Background: Between 30% and 60% of individuals with major depressive disorder will have treatment-resistant depression (TRD): depression that does not subside with pharmaceutical treatment. Repetitive transcranial magnetic stimulation (rTMS) is an emerging treatment for TRD.
Objective: To establish the efficacy and optimal protocol for rTMS among adults and youth with TRD.
Data Sources: Two systematic reviews were conducted: one to determine the efficacy of rTMS for adults with TRD and another to determine the effectiveness of rTMS for youth with TRD. For adults, MEDLINE, Cochrane Central Register of Controlled Trials, PubMed, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews, and Health Technology Assessment Database were searched from inception until January 10, 2014 with no language restrictions. Terms aimed at capturing the target diagnosis, such as depression and depressive disorder, were combined with terms describing the technology, such as transcranial magnetic stimulation and rTMS. Results were limited to studies involving human participants and designed as a randomized controlled trial. For youth, the search was altered to include youth only (aged 13-25 years) and all study designs. When possible, meta-analysis of response and remission rates was conducted.
Study Selection: Seventy-three articles were included in this review: 70 on adult and 3 on youth populations.
Results: Meta-analysis comparing rTMS and sham in adults found statistically significant results favoring rTMS for response (RR: 2.35 [95% CI, 1.70-3.25]) and remission (RR: 2.24 [95% CI, 1.53-3.27]). No statistically significant differences were found when comparing high- and low-frequency, unilateral and bilateral, low- and high-intensity rTMS or rTMS and electroconvulsive therapy (ECT). While meta-analysis of results from the youth literature was not possible, the limited evidence base suggests that rTMS may be effective for treating TRD in youth.
Conclusions: The evidence available on the use of rTMS for adults with TRD indicates that rTMS is approximately twice as effective as a sham procedure, although the optimal rTMS protocol remains unclear. Evidence also indicates that rTMS is as effective as ECT and appears promising as a treatment for youth with TRD; however, the evidence base is underdeveloped.
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
ABSTRACT
Background: Between 30% and 60% of individuals with major depressive disorder will have treatment-resistant depression (TRD): depression that does not subside with pharmaceutical treatment. Repetitive transcranial magnetic stimulation (rTMS) is an emerging treatment for TRD.
Objective: To establish the efficacy and optimal protocol for rTMS among adults and youth with TRD.
Data Sources: Two systematic reviews were conducted: one to determine the efficacy of rTMS for adults with TRD and another to determine the effectiveness of rTMS for youth with TRD. For adults, MEDLINE, Cochrane Central Register of Controlled Trials, PubMed, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews, and Health Technology Assessment Database were searched from inception until January 10, 2014 with no language restrictions. Terms aimed at capturing the target diagnosis, such as depression and depressive disorder, were combined with terms describing the technology, such as transcranial magnetic stimulation and rTMS. Results were limited to studies involving human participants and designed as a randomized controlled trial. For youth, the search was altered to include youth only (aged 13-25 years) and all study designs. When possible, meta-analysis of response and remission rates was conducted.
Study Selection: Seventy-three articles were included in this review: 70 on adult and 3 on youth populations.
Results: Meta-analysis comparing rTMS and sham in adults found statistically significant results favoring rTMS for response (RR: 2.35 [95% CI, 1.70-3.25]) and remission (RR: 2.24 [95% CI, 1.53-3.27]). No statistically significant differences were found when comparing high- and low-frequency, unilateral and bilateral, low- and high-intensity rTMS or rTMS and electroconvulsive therapy (ECT). While meta-analysis of results from the youth literature was not possible, the limited evidence base suggests that rTMS may be effective for treating TRD in youth.
Conclusions: The evidence available on the use of rTMS for adults with TRD indicates that rTMS is approximately twice as effective as a sham procedure, although the optimal rTMS protocol remains unclear. Evidence also indicates that rTMS is as effective as ECT and appears promising as a treatment for youth with TRD; however, the evidence base is underdeveloped.
Prim Care Companion CNS Disord 2015;17(6):doi:10.4088/PCC.15r01807
© Copyright 2015 Physicians Postgraduate Press, Inc.
aCommunity Health Sciences, University of Calgary, Calgary, Alberta, Canada
bInstitute for Public Health, University of Calgary, Calgary, Alberta, Canada
cInstitute of Health Economics, Edmonton, Alberta, Canada
*Corresponding author: Fiona M. Clement, PhD, 3D18, Teaching Research and Wellness Bldg, 3280 Hospital Drive NW, Calgary, Alberta, Canada T2N 4N1 ([email protected]).
Globally, more than 350 million people of all ages suffer from depression.1 Symptoms of depression include loss of focus, lack of energy, complaints of physical illness with no cause, and thoughts of suicide.2 On the basis of the epidemiologic data available,3,4 between 30% and 60% of people with major depressive disorder will have treatment-resistant depression (TRD), a type of depression in which the patient does not experience sufficient relief after adequate rounds of medication. The definition of TRD has not been standardized, and, in practice, varies from lack of response to 1 antidepressant to lack of response to at least 3 adequate antidepressant trials.4
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive procedure in which cerebral electrical activity is influenced by a rapidly changing magnetic field.5 The magnetic field is created by a plastic-encased coil that is placed over the patient’s scalp. This magnetic field can be directed onto specific areas of the brain and modulates cerebral activity using low or high frequencies. In contrast to electroconvulsive therapy (ECT), rTMS can induce cortical electrical activity without causing a seizure; it is subconvulsive and, therefore, does not require anesthesia.6
Recent systematic reviews and meta-analyses7-9 on rTMS have established the efficacy of rTMS for adults with TRD compared to sham. However, the optimal rTMS protocol, the efficacy of rTMS compared to ECT, and the effectiveness of rTMS in youth populations remain unknown.
Thus, the objective of this study was to determine the efficacy, safety, and optimal protocol of rTMS for TRD in adults and youth in comparison to sham procedures and ECT. This systematic review and meta-analysis provides a complete synthesis of the effectiveness of rTMS, the delivery of rTMS, and its place compared to relevant alternatives across all populations.
METHOD
Two systematic reviews were completed: one on the efficacy of rTMS for adults with TRD and one on the effectiveness of rTMS for youth with TRD. For adults, MEDLINE, Cochrane Central Register of Controlled Trials, PubMed, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews, and Health Technology Assessment Database were searched from inception until January 10, 2014 with no language restrictions. Terms aimed at capturing the target diagnosis, such as depression and depressive disorder, were combined with terms describing the technology, such as transcranial magnetic stimulation and rTMS. Results were limited to studies involving human participants and designed as a randomized controlled trial (RCT). For youth, the search was altered to include only subjects aged 13-25 years and all study designs.
All abstracts and full-text studies were screened by 2 reviewers/authors (L.E.L. and S.C.) using the inclusion and exclusion criteria outlined in Table 1. Abstracts selected for inclusion by either reviewer proceeded to full-text review; this abstract review was intentionally broad to ensure that all relevant literature was captured. Studies included after abstract review proceeded to full-text review. Studies were included if they met all inclusion criteria and failed to meet any of the exclusion criteria presented in Table 1. Any discrepancy between reviewers was resolved through consensus. After full-text review, published systematic reviews and meta-analysis on rTMS were hand-searched to ensure that all relevant articles were captured in the literature search.
For all studies, year of publication, country, patient selection, patient characteristics, definition of treatment resistance, description of technologies, protocols for control and treatment, outcomes measured, instruments used, definition of response, definition of remission, and follow-up time were extracted in duplicate using data extraction forms. The primary outcomes, response, and remission rates postintervention were also extracted from each study. During data extraction, each included study was assessed for quality using the Cochrane Risk of Bias Checklist10 for RCTs or the Downs and Black Checklist11 for all other study designs. Quality assessment was completed in duplicate, and discrepancies were resolved through discussion.

- While the optimal treatment protocol is yet to be established, repetitive transcranial magnetic stimulation (rTMS) is an effective treatment compared to sham with minor side effects.
- The performance of rTMS in comparison to electroconvulsive therapy is not well understood.
Meta-analysis was conducted when possible, with response and remission rates as the primary outcomes considered. The definitions of response and remission as defined by authors of the included articles were used. For each study, the number of participants who experienced remission and response were compared between the rTMS group and the comparator group. Meta-analyses were conducted using relative risk (RR) to express the efficacy of rTMS in relation to other comparators, and a random-effects model was used. Begg funnel plots were completed to assess the risk of publication bias. All analyses were completed using Stata/IC 13.1 (StataCorp, College Station, Texas).
RESULTS
Adult Populations
The search strategy identified 786 citations for the review of rTMS for adult populations. Of these, 602 were excluded and 184 articles proceeded to full-text review. An additional 114 articles were excluded during full-text review, resulting in 70 articles included in the final analysis (Figure 1). Although 5 published systematic reviews and meta-analyses8,12-15 were hand-searched for articles not captured in the original search, no additional articles were identified. On the basis of the Cochrane Risk of Bias Checklist, the included studies were determined to be of moderate quality, with areas of low, unclear, and high risk of bias (Figure 2).
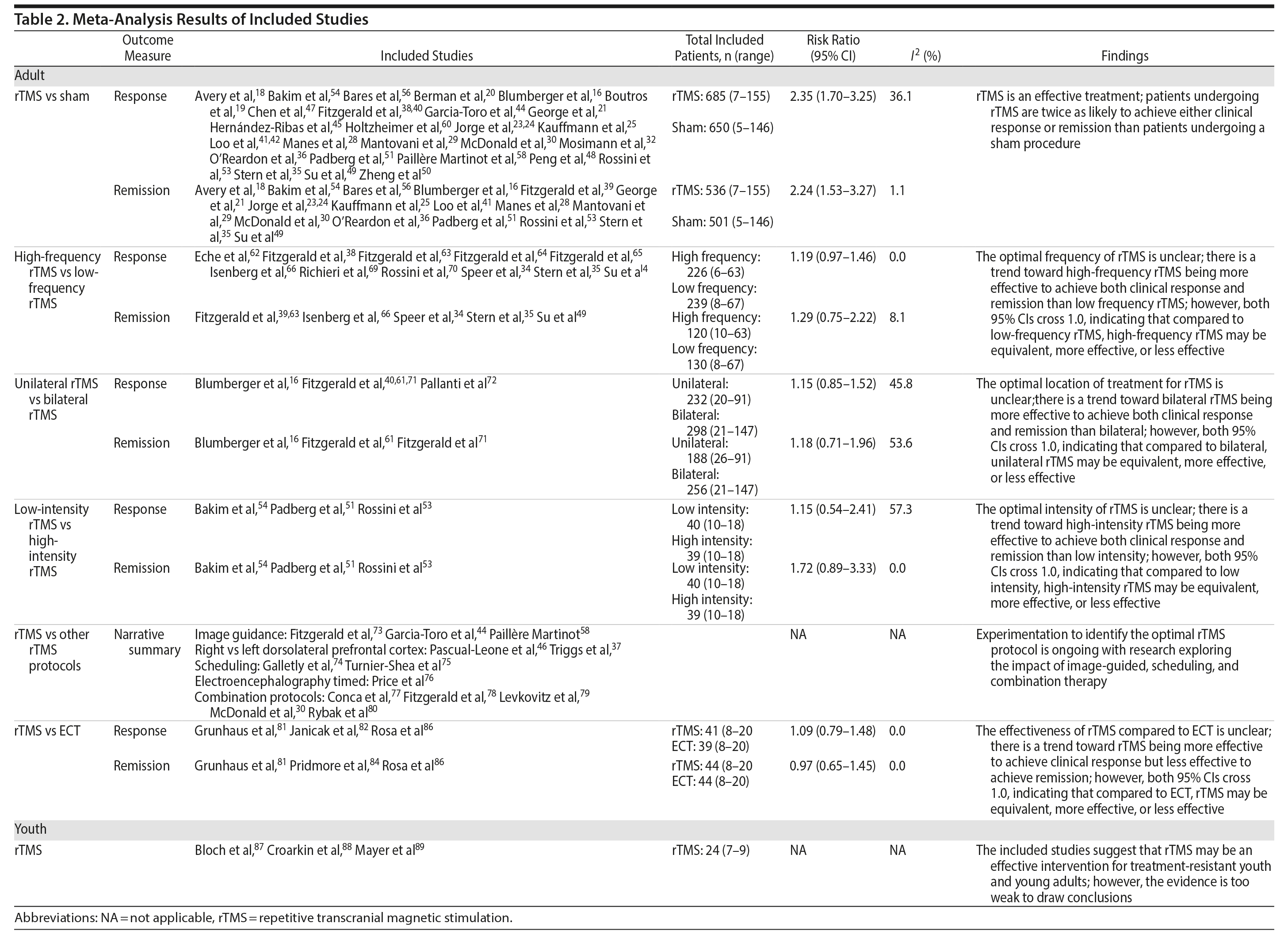
The 70 articles on rTMS in adult populations were further separated into 6 categories based on comparator: rTMS versus sham (45),16-61 high-frequency versus low-frequency rTMS (14),33-35,38,49,62-70 bilateral versus unilateral rTMS (5),16,40,61,71,72 high-intensity versus low-intensity rTMS (3),51,53,54 other rTMS protocols (13),30,37,44,46,58,73-80 and rTMS versus ECT (6).81-86 Seventeen of the included studies16,27,30,33-35,37,38,40,41,44,46,49,53,54,58,68 had 3 comparator arms (2 rTMS arms and a sham arm). These 17 studies were included in both the rTMS versus rTMS and the rTMS versus sham categories. The results for each category are summarized in Table 2.

rTMS Compared to Sham
Of the 45 studies comparing rTMS and sham, 1 was conducted in Canada,16 21 in the United States,17-37 5 in Australia,38-42 4 in Spain,43-46 3 in China,47-50 2 in Germany,51 2 in Italy,52,53 and the remaining 6 in various other countries (Turkey,54 Belgium,55 Czech Republic,56 Denmark,57 France,58 Iceland59). The studies were published between 199646 and 2013.29,34,45,47,55 Fifteen studies16,18,20,21,23,26,27,30,36,39,41,42,56,58,60 used an intention-to-treat analysis, 152 used a per-protocol analysis, and the remaining did not report on the type of analysis that was conducted.
The number of participants included in each study varied between 617 and 301,36 with a total of 1,903 participants included across 45 studies. Frequency of rTMS varied from 1 Hz25,29,36,39,44,56 to 20 Hz,19,20,28,31-34,43,47,49,52,54,55 and motor threshold varied from 80%17,19,20,28,31 to 120%.21,26,36,40 The number of rTMS sessions provided to each participant in the active arms varied from 5 to 30 over a period of 5 days to 6 weeks. The protocol used for the sham procedure was similar in all studies, with most using an rTMS machine turned on and at a 45° angle from the patient. Additional details can be found in Supplementary Table 1.
Thirty-one of the studies 16,18-21,23-25,28-30,32,35,36,38,39,41,42,44,45,47–51,53,54,56,58,60,61 comparing rTMS and sham provided adequate data on treatment response to permit pooling for meta-analysis and 1816,18,21,23-25,28-30,35,39,41,49,51,53,54,56 provided adequate data on treatment remission. As shown in Figures 3 and 4, the scale and threshold for both response and remission varied by article. The overall pooled RR for response was 2.35 (95% CI, 1.70-3.25) (Figure 3) and remission was 2.24 (95% CI, 1.53-3.27) (Figure 4). Thus, patients are twice as likely to achieve response or remission with rTMS than with a sham procedure. For response, there was evidence of publication bias (P value = .025), although visual inspection of the plot did not indicate any bias.
Optimal Protocol
Of the 35 studies comparing optimal protocols for rTMS, 1433-35,38,49,62-70 compared high-frequency and low-frequency rTMS, 516,40,61,71,72 compared unilateral and bilateral rTMS, 351,53,54 compared high-intensity and low-intensity rTMS, and 1330,37,44,46,58,73-80 compared standard rTMS with various other rTMS protocols (eg, image guided, session scheduling, combination protocols). Additional details can be found in Supplementary Tables 2-5.
Eleven34,35,38,49,62-66,69,70 of the 14 studies assessing high-frequency versus low-frequency rTMS provided adequate data on treatment response, and 6 of the studies34,35,49,63,65,66 provided adequate data on treatment remission to permit pooling. Comparing high-frequency and low-frequency rTMS, the overall pooled RR for response was 1.19 (95% CI, 0.97-1.46) and remission was 1.29 (95% CI, 0.75-2.22). These results suggest that there is a tendency for high-frequency rTMS to result in more cases of response and remission, but neither result is statistically significant (Table 2).
Of the studies comparing unilateral and bilateral rTMS, 516,40,61,71,72 provided adequate data on treatment response and 316,61,71 provided adequate data on treatment remission to permit pooling. The overall pooled RR for response was 1.15 (95% CI, 0.85-1.56) and remission was 1.18 (95% CI, 0.71-1.96). These results suggest that there is a tendency for bilateral frequency rTMS to result in more cases of response and remission, but neither result is statistically significant (Table 2).
All 3 of the studies comparing high- and low-intensity rTMS provided adequate data on treatment response and remission to permit pooling.51,53,54 For treatment response, the overall pooled RR for high-intensity versus low intensity rTMS was 1.15 (95% CI, 0.54-2.41) and remission was 1.72 (95% CI, 0.89-3.33). These results suggest that there is a tendency for high-intensity rTMS to result in more cases of response and remission, but neither result is statistically significant (Table 2).
Thirteen RCTs30,37,44,46,58,73-80 assessing rTMS compared to various other rTMS procedures were included. Meta-analyses could not be performed due to the significant heterogeneity in rTMS protocol properties, duration of follow-up periods, reported outcome measures, control or comparison groups, and study quality and size. Three of these studies44,58,73 investigated the use of image guidance in rTMS, 237,46 compared left and right cortex targeting, 274,75 compared the scheduling of rTMS sessions, 176 compared standard rTMS to rTMS using electroencephalogram activity, and 530,77-80 assessed the efficacy of combination protocols for rTMS treatment. Among the 13 RCTs30,37,44,46,58,73-80 that assessed the efficacy of rTMS compared to various other rTMS procedures, there was significant variability in the reporting of and criteria used to define treatment efficacy (ie, response and/or remission). The majority of studies were small (n ≤ 100 patients). Ten studies30,37,44,58,74,75-78,80 reported no differences in treatment efficacy (ie, rates of response and/or remission, mean change in outcome score) between groups, 3 studies46,73,79 reported statistically significant differences in treatment efficacy between groups, and no studies reported worsening of TRD following any of the investigated rTMS treatments. Of the 3 studies46,73,79 that reported statistically significant differences in outcomes between treatment groups, 1 study73 reported higher rates of response and remission using a neuronavigational method for rTMS localization over the standard 5-cm method, 1 multiple crossover study46 found that left-sided rTMS resulted in lower mean Hamilton Depression Rating Scale (HDRS) scores in comparison to right-sided, and 1 study79 reported superior efficacy of high-intensity and left-sided (unilateral) rTMS compared to low-intensity and bilateral rTMS.
rTMS Compared to Electroconvulsive Therapy
Six RCTs81-86 comparing rTMS with ECT were included. Two studies83,84 were conducted in Australia, 186 was conducted in Brazil, 1 was conducted in Iran,85 181 was conducted in Israel, and 182 was conducted in the United States. Five of the 6 RCTs81,82,84-86 compared an rTMS arm to an ECT arm, while the remaining RCT83 compared a combination of rTMS and ECT to ECT alone. Additional details can be found in Supplementary Table 6.
Three rTMS versus ECT studies81,82,86 provided adequate data on treatment response to permit pooling. All 3 articles used the HDRS to determine treatment response, which was defined as a minimum 50% reduction in the HDRS score. The overall pooled RR for rTMS versus ECT is 1.09 (95% CI, 0.79-1.48) (Table 2). However, the results are not statistically significant different; rTMS may be less or more effective compared to ECT.
Three of the rTMS versus ECT studies81,84,86 provided adequate data on treatment remission to permit pooling. While all 3 of these studies used the HDRS to define remission, 281,84 used a threshold score of 8 and 186 used a threshold score of 7.
The overall pooled RR for rTMS versus ECT remission rate is 0.97 (95% CI, 0.65-1.45). This result suggests that there is no statistically significant difference in remission rates of patients treated with rTMS compared to those treated with ECT.
Youth Populations
The systematic review of rTMS effectiveness with youth populations identified 140 abstracts. During abstract review, 114 studies were excluded, with 26 proceeding to full-text review. After being reviewed in full-text, 23 were excluded and 3 were included (Figure 1). The included studies were conducted in Israel,87 the United States,88 and Australia,89 and all 3 were designed as prospective cohort studies, were small including 788,89 or 987 participants, and were conducted between 200887 and 2012.88,89 Bloch et al87 and Croarkin et al88 recruited participants from medical centers and reported short-term outcomes after rTMS treatment (1 month posttreatment and 5 weeks posttreatment, respectively). Mayer et al89 recruited participants from the Bloch et al87 trial and reported patients’ long-term outcomes (6 years posttreatment) based on their original treatment allocation. Bloch et al,87 Croarkin et al,88 and Mayer et al89 were given quality assessment scores of 17, 16, and 15, respectively, out of a possible total of 23 points on the modified Downs and Black Checklist. Areas where quality was most often lacking were related to whether an attempt was made to blind study subjects or those measuring intervention outcomes, respectively. Additional study details are available in Supplementary Table 7.
Bloch et al87 used 10 Hz rTMS at 80% motor threshold for 14 sessions and assessed the outcomes at baseline; days 7, 10, and 14; and 1 month posttreatment. At 1 month posttreatment, 3 of 9 participants experienced clinical response (at least a 30% reduction in the Children’s Depression Rating Scale).87 This study also found statistically significant reductions in depression measured using the Beck Depression Inventory at days 7 and 10 and 1 month posttreatment when compared to baseline (P < .05).87 Using the Screen for Child Anxiety-Related Disorders Questionnaire, participants’ anxiety levels were significantly lower at the end of treatment and 1 month posttreatment (P < .05).87 Statistically significant results were not found with the Suicide Ideation Questionnaire at any time point, indicating that there is no evidence to suggest that rTMS has an effect on suicidal ideations and behaviors.87
Mayer et al89 reported long-term (3-year) outcomes of the patients in the study by Bloch et al87; no additional rTMS treatment was received. No statistically significant differences between long-term outcomes and outcomes at the end of treatment were reported. This finding suggests that participants experienced initial improvement in their depression severity and did not experience worsening or improvement in depression severity over time.89
Croarkin et al88 used 10 Hz rTMS at 120% motor threshold for 30 sessions over a period of 6 to 8 weeks and assessed outcome measures at baseline and at weeks 2, 4, and 6. Clinical response and remission were not reported. However, the mean Children’s Depression Rating Scale score was reduced from 69.3 (SD = 8.6) at baseline to 42.1 (SD = 10.7) (average of week 2, 4, and 6 outcomes reported). This study also reported increases in cortical activity over time when baseline is compared to week 5.
DISCUSSION
The 2 systematic reviews identified 70 relevant RCTs and 3 observational studies. Of these studies, 45 compared rTMS and sham, 35 compared optimal rTMS protocols, 6 compared rTMS and ECT, and 3 assessed the effectiveness of rTMS for youth populations. The body of evidence available on the use of rTMS for adults indicates that rTMS is approximately twice as effective as the sham procedure. However, the optimal frequency, location, and intensity of rTMS for adults remain unclear. There is likely no difference in efficacy between ECT and rTMS; rTMS may be more effective to achieve response but less effective to achieve remission; however, neither result is statistically significant. There is substantial experimentation still required to identify and improve the optimal rTMS protocol. Active research is ongoing with the use of image-guided techniques, scheduling of treatment, and timing of treatment. However, none of these research areas are developed enough to clarify the role of these variables in the effective use of rTMS.
Compared to the breadth of adult literature available on the use of rTMS for TRD, there is very little literature on youth and adolescent populations. Three articles on the treatment of youth with TRD were included in this research, all of which were prospective cohort studies. Using the Children’s Depression Rating Scale, both of the studies assessing short-term outcomes found a reduction in depression severity after rTMS treatment; 187 reported statistically significant reductions, while the other88 did not conduct tests of significance. The former87 found statistically significant reductions in depression severity at days 7 and 10 and 1 month posttreatment. Other outcome measures such as the Beck Depression Inventory and the Screen for Child Anxiety-Related Disorders Questionnaire also suggested statistically significant reduction in depression severity.
The results from the 3 included articles suggest that rTMS may be an effective method for alleviating severe depression in youth who have failed to respond to other treatments. However, the limited number and the low to moderate quality of the studies on this topic restrict the ability to draw generalized conclusions about the use of rTMS in this population. The rTMS protocols were heterogeneous among the included studies, precluding inference of the most appropriate protocol for this patient population. Furthermore, the small sample size in each article, with a total of 25 participants included in all 3 studies, does not provide a robust evidence base.
With depression affecting a sizeable number of youth and adolescents, finding acceptable, efficacious treatments for this patient population is of particular importance. The included literature suggests that rTMS may be an effective treatment option for youth and young adults with depression. However, with limited literature and data available, further studies, particularly large-scale high-quality studies with this patient population, are required before conclusive inferences can be drawn.
A number of limitations from both the adult and youth systematic reviews merit comment. Within the adult literature, there is significant heterogeneity in rTMS protocols, duration of follow-up periods, reported outcome measures, control or comparison groups, and study quality and size. When possible, studies were divided by comparator. However, it was not possible to further divide them on the basis of small protocol differences, and this may limit the robustness of our findings. Yet, given that no statistically significant differences were found between protocol differences such as high and low frequency, unilateral and bilateral treatment, and high and low intensity, it is unlikely that pooling the mixed protocols would have introduced significant bias.
Broadly, the included studies on the efficacy of rTMS for adult populations were of moderate quality, with most having a combination of unclear and low risks of bias and few having high risks of bias, as assessed by the Cochrane Risk of Bias Checklist. Blinding of participants and treatment providers was an area in which the included studies often suffered from a lack of clarity. Methods of random sequence generation were also largely unclear in the included studies. However, given that very few studies had a “high” risk in this area and most were “unclear,” these areas of bias could have been due to a lack of detail in method descriptions rather than an area of bias.
In both of the systematic reviews on effectiveness/efficacy (youth and adult), treatment response and remission were the primary outcomes assessed. These outcomes were selected because they were most frequently reported among the included studies and give a broad sense of patient improvement or worsening. Ideally, outcomes such as function and quality of life would be the primary outcomes assessed, as these outcomes would more closely determine the impact of rTMS treatment on a patient’s life. However, measures of function and/or quality of life are infrequently found in the literature, resulting in insufficient data to pool for meta-analysis. Thus, a major limitation is that the outcomes available in the literature are not directly measuring improvement in patient quality of life.
Both the adult and youth reviews were also limited by a lack of studies reporting long-term data. The vast majority of studies on rTMS assess only the short-term impact of treatment (4-6 weeks). Due to limited long-term data, it is not possible to draw conclusions about the length of treatment effect or the long-term safety of treatment and its impact on outcomes such as return to work or ability to complete daily tasks. Studies reporting long-term patient outcomes such as relapse, reoccurrence, participation in life, and major side effects are required.
Lastly, the majority of the included studies were conducted in the United States and Australia. With no reason to suspect that the patient mix and underlying etiology of major depressive disorder and TRD are substantially different in these countries, we anticipate that our findings are broadly generalizable to other countries.
In summary, the evidence available on the use of rTMS for adults with TRD indicates that rTMS is approximately twice as effective as a sham procedure and most likely as effective as ECT. However, the optimal rTMS protocol remains unclear, with no statistically significant differences between frequency, intensity, or location. rTMS appears promising as an effective treatment for youth with TRD, although the evidence base is underdeveloped.
Submitted: February 24, 2015; accepted June 1, 2015.
Published online: November 5, 2015.
Author contributions: Design of the study (all authors), collection of data (Mss Leggett, Coward, and Lorenzetti), management of data (Mss Leggett, Coward, and Soril and Dr Clement), analysis of data (Mss Leggett, Coward, and Soril and Dr Clement), interpretation of data (Mss Leggett and Coward), preparation of the manuscript (all authors), review of the manuscript (all authors), and approval of the manuscript (all authors).
Potential conflicts of interest: None reported.
Funding/support: This work was supported by a financial contribution from Alberta Health.
Role of the sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation of the manuscript. The sponsor did review and approve the manuscript content for publication.
Disclaimer: The views expressed herein do not necessarily represent the official policy of Alberta Health.
Supplementary material: See accompanying pages.
REFERENCES
1. Depression. World Health Organization Web site. http://www.who.int/mediacentre/factsheets/fs369/en/. Updated October 2012. Accessed August 1, 2014.
2. Facts about depression and bipolar disorder. 2014. Canadian Mental Health Association Web site. http://www.cmha.ca/mental_health/facts-about-depression-and-bipolar-disorder/#.VaPZKvlVhBd. Accessed August 1, 2014.
3. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510. PubMed doi:10.1176/appi.ajp.2010.10081187
4. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659. PubMed doi:10.1016/S0006-3223(03)00231-2
5. Kennedy SH, Milev R, Giacobbe P, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults, 4: neurostimulation therapies. J Affect Disord. 2009;117(suppl 1):S44-S53. PubMed doi:10.1016/j.jad.2009.06.039
6. McLoughlin DM, Mogg A, Eranti S, et al. The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis. Health Technol Assess. 2007;11(24):1-54. PubMed
7. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239. PubMed doi:10.1017/S0033291713000512
8. Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551. PubMed doi:10.1038/npp.2012.237
9. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489, quiz 489. PubMed doi:10.4088/JCP.13r08815
10. Higgins J, Altman DG. Assessing risk of bias in included studies. In: Higgins J, Altman DJ, eds. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, England: John Wiley & Sons Ltd; 2008;187-241.
11. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed doi:10.1136/jech.52.6.377
12. Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2013;43(11):2245-2254. PubMed doi:10.1017/S0033291712002802
13. Herrmann LL, Ebmeier KP. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J Clin Psychiatry. 2006;67(12):1870-1876. PubMed doi:10.4088/JCP.v67n1206
14. Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013;74(2):e122-e129. PubMed doi:10.4088/JCP.12r07996
15. Fitzgerald P. Repetitive transcranial magnetic stimulation and electroconvulsive therapy: complementary or competitive therapeutic options in depression? Australas Psychiatry. 2004;12(3):234-238. PubMed doi:10.1080/j.1039-8562.2004.02113.x
16. Blumberger DM, Mulsant BH, Fitzgerald PB, et al. A randomized double-blind sham-controlled comparison of unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant major depression. World J Biol Psychiatry. 2012;13(6):423-435. PubMed doi:10.3109/15622975.2011.579163
17. Avery DH, Claypoole K, Robinson L, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. J Nerv Ment Dis. 1999;187(2):114-117. PubMed doi:10.1097/00005053-199902000-00009
18. Avery DH, Holtzheimer PE 3rd, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187-194. PubMed doi:10.1016/j.biopsych.2005.07.003
19. Boutros NN, Gueorguieva R, Hoffman RE, et al. Lack of a therapeutic effect of a 2-week sub-threshold transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Res. 2002;113(3):245-254. PubMed doi:10.1016/S0165-1781(02)00267-6
20. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47(4):332-337. PubMed doi:10.1016/S0006-3223(99)00243-7
21. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507-516. PubMed doi:10.1001/archgenpsychiatry.2010.46
22. Holtzheimer PE, Avery D, Schlaepfer TE. Antidepressant effects of repetitive transcranial magnetic stimulation. Br J Psychiatry. 2004;184(6):541-542. PubMed doi:10.1192/bjp.184.6.541-a
23. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276. PubMed doi:10.1001/archgenpsychiatry.2007.45
24. Jorge RE, Robinson RG, Tateno A, et al. Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry. 2004;55(4):398-405. PubMed doi:10.1016/j.biopsych.2003.08.017
25. Kauffmann CD, Cheema MA, Miller BE. Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety. 2004;19(1):59-62. PubMed doi:10.1002/da.10144
26. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522-534. PubMed doi:10.1038/npp.2008.118
27. Loo C, Mitchell P, Sachdev P, et al. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry. 1999;156(6):946-948. PubMed doi:10.1176/ajp.156.6.946
28. Manes F, Jorge R, Morcuende M, et al. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. 2001;13(2):225-231. PubMed doi:10.1017/S1041610201007608
29. Mantovani A, Aly M, Dagan Y, et al. Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression.. J Affect Disord. 2013;144(1-2):153-159. doi:10.1016/j.jad.2012.05.038
30. McDonald WM, Easley K, Byrd EH, et al. Combination rapid transcranial magnetic stimulation in treatment refractory depression. Neuropsychiatr Dis Treat. 2006;2(1):85-94. PubMed
31. Moser DJ, Jorge RE, Manes F, et al. Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology. 2002;58(8):1288-1290. PubMed doi:10.1212/WNL.58.8.1288
32. Mosimann UP, Schmitt W, Greenberg BD, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126(2):123-133. PubMed doi:10.1016/j.psychres.2003.10.006
33. Speer AM, Benson BE, Kimbrell TK, et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J Affect Disord. 2009;115(3):386-394. PubMed doi:10.1016/j.jad.2008.10.006
34. Speer AM, Wassermann EM, Benson BE, et al. Antidepressant efficacy of high and low frequency rTMS at 110% of motor threshold versus sham stimulation over left prefrontal cortex. Brain Stimulat. 2014;7(1):36-41. PubMed doi:10.1016/j.brs.2013.07.004
35. Stern WM, Tormos JM, Press DZ, et al. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double-blind, randomized, placebo-controlled trial. J Neuropsychiatry Clin Neurosci. 2007;19(2):179-186. PubMed doi:10.1176/jnp.2007.19.2.179
36. O’ Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216. PubMed doi:10.1016/j.biopsych.2007.01.018
37. Triggs WJ, Ricciuti N, Ward HE, et al. Right and left dorsolateral pre-frontal rTMS treatment of refractory depression: a randomized, sham-controlled trial. Psychiatry Res. 2010;178(3):467-474. PubMed doi:10.1016/j.psychres.2010.05.009
38. Fitzgerald PB, Brown TL, Marston NAU, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60(10):1002-1008. PubMed
39. Fitzgerald PB, Benitez J, de Castella A, et al. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163(1):88-94. PubMed doi:10.1176/appi.ajp.163.1.88
40. Fitzgerald PB, Hoy KE, Herring SE, et al. A double blind randomized trial of unilateral left and bilateral prefrontal cortex transcranial magnetic stimulation in treatment resistant major depression. J Affect Disord. 2012;139(2):193-198. PubMed doi:10.1016/j.jad.2012.02.017
41. Loo CK, Mitchell PB, McFarquhar TF, et al. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37(3):341-349. PubMed doi:10.1017/S0033291706009597
42. Loo CK, Mitchell PB, Croker VM, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33(1):33-40. PubMed doi:10.1017/S0033291702006839
43. Garcia-Toro M, Mayol A, Arnillas H, et al. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J Affect Disord. 2001;64(2-3):271-275. PubMed doi:10.1016/S0165-0327(00)00223-8
44. Garcia-Toro M, Salva J, Daumal J, et al. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry Res. 2006;146(1):53-57. PubMed doi:10.1016/j.pscychresns.2004.08.005
45. Hernández-Ribas R, Deus J, Pujol J, et al. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimulat. 2013;6(1):54-61. PubMed doi:10.1016/j.brs.2012.01.001
46. Pascual-Leone A, Rubio B, Pallardó F, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233-237. PubMed doi:10.1016/S0140-6736(96)01219-6
47. Chen SJ, Chang CH, Tsai HC, et al. Superior antidepressant effect occurring 1 month after rTMS: add-on rTMS for subjects with medication-resistant depression. Neuropsychiatr Dis Treat. 2013;9:397-401. PubMed doi:10.2147/NDT.S40466
48. Peng H, Zheng H, Li L, et al. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J Affect Disord. 2012;136(3):249-257. PubMed doi:10.1016/j.jad.2011.12.006
49. Su TP, Huang CC, Wei IH. Add-on rTMS for medication-resistant depression: a randomized, double-blind, sham-controlled trial in Chinese patients. J Clin Psychiatry. 2005;66(7):930-937. PubMed doi:10.4088/JCP.v66n0718
50. Zheng H, Zhang L, Li L, et al. High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1189-1195. PubMed doi:10.1016/j.pnpbp.2010.06.009
51. Padberg F, Zwanzger P, Keck ME, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27(4):638-645. PubMed
52. Bortolomasi M, Minelli A, Fuggetta G, et al. Long-lasting effects of high frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res. 2007;150(2):181-186. PubMed doi:10.1016/j.psychres.2006.04.010
53. Rossini D, Lucca A, Zanardi R, et al. Transcranial magnetic stimulation in treatment-resistant depressed patients: a double-blind, placebo-controlled trial. Psychiatry Res. 2005;137(1-2):1-10. PubMed doi:10.1016/j.psychres.2005.06.008
54. Bakim B, Uzun UE, Karamustafalioglu O, et al. The combination of antidepressant drug therapy and high-frequency repetitive transcranial magnetic stimulation in medication-resistant depression. Klinik Psikofarmakoloji Bulteni. 2012;22:244-253.
55. Baeken C, Vanderhasselt MA, Remue J, et al. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J Affect Disord. 2013;151(2):625-631. PubMed doi:10.1016/j.jad.2013.07.008
56. Bares M, Kopecek M, Novak T, et al. Low frequency (1-Hz), right prefrontal repetitive transcranial magnetic stimulation (rTMS) compared with venlafaxine ER in the treatment of resistant depression: a double-blind, single-centre, randomized study. J Affect Disord. 2009;118(1-3):94-100. PubMed doi:10.1016/j.jad.2009.01.032
57. Bretlau LG, Lunde M, Lindberg L, et al. Repetitive transcranial magnetic stimulation (rTMS) in combination with escitalopram in patients with treatment-resistant major depression: a double-blind, randomised, sham-controlled trial. Pharmacopsychiatry. 2008;41(2):41-47. PubMed doi:10.1055/s-2007-993210
58. Paillרre Martinot ML, Galinowski A, Ringuenet D, et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. Int J Neuropsychopharmacol. 2010;13(1):45-59. PubMed doi:10.1017/S146114570900008X
59. Möller AL, Hjaltason O, Ivarsson O, et al. The effects of repetitive transcranial magnetic stimulation on depressive symptoms and the P(300) event-related potential. Nord J Psychiatry. 2006;60(4):282-285. PubMed doi:10.1080/08039480600790119
60. Holtzheimer PE 3rd, Russo J, Claypoole KH, et al. Shorter duration of depressive episode may predict response to repetitive transcranial magnetic stimulation. Depress Anxiety. 2004;19(1):24-30. PubMed doi:10.1002/da.10147
61. Fitzgerald PB, Hoy K, Gunewardene R, et al. A randomized trial of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in treatment-resistant major depression. Psychol Med. 2011;41(6):1187-1196. PubMed doi:10.1017/S0033291710001923
62. Eche J, Mondino M, Haesebaert F, et al. Low- vs high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front Psychiatry. 2012;3:13. doi:10.3389/fpsyt.2012.00013 PubMed
63. Fitzgerald PB, Huntsman S, Gunewardene R, et al. A randomized trial of low-frequency right-prefrontal-cortex transcranial magnetic stimulation as augmentation in treatment-resistant major depression. Int J Neuropsychopharmacol. 2006;9(6):655-666. PubMed doi:10.1017/S1461145706007176
64. Fitzgerald PB, Sritharan A, Daskalakis ZJ, et al. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27(5):488-492. PubMed doi:10.1097/jcp.0b013e318151521c
65. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234. PubMed doi:10.1002/da.20454
66. Isenberg K, Downs D, Pierce K, et al. Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann Clin Psychiatry. 2005;17(3):153-159. PubMed doi:10.1080/10401230591002110
67. Miniussi C, Bonato C, Bignotti S, et al. Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: an efficacious therapy for major drug-resistant depression? Clin Neurophysiol. 2005;116(5):1062-1071. PubMed doi:10.1016/j.clinph.2005.01.002
68. Padberg F, Zwanzger P, Thoma H, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88(3):163-171. PubMed doi:10.1016/S0165-1781(99)00092-X
69. Richieri R, Boyer L, Padovani R, et al. Equivalent brain SPECT perfusion changes underlying therapeutic efficiency in pharmacoresistant depression using either high-frequency left or low-frequency right prefrontal rTMS. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):364-370. PubMed doi:10.1016/j.pnpbp.2012.07.012
70. Rossini D, Lucca A, Magri L, et al. A symptom-specific analysis of the effect of high-frequency left or low-frequency right transcranial magnetic stimulation over the dorsolateral prefrontal cortex in major depression. Neuropsychobiology. 2010;62(2):91-97. PubMed doi:10.1159/000315439
71. Fitzgerald PB, Hoy KE, Singh A, et al. Equivalent beneficial effects of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in a large randomized trial in treatment-resistant major depression. Int J Neuropsychopharmacol. 2013;16(9):1975-1984. PubMed doi:10.1017/S1461145713000369
72. Pallanti S, Bernardi S, Di Rollo A, et al. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience. 2010;167(2):323-328. PubMed doi:10.1016/j.neuroscience.2010.01.063
73. Fitzgerald PB, Hoy K, McQueen S, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34(5):1255-1262. PubMed doi:10.1038/npp.2008.233
74. Galletly C, Gill S, Clarke P, et al. A randomized trial comparing repetitive transcranial magnetic stimulation given 3 days/week and 5 days/week for the treatment of major depression: is efficacy related to the duration of treatment or the number of treatments? Psychol Med. 2012;42(5):981-988. PubMed doi:10.1017/S0033291711001760
75. Turnier-Shea Y, Bruno R, Pridmore S. Daily and spaced treatment with transcranial magnetic stimulation in major depression: a pilot study. Aust N Z J Psychiatry. 2006;40(9):759-763. PubMed doi:10.1080/j.1440-1614.2006.01880.x
76. Price GW, Lee JWY, Garvey CA, et al. The use of background EEG activity to determine stimulus timing as a means of improving rTMS efficacy in the treatment of depression: a controlled comparison with standard techniques. Brain Stimulat. 2010;3(3):140-152. PubMed doi:10.1016/j.brs.2009.08.004
77. Conca A, Di Pauli J, Beraus W, et al. Combining high and low frequencies in rTMS antidepressive treatment: preliminary results. Hum Psychopharmacol. 2002;17(7):353-356. PubMed doi:10.1002/hup.422
78. Fitzgerald PB, Hoy K, McQueen S, et al. Priming stimulation enhances the effectiveness of low-frequency right prefrontal cortex transcranial magnetic stimulation in major depression. J Clin Psychopharmacol. 2008;28(1):52-58. PubMed doi:10.1097/jcp.0b013e3181603f7c
79. Levkovitz Y, Harel EV, Roth Y, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimulat. 2009;2(4):188-200. PubMed doi:10.1016/j.brs.2009.08.002
80. Rybak M, Bruno R, Turnier-Shea Y, et al. An attempt to increase the rate and magnitude of the antidepressant effect of transcranial magnetic stimulation (TMS): a pilot study. Ger J Psychiatry. 2005;8:59-65.
81. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53(4):324-331. PubMed doi:10.1016/S0006-3223(02)01499-3
82. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667. PubMed doi:10.1016/S0006-3223(01)01354-3
83. Pridmore S. Substitution of rapid transcranial magnetic stimulation treatments for electroconvulsive therapy treatments in a course of electroconvulsive therapy. Depress Anxiety. 2000;12(3):118-123. PubMed doi:10.1002/1520-6394(2000)12:3<118::AID-DA2>3.0.CO;2-G
84. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol. 2000;3(2):129-134. PubMed doi:10.1017/S1461145700001784
85. Keshtkar M, Ghanizadeh A, Firoozabadi A. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for the treatment of major depressive disorder, a randomized controlled clinical trial. J ECT. 2011;27(4):310-314. PubMed doi:10.1097/YCT.0b013e318221b31c
86. Rosa MA, Gattaz WF, Pascual-Leone A, et al. Comparison of repetitive transcranial magnetic stimulation and electroconvulsive therapy in unipolar non-psychotic refractory depression: a randomized, single-blind study. Int J Neuropsychopharmacol. 2006;9(6):667-676. PubMed doi:10.1017/S1461145706007127
87. Bloch Y, Grisaru N, Harel EV, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. 2008;24(2):156-159. PubMed doi:10.1097/YCT.0b013e318156aa49
88. Croarkin PE, Wall CA, Nakonezny PA, et al. Increased cortical excitability with prefrontal high-frequency repetitive transcranial magnetic stimulation in adolescents with treatment-resistant major depressive disorder. J Child Adolesc Psychopharmacol. 2012;22(1):56-64. PubMed doi:10.1089/cap.2011.0054
89. Mayer G, Aviram S, Walter G, et al. Long-term follow-up of adolescents with resistant depression treated with repetitive transcranial magnetic stimulation. J ECT. 2012;28(2):84-86. PubMed doi:10.1097/YCT.0b013e318238f01a
90. Mayer G, Faivel N, Aviram S, et al. Repetitive transcranial magnetic stimulation in depressed adolescents: experience, knowledge, and attitudes of recipients and their parents. J ECT. 2012;28(2):104-107. PubMed doi:10.1097/YCT.0b013e318250058c
Please sign in or purchase this PDF for $40.00.
Save
Cite