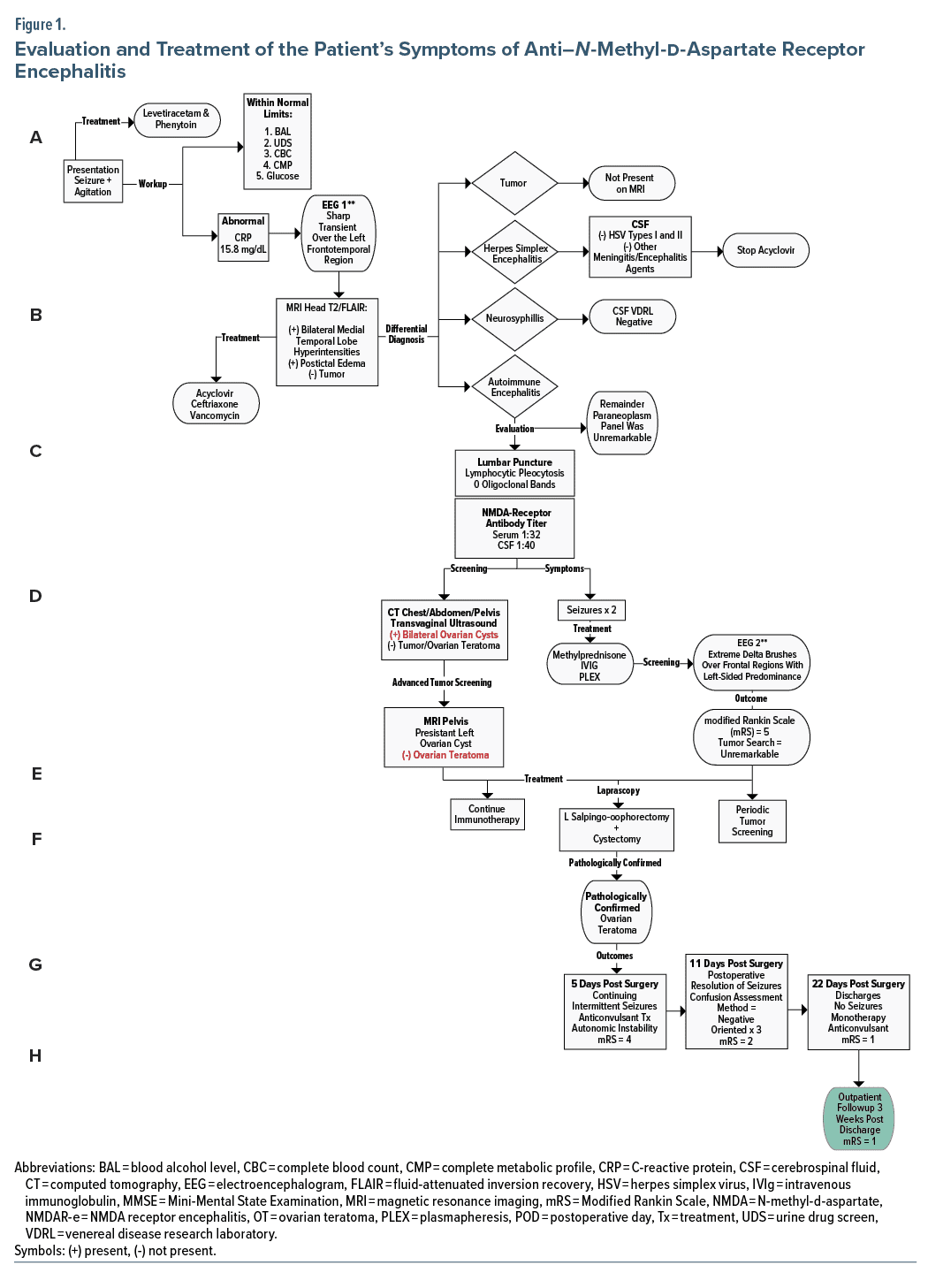
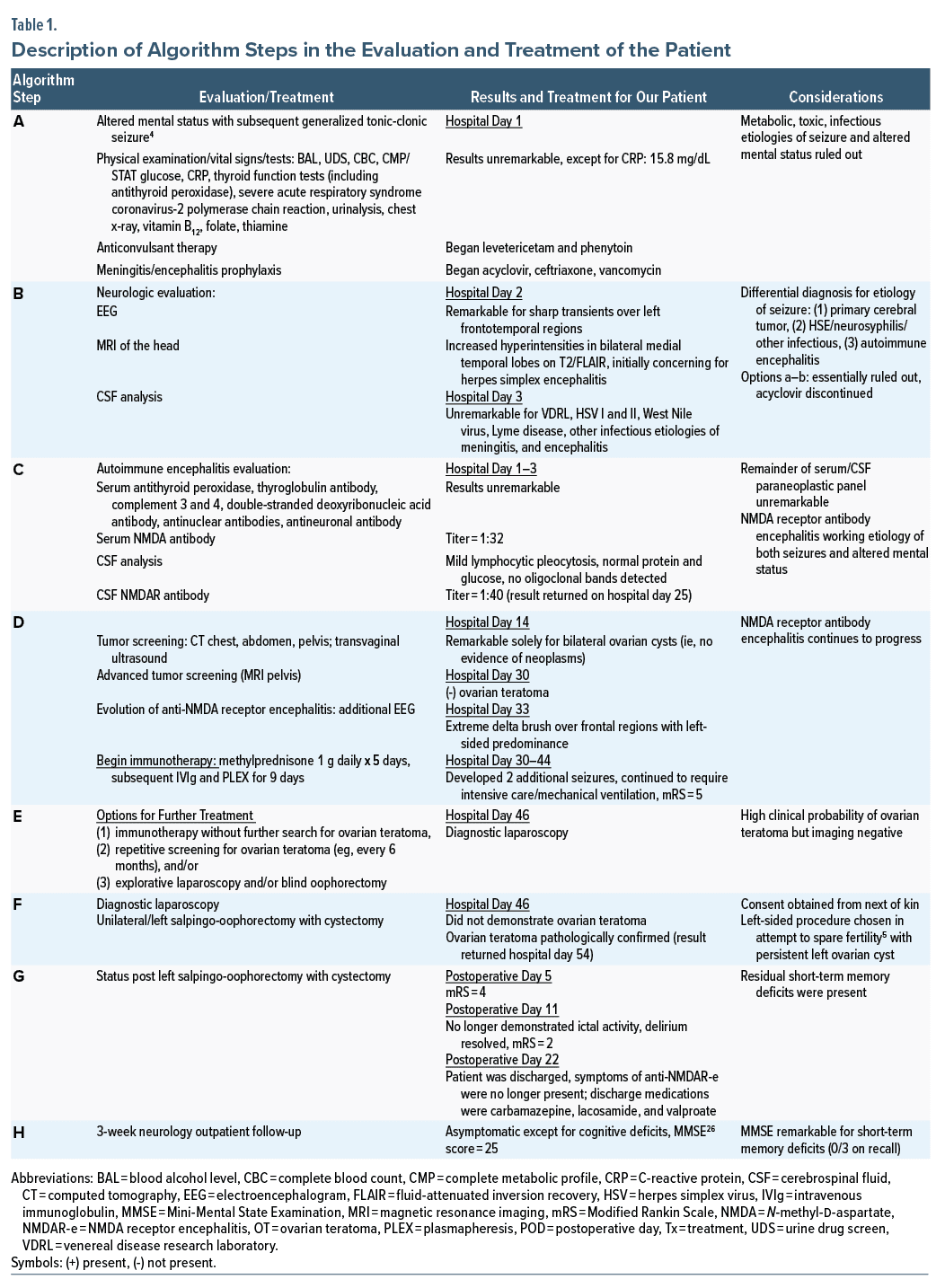
The most common and first described autoimmune encephalitis syndrome is anti–N-methyl-d-aspartate receptor (NMDAR) encephalitis.1 An underlying tumor is found in 25%–40% of patients and corresponds to ovarian teratoma (OT) in 90% of these tumors2; however, negative imaging results can occur.3 Thus, we present a patient with this scenario and the dilemmas involved in treatment.
Case Report
The patient was a 27-year-old Black woman, with no prior medical, psychiatric, or substance misuse history, who presented to the emergency department (ED) following an unwitnessed seizure at home and a recurrence in the ED. Although the patient was unable to provide a history due to altered mental status, her mother reported that she was in her usual state of health until 3 days prior to admission when she complained of fatigue, dizziness, and a worsening headache over the ensuing days prior to presentation. Due to confusion and agitation, our team was consulted.
On examination, the patient was oriented × 1, endorsed no hallucinations or delusions, and scored positive for delirium on the Confusion Assessment Method.7 We began olanzapine 5 mg 3 times daily with no improvement in agitation; however, she subsequently manifested orofacial dyskinesias without ictal activity on electroencephalogram. Shortly thereafter, the patient was intubated and transferred to the intensive care unit wherein dexmedetomidine and propofol were initiated (Figure 14–6 and Table 1).
On hospital day 71, the patient was discharged with the following medications: carbamazepine 200 mg twice daily, lacosamide 300 mg twice daily, and valproate 875 mg twice daily. Three weeks later, the patient had an outpatient neurology follow-up. At that time, she reported continued apraxia and short-term memory impairment though without further seizures on carbamazepine monotherapy. After this visit, the patient was lost to follow-up.
Discussion
Anti-NMDAR encephalitis is characterized by a constellation of neurologic and psychiatric features along with positive NMDAR antibody. Given that about 80% of patients with anti-NMDAR encephalitis are women, and up to 60% have a concomitant tumor, typically OT, tumor screening is imperative.8 As there is no serum tumor marker for OT, recommended screening includes ultrasound and pelvic computed tomography and magnetic resonance imaging.9 The best management of women with anti-NMDAR encephalitis and high clinical probability of OT but negative imaging studies is unclear. The options include immunotherapy without further search for OT, repetitive screening for OT (eg, every 6 months), explorative laparoscopy, and blind oophorectomy.9 Notably, while exploratory laparoscopies and blind oophorectomies demonstrate OT in some patients, no tumor may be detected in others.1
The detection of an underlying tumor is dependent of age (≥ 18 years), sex (female), and ethnic background (Black women).1 Known independent predictors of poor outcome include the higher severity of anti-NMDAR encephalitis and delayed initiation of both immunotherapy and tumor removal.10 Furthermore, patients whose tumors are resected have a better outcome (modified Rankin Scale score ≤ 2)11 and decreased risk of relapse compared with those patients whose tumor was not removed.1,8 Based on the above, our patient’s demographics and risk factors were predictive of a poor outcome without further treatment. Thus, the gynecology service decided to proceed with a left salpingo-oopherectomy (see Figure 1/Table 1 algorithm steps E and F).
Conclusion
Cases of “microteratomas” not previously identified by any imaging modalities, as in our patient, have been reported.9,12,13 Fortunately, unilateral salpingo-oopherectomy identified an OT in our patient; however, in cases in which imaging fails to identify a teratoma, some investigators advocate for bilateral oophorectomy, especially if patients do not respond to immunotherapy.1,14 While the latter approach may be the best opportunity for survival, we advocate for further research in potential fertility-sparing options in these cases.
Article Information
Published Online: January 11, 2024. https://doi.org/10.4088/PCC.23cr03510
© 2024 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2024;26(1):23cr03510
Submitted: February 13, 2023; accepted June 15, 2023.
To Cite: Spiegel DR, Mclean A, Ralston M. Seizures in a young woman due to N-methyl-d-aspartate receptor antibody encephalitis with unremarkable imaging evaluations: a proposed treatment algorithm. Prim Care Companion CNS Disord. 2024;26(1):23cr03510.
Author Affiliations: Department of Psychiatry and Behavioral Sciences, Eastern Virginia Medical School, Norfolk, Virginia (all authors).
Corresponding Author: David R. Spiegel, MD, Eastern Virginia Medical School, Department of Psychiatry and Behavior Sciences, 825 Fairfax Avenue, Norfolk, Virginia 23507 ([email protected]).
Relevant Financial Relationships: Dr Spiegel is in the speaker’s bureau for Allergen, Alkermes, Otsuka, and IntraCellular but has no conflict of interest in preparation of this manuscript. The remainder of the authors have no disclaimer/conflict of interest to report.
Funding/Support: None.
Patient Consent: Consent was verbally received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (14)

- Linnoila JJ, Rosenfeld MR, Dalmau J. Neuronal surface antibody-mediated autoimmune encephalitis. Semin Neurol. 2014;34(4):458–466. PubMed CrossRef
- Nougaret S, Cunha TM. From ovary to brain: Specific imaging features of ovarian teratomas associated with anti-N–methyl-d-aspartate receptor encephalitis. Diagn Interv Imaging. 2021;102(7–8):403–404. PubMed CrossRef
- Vargas RJ, Farid H, Goldenson RP, et al. Ovarian teratomas and anti–N-methyl-d-aspartate receptor encephalitis: why sonography first? J Ultrasound Med. 2016;35(4):852–854. PubMed CrossRef
- Steriade C, Britton J, Dale RC, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: Conceptual definitions. Epilepsia. 2020;61(7):1341–1351. PubMed CrossRef
- Flannery P, Yang I, Keyvani M, et al. Acute psychosis due to anti- N–methyl-d-aspartate receptor receptor encephalitis following COVID-19 vaccination: a case report. Front Neurol. 2021;12:764197. PubMed CrossRef
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the Confusion Assessment Method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. PubMed CrossRef
- Dai Y, Zhang J, Ren H, et al. Surgical outcomes in patients with anti–N-methyl-d-aspartate receptor encephalitis with ovarian teratoma. Am J Obstet Gynecol. 2019;221(5):485.e1–485.e10. PubMed CrossRef
- Cirkel C, Cirkel A, Royl G, et al. On the quest for hidden ovarian teratomas in therapy-refractory anti-NMDA receptor encephalitis: a case report. Neurol Res Pract. 2022;4(1):15. PubMed CrossRef
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. PubMed CrossRef
- Rankin J. Cerebral vascular accidents in patients over the age of 60, II: prognosis. Scott Med J. 1957;2(5):200–215. PubMed CrossRef
- Maramattom BV, Jacob A. N–methyl-d-aspartate receptor encephalitis: a new addition to the spectrum of autoimmune encephalitis. Ann Indian Acad Neurol. 2011;14(3):153–157. PubMed CrossRef
- Cleverly K, Gambadauro P, Navaratnarajah R. Paraneoplastic anti- N–methyl-d-aspartate receptor encephalitis: have you checked the ovaries? Acta Obstet Gynecol Scand. 2014;93(7):712–715. PubMed CrossRef
- Bartolini L. Practice current: how do you treat anti-NMDA receptor encephalitis? Neurol Clin Pract. 2016;6(1):69–72. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite