2. Kane JM, Correll CU. Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci. 2010;12(3):345-357. PubMed
3. Weiden PJ. Switching in the era of atypical antipsychotics: an updated review. Postgrad Med. 2006;Spec No:27-44. PubMed
4. Faries DE, Ascher-Svanum H, Nyhuis AW, et al. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9:54. PubMed doi:10.1186/1471-244X-9-54
5. Nyhuis AW, Faries DE, Ascher-Svanum H, et al. Predictors of switching antipsychotic medications in the treatment of schizophrenia. BMC Psychiatry. 2010;10:75. PubMed doi:10.1186/1471-244X-10-75
6. Johnson DA. Observations on the use of long-acting depot neuroleptic injections in the maintenance therapy of schizophrenia. J Clin Psychiatry. 1984;45(5 pt 2):13-21. PubMed
7. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
8. Altamura AC, Aguglia E, Bassi M, et al. Rethinking the role of long-acting atypical antipsychotics in the community setting. Int Clin Psychopharmacol. 2012;27(6):336-349. 10.1097/YIC.0b013e328357727a PubMed
9. National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Prevention and Management. Clinical guideline [CG178]. February 2014. https://www.nice.org.uk/guidance/cg178
10. Grupo de trabajo de la Gu×a de Práctica Cl×nica sobre la Esquizofrenia y el Trastorno Psicótico Incipiente. Fײrum de Salut Mental, coordinación. Gu×a de Práctica Cl×nica sobre la Esquizofrenia y el Trastorno Psicótico Incipiente. Madrid: Plan de Calidad para el Sistema Nacional de Salud del Ministerio de Sanidad y Consumo. Agרncia d’ Avaluació de Tecnologia i Recerca Mרdiques; 2009. Gu×a de Práctica Cl×nica: AATRM. Nº 2006/05-2. http://www.guiasalud.es/GPC/GPC_495_Esquizofr_compl_cast_2009.pdf
11. Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed doi:10.1177/2045125314540297
12. Heres S, Lambert M, Vauth R. Treatment of early episode in patients with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413. PubMed doi:10.1016/S0924-9338(14)70001-X
13. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301. PubMed doi:10.1016/j.eurpsy.2009.12.020
14. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. PubMed doi:10.1176/appi.ajp.2011.10081224
15. Suzuki T. A further consideration on long-acting injectable versus oral antipsychotics in the treatment of schizophrenia: a narrative review and critical appraisal. Expert Opin Drug Deliv. 2016;13(2):253-264. PubMed doi:10.1517/17425247.2016.1115479
16. Johnson DAW. Historical perspective on antipsychotic long-acting injections. Br J Psychiatry suppl. 2009;52:S7-S12. PubMed doi:10.1192/bjp.195.52.s7
17. De Risio A, Lang AP. History and therapeutic rationale of long acting antipsychotics. Curr Clin Pharmacol. 2014;9(1):39-52. PubMed doi:10.2174/15748847113089990057
18. Samtani MN, Gopal S, Gassmann-Mayer C, et al. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25(10):829-845. PubMed doi:10.2165/11591690-000000000-00000
19. Rossi G, Frediani S, Rossi R, et al. Long-acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations. BMC Psychiatry. 2012;12:122. PubMed doi:10.1186/1471-244X-12-122
20. Pechlivanoglou P, Vehof J, van Agthoven M, et al. Diffusion of a new drug: a comparative analysis of adoption, treatment complexity, and persistence of risperidone long-acting injectable therapy in the Netherlands. Clin Ther. 2010;32(1):108-118. PubMed doi:10.1016/j.clinthera.2010.01.008
21. Gaviria AM, Franco JG, Aguado V, et al. A non-interventional naturalistic study of the prescription patterns of antipsychotics in patients with schizophrenia from the Spanish province of Tarragona. PLoS One. 2015;10(10):e0139403. PubMed doi:10.1371/journal.pone.0139403
22. Lang K, Meyers JL, Korn JR, et al. Medication adherence and hospitalization among patients with schizophrenia treated with antipsychotics. Psychiatr Serv. 2010;61(12):1239-1247. PubMed doi:10.1176/ps.2010.61.12.1239
23. Koola MM, Wehring HJ, Kelly DL. The potential role of long-acting injectable antipsychotics in people with schizophrenia and comorbid substance use. J Dual Diagn. 2012;8(1):50-61. PubMed doi:10.1080/15504263.2012.647345
24. Aggarwal NK, Sernyak MJ, Rosenheck RA. Prevalence of concomitant oral antipsychotic drug use among patients treated with long-acting, intramuscular, antipsychotic medications. J Clin Psychopharmacol. 2012;32(3):323-328. PubMed doi:10.1097/JCP.0b013e31825244f6
25. Fenton WS, McGlashan TH. Natural history of schizophrenia subtypes, I: longitudinal study of paranoid, hebephrenic, and undifferentiated schizophrenia. Arch Gen Psychiatry. 1991;48(11):969-977. PubMed doi:10.1001/archpsyc.1991.01810350009002
26. Ascher-Svanum H, Peng X, Faries D, et al. Treatment patterns and clinical characteristics prior to initiating depot typical antipsychotics for nonadherent schizophrenia patients. BMC Psychiatry. 2009;9:46. PubMed doi:10.1186/1471-244X-9-46
27. Cordiner M, Shajahan P, McAvoy S, et al. Effectiveness of long-acting antipsychotics in clinical practice, 1: a retrospective, 18-month follow up and comparison between paliperidone palmitate, risperidone long-acting injection and zuclopenthixol decanoate. Ther Adv Psychopharmacol. 2016;6(1):22-32. PubMed doi:10.1177/2045125315623168
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. PubMed doi:10.1007/s40263-016-0350-7
This PDF is free for all visitors!
Save
Cite