ABSTRACT
Objective: To compare the safety and efficacy of conventional anticoagulants with new oral anticoagulants (NOACs) for management of cerebral venous sinus thrombosis (CVST).
Methods: This was a retrospective, prospective cohort study of patients who presented with CVST to a tertiary stroke center in the Middle East from January 2012 to October 2019. Patients with a diagnosis of CVST were identified, and data were analyzed for demographic characteristics. Specific consideration was given to compare the efficacy and safety of different anticoagulation treatments.
Results: A total of 36 patients were included in the final analysis, with 15 (41%) men and 21 (59%) women and a male to female ratio of 1:1.4. Most of the patients (n = 22, 61%) were Saudi. Their ages ranged between 15 and 82 years (mean ± SD age of 34.22 ± 13.16 years). Headache was the most common feature, present in 22 (61%) of the patients, followed by unilateral weakness in 15 (41%) and cranial nerve palsies in 11 (30%). The most common etiology was prothrombotic state (both hereditary and acquired thrombophilia: n = 16, 45%). Other etiologies were postpartum state/oral contraceptive pill usage in 7 (19%), infections in 7 (19%), and trauma in 3 (8%). Most of the patients (n = 24, 67%) still received conventional anticoagulation (warfarin/low molecular weight heparin), but 9 (25%) of the patients consented to start NOACs. Efficacy (as measured by clinical improvement plus rate of recanalization of previously thrombosed venous sinuses) showed no statistically significant difference, although it proved to be better tolerated, as none of the patients stopped the treatment due to adverse events and risk of major bleeding was significantly low in the NOAC group. Nine patients in the warfarin group stopped medication, while none in the NOAC group did so (P = .034).
Conclusion: NOACs were found to be at least as good as conventional anticoagulation for the management of CVST. However, efficacy was almost similar, a finding that is consistent with most of the published case series and the few recently published prospective studies. Larger prospective and population-based studies are needed to clarify our preliminary results.
Prim Care Companion CNS Disord 2021;23(6):21m02927
To cite: Shahid R, Zafar A, Nazish S, et al. An observational study comparing the safety and efficacy of conventional anticoagulation versus new oral anticoagulants in the management of cerebral venous sinus thrombosis. Prim Care Companion CNS Disord. 2021;23(6):21m02927.
To share: https://doi.org/10.4088/PCC.21m02927
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, King Fahd University Hospital, Dammam, Saudi Arabia
bDepartment of Medicine and Neurology, University of Alberta, Edmonton, Alberta, Canada
cHamad General Hospital, Doha, Qatar
*Corresponding author: Rizwana Shahid, MBBS, FCPS, Department of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, King Fahd University Hospital, Dammam, 31952, Saudi Arabia ([email protected]).
The first case of cerebral venous sinus thrombosis (CVST) was recognized in 1825 by Ribes,1 who diagnosed cerebral venous thrombosis (CVT) in a 45-year-old man who died after a 6-month history of severe headache, epilepsy, and delirium. The initial cases were mainly confirmed at autopsy. However, advancement in radiologic techniques has improved the recognition of the condition, and this seems to be a major contributor toward a seemingly increasing incidence of the condition over the past few decades.2
Accurate and prompt diagnosis of CVT is crucial because timely and appropriate therapy can reverse the disease process and significantly reduce the risk of acute complications and long-term sequelae, as CVST is associated with a mortality rate of 8% if untreated.3
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are considered the best diagnostic methods for CVST.4,5 Anticoagulant therapy is considered the standard treatment for CVST.6–8 However, the decision to start anticoagulation can be challenging in some cases in which there is significant hemorrhagic components on neuroimaging at presentation that might be worsened with the standard use of anticoagulants.5 However, the general consensus among neurologists is to start anticoagulants as soon as possible and monitor closely with neuroimaging for any progression in the hemorrhagic transformation. The new oral anticoagulants (NOACs) are recently being used off label for the treatment of CVST, since these medications are much easier to monitor and might carry lower risk of intracerebral hemorrhage as compared to vitamin K antagonists. NOACs have been approved for secondary prevention of stroke from atrial fibrillation and for prevention and treatment of deep vein thrombosis; however, data are sparse to date regarding the use of NOACs in CVST. Based on the limited data on the use of anticoagulation in CVST, it appears safe and effective.5,9–13 Currently, a multicenter prospective trial is underway to assess the efficacy and safety of dabigatran in CVST.14
The objective of this study was to compare the safety and efficacy of conventional anticoagulants with NOACs for management of CVST in a tertiary stroke center in the Middle East.
METHODS
Data Extraction
This retrospective and prospective study comprised a cohort of patients who presented with the diagnosis of CVST to the stroke service of one of the main tertiary hospitals in the region (King Fahd University Hospital, Dammam, Saudi Arabia) between January 2012 and October 2019. For patients admitted prior to 2016, data were collected retrospectively through the electronic data bank system of the hospital. Data were collected prospectively for patients admitted from 2017 to 2019.
The following inclusion and exclusion criteria were used for enrollment of the patients.
Inclusion Criteria:
- Patients with confirmed diagnosis of CVST based on clinical and radiologic findings
- Patients ready to consent to try a new treatment modality, which was not US Food and Drug Administration approved for CVST but was already being used off label.
Exclusion Criteria:
- Patients aged < 18 years
- Patients with underlying comorbid conditions that necessitate the use of warfarin as an anticoagulant.
Ethics approval for this study was received from the Institutional Review Board of Imam Abdulrahman Bin Faisal University (#IRB-2019–01-218).
Diagnosis and Confirmation of CVST
The diagnosis of CVST was made based on both clinical presentation and neuroimaging findings. The main clinical symptoms that are suggestive of CVT are headache, new-onset seizures, focal neurologic deficits, cranial nerve palsies, blurred vision, and alteration of conscious level. The neuroimaging modalities used are computerized tomography venogram (CTV), MRI, and magnetic resonance venogram, as well as conventional 4-vessel cerebral angiogram in a few cases if indicated.
Sample Analysis
A structured proforma was designed comprising details regarding demographic characteristics such as age; sex; nationality; symptoms and signs at time of presentation such as headache, seizure, blurred vision, focal weakness or numbness, speech difficulty, cranial nerve palsies, and altered sensorium; blood investigations; and details of imaging modalities and radiologic findings (location of thrombus, number of involved venous sinuses, venous infarction, and hemorrhagic transformation). Patients were educated about the available treatment options and informed about the off-label use of NOACs. The treatment decision was left to the patient when he/she was able to make the decision; otherwise, a conventional anticoagulant was started. Demographic data about various treatments involving the anticoagulation either with low molecular weight heparin (LMWH)/warfarin or NOACs were recorded. Any adverse event or clinical worsening was recorded during admission and in follow-up clinical visits. The efficacy of anticoagulation or NOAC was defined as complete or partial resolution of the thrombus on repeat brain imaging at 6 months if available.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22 (IBM Corp, Armonk, New York). Means and standard deviations were computed for age and scaled data. Frequencies and percentages were calculated for categorical data. A P value < .005 was considered statistically significant. Comparisons were made between the use of conventional anticoagulation and NOACs in both clinical and neuroimaging aspects at baseline and follow-up.
RESULTS
A total of 36 patients with the diagnosis of CVST were included in the study in a retrospective/prospective manner. Fifteen (41%) were men, while 21 (59%) were women, with a male to female ratio of 1:1.4.
Most of the patients (n = 22, 61%) were Saudi. In 19% of the patients, nationality was not mentioned; while almost 19% belonged to other ethnic groups, most were from the Indo-Pak region. Their ages ranged between 18 and 82 years (mean ± SD = 34.22 ± 13.88 years). Almost two-thirds of the patients (22/36, 61%) had headache as the first symptom at presentation, followed by unilateral weakness in 15 (41%) and cranial nerve palsies in almost 30% (11 patients). Baseline demographics and characteristics are summarized in Table 1.
Neurologic examination showed no abnormal findings in 13 (36%) patients, while the most common abnormality on neurologic examination was cranial nerve palsies, which was detected in 14 (66.6%), followed by unilateral body weakness in 12 (57%) and visual field defects in 6 (28%). In 7 patients (19.4%), no risk factor was identified, while 12 patients (33%) had more than 1 predisposing factor for CVST. The most common contributing factor was thrombophilia, both hereditary and acquired, which was present in 16 (44.4%) of the patients. Protein S deficiency was most common among hereditary factors, while antiphospholipid syndrome accounted for most of the cases of acquired hypercoagulable state (7 patients, 22.2%).
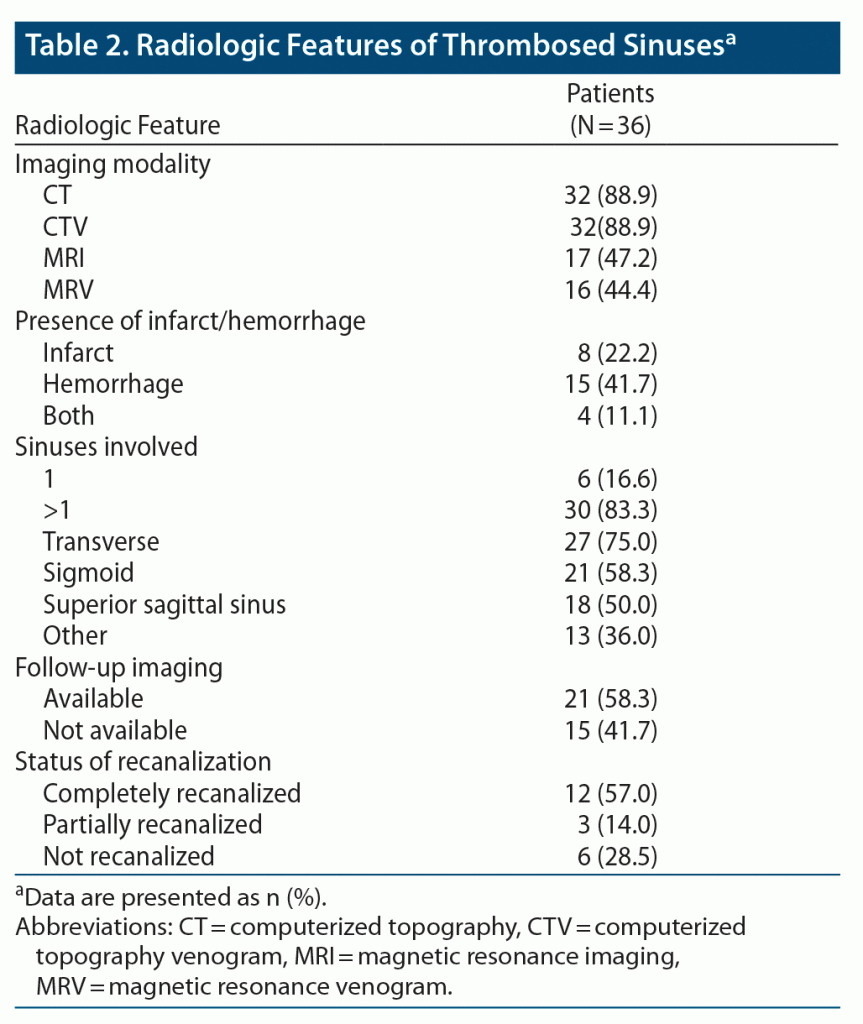
Most of our patients had head CT with CTV as the initial diagnostic modality (n = 32, 89%). Also, most of the patients had multiple sinuses involved, the most common being the transverse sinus in 27 (75%), followed by sigmoid and superior sagittal sinus. The radiologic characteristics of the patients are summarized in Table 2.
Of 36 patients, 15 (41.7%) were on warfarin. Twelve patients were started directly on warfarin after the initial heparinization, while 3 patients were switched to warfarin from LMWH. Ten patients were initially started on LMWH, but 3 were switched to warfarin due to patient noncompliance with using an injectable. The warfarin dose was adjusted to achieve an international normalized ratio (INR) of 2 to 3.
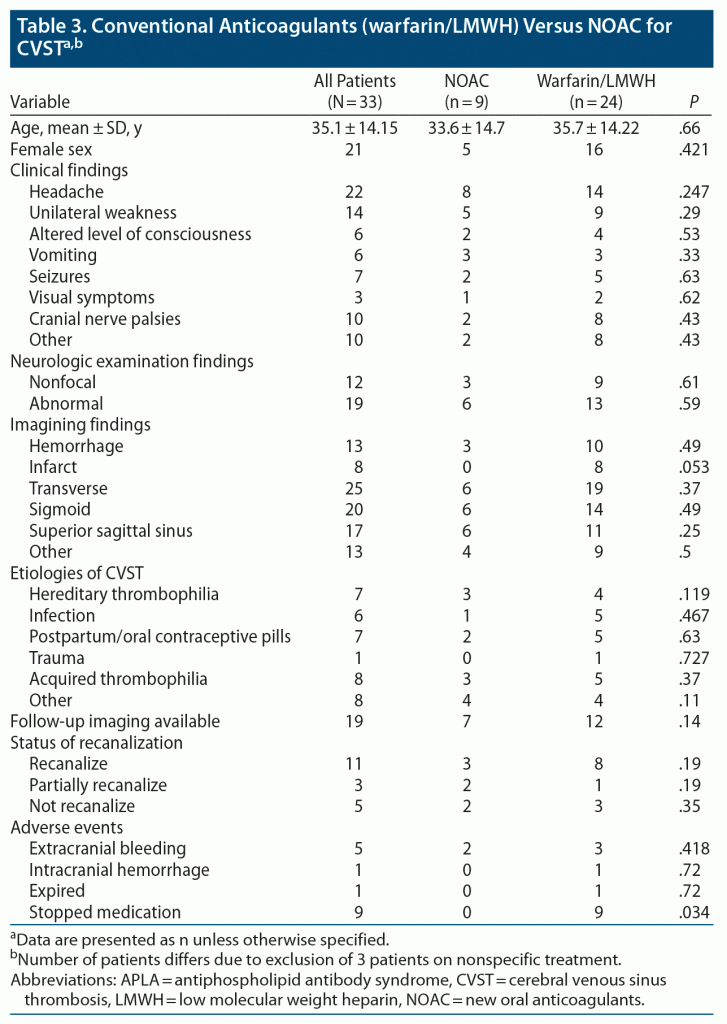
Nine (25%) patients were on NOACs and 3 received other treatments, which included antibiotics in 2 cases and steroids in 1. Of 9 patients on NOACs, 7 received dabigatran, 1 received rivaroxaban, and 1 received apixaban. Follow-up imaging was available for 21 (58%) patients. Twelve (57%) patients had complete recanalization: 8 in the warfarin group, 3 taking NOACs, and 1 on antibiotics plus aspirin. Partial recanalization was observed in 3 (14%) patients: 2 in the NOAC group and 1 in the warfarin group. In 6 (28.5%) patients, no recanalization was observed (2 in the NOAC group, 3 in the warfarin group, and 1 on nonspecific treatment). The baseline demographics did not differ significantly between the 2 groups. The only difference was more patients in the warfarin group had infarcts on baseline imaging. Of 36 patients, 33 were included in the comparison, as the 3 patients who received nonspecific treatment were excluded from the final analysis. Nineteen (57%) patients had follow-up imaging; in most of the cases, it was performed at 6-month intervals. As NOAC is a relatively new treatment modality for CVST, there were only a few studies10,11,15 available for comparison. Comparison demographic characteristics are provided in detail in Table 3.
In both groups, 11 patients had complete recanalization, while partial recanalization was observed in 3 patients. Regarding adverse events, 3 patients on conventional anticoagulants had extracranial hemorrhage, mainly gastrointestinal; 1 patient had significant menorrhagia, leading to uterine artery embolization; and 1 patient had cerebellar hemorrhage on warfarin. Premature discontinuation of warfarin was the most common adverse event, observed in 9 patients. The main reason for stopping warfarin and switching to an NOAC was difficulty in managing INR and need for repeated blood monitoring.
Three patients on LMWH initially switched to warfarin due to difficulty in managing an injectable and later stopped the medication, while none of the patients in the NOAC group stopped the treatment, with a P value of compliance of .034.
DISCUSSION
The findings of this study are consistent with a few published case series,11,12 wherein off-label use of NOAC for CVST proved to be as efficacious as conventional anticoagulants. So far, there are only a few prospective studies,14,15 wherein the comparison was made between the 2 treatment arms and NOAC was reported to be at least noninferior in comparison to conventional anticoagulants in terms of efficacy. In a prospective comparison study by Wasay at al,15 at 6-month follow-up (95/107 discharged patients, 89%), clinical neurologic worsening was reported in 1 patient (warfarin group), no patient required switching to the other group, and no patient required discontinuation of therapy by the physician. In conclusion, NOACs for the treatment of CVST were considered safe and may be as effective as warfarin in patients with CVT.
Heparin followed by warfarin is still the mainstay of treatment,6,16,17 although there are certain factors associated with warfarin such as risk of major bleeding, need for constant monitoring, and drug and dietary interactions, which are the reason for poor compliance and limiting factors in the majority of patients.18,19 Because of decreased compliance with warfarin due to the above-mentioned factors, there is an emerging need for alternate anticoagulant therapy in CVST. NOACs have already been approved for other thromboembolic conditions, including embolic stroke secondary to nonvalvular atrial fibrillation, and are being used off label as alternative treatment for CVST. Wasay et al15 compared NOAC versus warfarin in the treatment of CVST and found it to be at least as efficacious as conventional anticoagulants. Our study’s findings are consistent with that study,15 as we found better tolerance and almost similar efficacy among the 2 treatment arms.
For LMWH, its injectable nature is the limiting factor and a cause of reluctance in most patients. Thus, many neurovascular consultants in our hospital are now prescribing NOAC off label with informed consent to patients given the results of the RE-SPECT CVT study presented at the World Stroke Congress in 2018.19 The results of the RE-SPECT CVT study provide insight into the role of anticoagulation in patients with CVT of mild-moderate severity. In that study,19 no recurrent venous thromboembolism events were observed in either treatment group. The rate of bleeding was low, with 2 patients in the warfarin arm (3.3%) and 1 (1.7%) in the dabigatran arm developing a major bleed; no mortality in either treatment arm was noted. The trial showed that the risk of recurrent venous thromboembolism in CVT patients of mild to moderate severity under anticoagulant therapy with dabigatran for 6 months was low and associated with few major or clinically relevant bleeding events.19
Hematologic disorders and systemic infections have been found consistently to be the most common etiologic factors in most CVST studies.15,20 Our study also found these disorders and infections to be the most common etiologies for CVT in both the NOAC and warfarin groups. CVT is typically considered multifactorial, and it has been recommended to look for other etiologic factors as well, even in the presence of 1 risk factor, especially for congenital thrombophilia. In our study, 33% of the patients were found to have more than 1 etiologic factor, which is consistent with most of the international studies including the International Study on Cerebral Vein and Dural Sinus Thrombosis20 in which 44% had more than 1 etiology.
Specific treatment for CVST is anticoagulation, even in the presence of hemorrhage. Almost a decade ago, the only possible treatment options were the vitamin K–dependent antagonist (warfarin) versus LMWH. Both treatment options have certain limitations, as discussed previously, so with the emergence of new oral anticoagulants and their proven efficacy in certain thromboembolic conditions, it is postulated that these may be a better alternative to conventional anticoagulants. Hence, they are being increasingly used off label around the world for the treatment of CVST because of their better tolerance and decreased risk of intracranial hemorrhage. In our study, 25% of patients were treated with NOACs.
Recanalization has been defined differently across various studies; therefore, it is difficult to provide uniform results. The rate of recanalization (complete or partial) between different studies has been reported in the range of 73%–93%.21–23 In our study, the overall rate of recanalization was 15/21 (71%), defined as complete and partial. In Wasay et al,15 only 12 patients had follow-up imaging results available; of these, 8 (66%) patients had recanalization.
Adverse events were reported more frequently in the warfarin/LMWH group as was poor compliance leading to stopping the medication. None of the patients in the NOAC group stopped the medication or needed to be switched to a different AC, while in the warfarin group, 9 patients stopped the medication due to either side effects or difficulty in managing the target INR. One patient died in the warfarin group, but this outcome seems secondary to his extensive disease rather than a complication of the anticoagulant therapy.
Although NOACs did not appear to be superior to the conventional anticoagulants in the management of CVST in our study, we assume this to be secondary to unavailability of follow-up data in most of the patients and a very small sample size among the groups; but the results did prove the noninferiority of NOACs in comparison to conventional anticoagulants. Our study findings validate the results of the RE-SPECT CVT study,19 as well data by Wasay et al15 in a real-world setting and support the growing trend of NOAC use for this potentially devastating condition. Further large-scale, prospective studies are recommended to clarify our results.
There are certain limitations of our study including retrospective data collection, inadequate radiologic data at follow-up to assess the status of recanalization, and nonblinded and nonadjudicated outcome assessments. The small sample size is another limitation that precludes us from making firm conclusions.
CONCLUSION
NOAC proved to be a good alternative in case of any contraindication or unavailability of conventional anticoagulation for the management of CVST in our study sample. However, efficacy was almost similar, which is consistent with most of the published case series and few recently published prospective studies. Further studies are needed to evaluate if they are superior in tolerance. Larger prospective and population-based studies are needed to clarify our preliminary results.
Submitted: January 17, 2021; accepted April 22, 2021.
Published online: November 24, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: This study made use of the computational resources and technical services of the Scientific and High Performance Computing Center at Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
Clinical Points
- Cerebral venous sinus thrombosis (CVST) is a potentially serious disease with acute complications if not diagnosed and treated promptly.
- Anticoagulants are the first line of management, even in the presence of hemorrhagic lesions on brain imaging.
- New oral anticoagulants have proved to be at least noninferior compared to conventional anticoagulants in the management of CVST in many studies.
- New oral anticoagulants can be an alternate option for patients with contraindication/ reluctance to take warfarin/low molecular weight heparin to avoid repeated blood testing and injectables.
References (23)

- Ribes MF. Des recherches faites sur la phlebite. In: Revue Medical Francais et Etrangere er Journal de clinique del′ Hotel Dieu et de la Charite de Paris. 1825;3:5–41.
- Luo Y, Tian X, Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Front Aging Neurosci. 2018;10:2. PubMed CrossRef
- Leach JL, Strub WM, Gaskill-Shipley MF. Cerebral venous thrombus signal intensity and susceptibility effects on gradient recalled-echo MR imaging. AJNR Am J Neuroradiol. 2007;28(5):940–945. PubMed
- Röttger C, Trittmacher S, Gerriets T, et al. Reversible MR imaging abnormalities following cerebral venous thrombosis. AJNR Am J Neuroradiol. 2005;26(3):607–613. PubMed
- Manzione J, Newman GC, Shapiro A, et al. Diffusion- and perfusion-weighted MR imaging of dural sinus thrombosis. AJNR Am J Neuroradiol. 2000;21(1):68–73. PubMed
- Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192. PubMed CrossRef
- Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597–600. PubMed CrossRef
- Stam J, Lensing AWA, Vermeulen M, et al. Heparin treatment for cerebral venous and sinus thrombosis. Lancet. 1991;338(8775):1154. PubMed CrossRef
- Ferro JM, Bousser MG, Canhão P, et al; European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–1213. PubMed CrossRef
- Hon SF, Li HL, Cheng PW. Use of direct thrombin inhibitor for treatment of cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2012;21(8):915.e11–915.e15. PubMed CrossRef
- Geisbüsch C, Richter D, Herweh C, et al. Novel factor xa inhibitor for the treatment of cerebral venous and sinus thrombosis: first experience in 7 patients. Stroke. 2014;45(8):2469–2471. PubMed CrossRef
- Mendonça MD, Barbosa R, Cruz-e-Silva V, et al. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: a series of 15 patients. Int J Stroke. 2015;10(7):1115–1118. PubMed CrossRef
- Rao SK, Ibrahim M, Hanni CM, et al. Apixaban for the treatment of cerebral venous thrombosis: a case series. J Neurol Sci. 2017;381:318–320. PubMed CrossRef
- Ferro JM, Coutinho JM, Dentali F, et al; RE-SPECT CVT Study Group. Safety and efficacy of dabigatran etexilate vs dose-adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019;76(12):1457–1465. PubMed CrossRef
- Wasay M, Khan M, Rajput HM, et al. New oral anticoagulants versus warfarin for cerebral venous thrombosis: a multi-center, observational study. J Stroke. 2019;21(2):220–223. PubMed CrossRef
- Nagaraja D, Rao BSS, Taly AB, et al. Randomized controlled trial of heparin in puerperal cerebral venous/sinus thrombosis. NIMHANS J. 1995;13(2):111–115.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139(11):893–900. PubMed CrossRef
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120(8):700–705. PubMed CrossRef
- Ferro JM, Dentali F, Coutinho JM, et al. Rationale, design, and protocol of a randomized controlled trial of the safety and efficacy of dabigatran etexilate versus dose-adjusted warfarin in patients with cerebral venous thrombosis. Int J Stroke. 2018;13(7):766–770. PubMed CrossRef
- Ferro JM, Canhão P, Stam J, et al; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. PubMed CrossRef
- Shahid R, Zafar A, Nazish S, et al. Etiologic and clinical features of cerebral venous sinus thrombosis in Saudi Arabia. J Neurosci Rural Pract. 2019;10(2):278–282. PubMed CrossRef
- Dentali F, Gianni M, Crowther MA, et al. Natural history of cerebral vein thrombosis: a systematic review. Blood. 2006;108(4):1129–1134. PubMed CrossRef
- Arauz A, Vargas-González JC, Arguelles-Morales N, et al. Time to recanalisation in patients with cerebral venous thrombosis under anticoagulation therapy. J Neurol Neurosurg Psychiatry. 2016;87(3):247–251. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite