Objective: Use of second-generation antipsychotics (SGAs) for treatment of depression has increased, and patients with depression and comorbid diabetes or cardiovascular disease are more likely to use SGAs than those without these conditions. We compared SGA and non-SGA depression pharmacotherapies on the risk of diabetes hospitalization or treatment intensification in adults with depression and preexisting diabetes.
Methods: This was a retrospective cohort study of US commercially insured adults (2009-2015 Truven MarketScan Commercial Claims and Encounters Database) aged 18-64 years old with type 2 diabetes mellitus and unipolar depression previously treated with a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor. New users of SGAs versus non-SGAs, as well as specific treatments (aripiprazole, quetiapine, bupropion, mirtazapine, and tricyclic antidepressants [TCAs]) were matched on class/medication-specific high-dimensional propensity score. Cox proportional hazard models were used to compare the risk of diabetes-related hospitalization or treatment intensification.
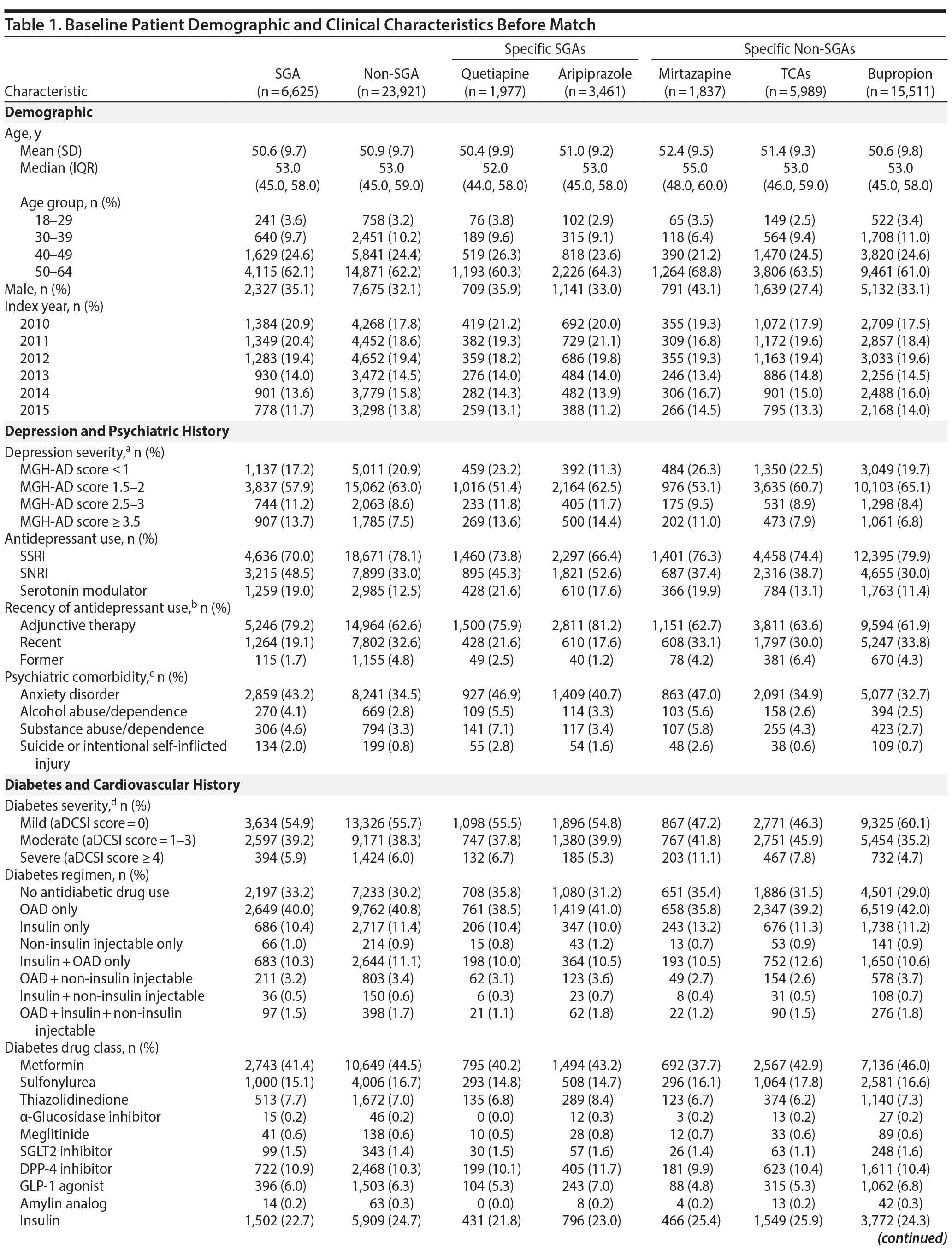
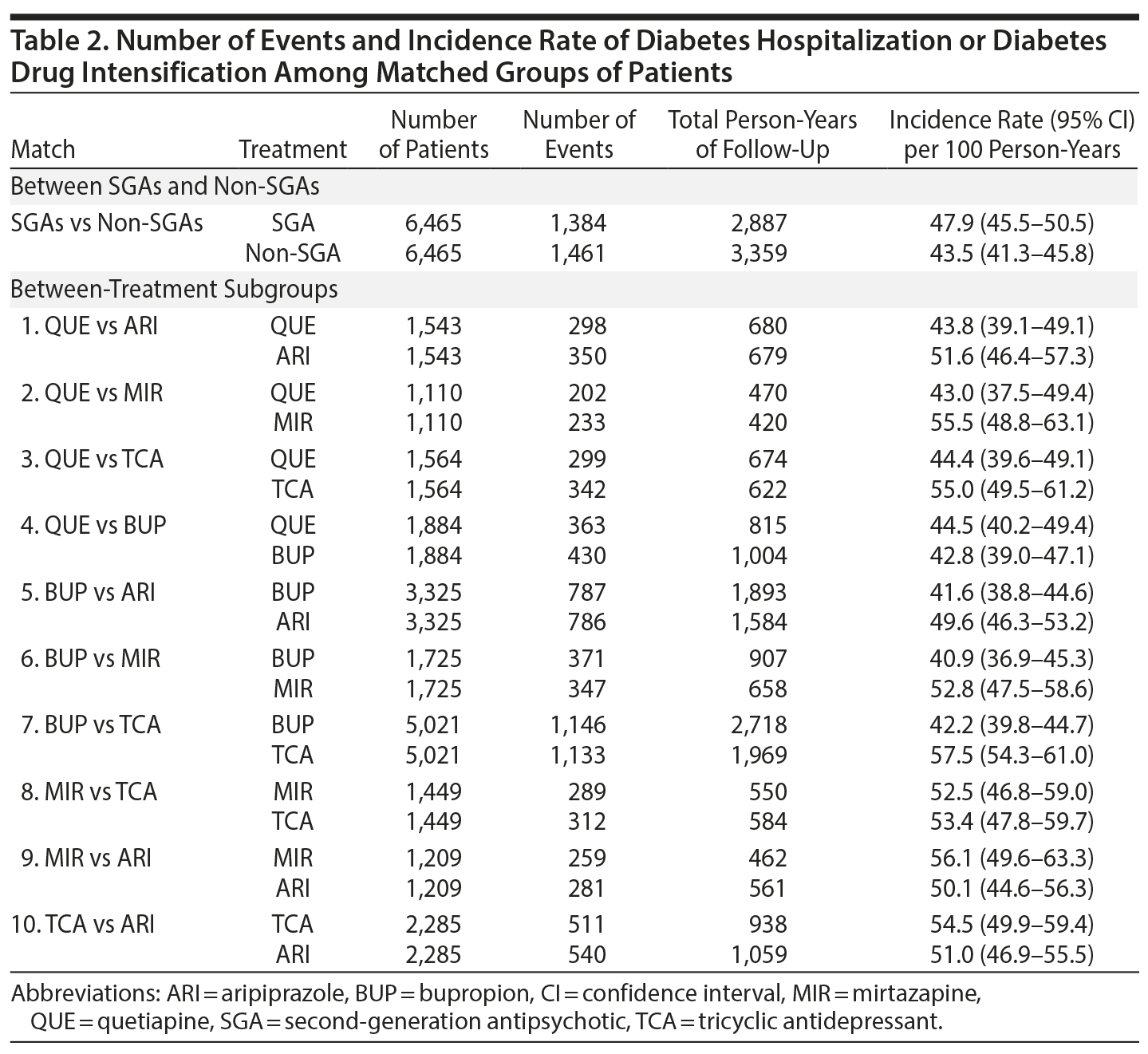
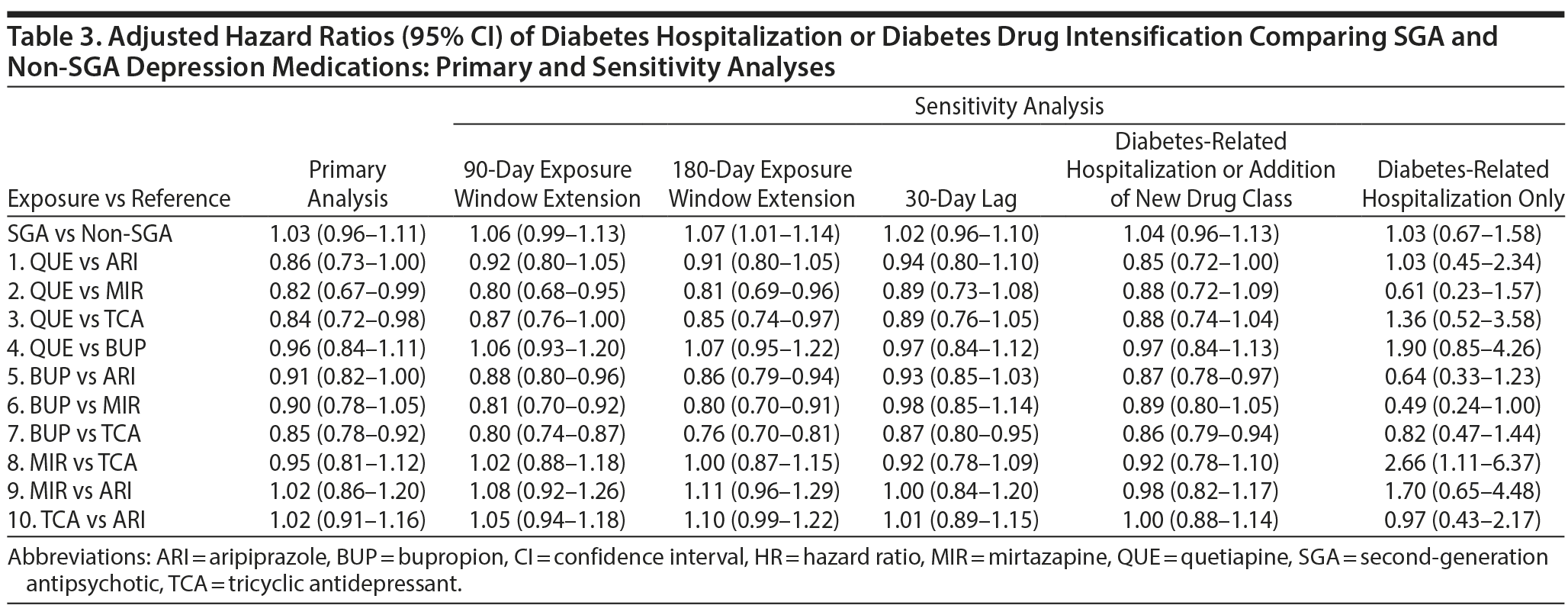
Results: We identified 6,625 SGA (aripiprazole = 3,461; quetiapine = 1,977; other = 1,187) and 23,921 non-SGA patients for inclusion (bupropion = 15,511; mirtazapine = 1,837; TCAs = 5,989; other = 584) with a mean age of 51 years. In the matched cohort, the rate of diabetes-related hospitalization or drug intensification was 47.9 per 100 person-years in the SGA group and 43.5 per 100 person-years in the non-SGA group (adjusted hazard ratio [aHR] = 1.03; 95% CI, 0.96-1.11). When comparing treatment subgroups, the risk of events was lower for bupropion versus TCAs (aHR = 0.85; 95% CI, 0.76-0.98), quetiapine versus mirtazapine (aHR = 0.82; 95% CI, 0.67-0.99), and quetiapine versus TCAs (aHR = 0.84; 95% CI, 0.72-0.98). For other comparisons, differences were small and not statistically significant.
Conclusions: While drug-specific effects on risk of diabetes hospitalization or treatment intensification most likely guide clinical decision making, we observed only modest differences in risk. The overall impact of SGAs on diabetes control depends not only on direct effects on glucose metabolism but also on effectiveness of depression symptom relief. Future studies evaluating other diabetes outcomes (glycosylated hemoglobin, diabetes complications) are needed.
Objective: Use of second-generation antipsychotics (SGAs) for treatment of depression has increased, and patients with depression and comorbid diabetes or cardiovascular disease are more likely to use SGAs than those without these conditions. We compared SGA and non-SGA depression pharmacotherapies on the risk of diabetes hospitalization or treatment intensification in adults with depression and preexisting diabetes.
Methods: This was a retrospective cohort study of US commercially insured adults (2009-2015 Truven MarketScan Commercial Claims and Encounters Database) aged 18-64 years old with type 2 diabetes mellitus and unipolar depression previously treated with a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor. New users of SGAs versus non-SGAs, as well as specific treatments (aripiprazole, quetiapine, bupropion, mirtazapine, and tricyclic antidepressants [TCAs]) were matched on class/medication-specific high-dimensional propensity score. Cox proportional hazard models were used to compare the risk of diabetes-related hospitalization or treatment intensification.
Results: We identified 6,625 SGA (aripiprazole = 3,461; quetiapine = 1,977; other = 1,187) and 23,921 non-SGA patients for inclusion (bupropion = 15,511; mirtazapine = 1,837; TCAs = 5,989; other = 584) with a mean age of 51 years. In the matched cohort, the rate of diabetes-related hospitalization or drug intensification was 47.9 per 100 person-years in the SGA group and 43.5 per 100 person-years in the non-SGA group (adjusted hazard ratio [aHR] = 1.03; 95% CI, 0.96-1.11). When comparing treatment subgroups, the risk of events was lower for bupropion versus TCAs (aHR = 0.85; 95% CI, 0.76-0.98), quetiapine versus mirtazapine (aHR = 0.82; 95% CI, 0.67-0.99), and quetiapine versus TCAs (aHR = 0.84; 95% CI, 0.72-0.98). For other comparisons, differences were small and not statistically significant.
Conclusions: While drug-specific effects on risk of diabetes hospitalization or treatment intensification most likely guide clinical decision making, we observed only modest differences in risk. The overall impact of SGAs on diabetes control depends not only on direct effects on glucose metabolism but also on effectiveness of depression symptom relief. Future studies evaluating other diabetes outcomes (glycosylated hemoglobin, diabetes complications) are needed.
Prim Care Companion CNS Disord 2018;20(3):17m02220
To cite: Xing S, Kim S, Schumock GT, et al. Risk of diabetes hospitalization or diabetes drug intensification in patients with depression and diabetes using second-generation antipsychotics compared to other depression therapies. Prim Care Companion CNS Disord. 2018;20(3):17m02220.
To share: https://doi.org/10.4088/PCC.17m02220
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Pharmacy Systems, Outcomes and Policy, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois
bDepartment of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois
cDepartment of Psychiatry, College of Medicine, University of Illinois at Chicago, Chicago, Illinois
dDepartment of Bioengineering, College of Engineering and College of Medicine, University of Illinois at Chicago, Chicago, Illinois
*Corresponding author: Todd A. Lee, PharmD, PhD, Department of Pharmacy Systems, Outcomes and Policy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St, Room 287, MC 871, Chicago, IL 60612 ([email protected]).
Use of second-generation antipsychotics (SGAs) for the treatment of depression has increased in the United States.1 In 2009-2010, 12.5% of adult outpatient nonpsychotic depression visits included a prescription for an SGA.1 Despite the metabolic risks previously documented with SGA use,2,3 patients with comorbid diabetes, hyperlipidemia, or cardiovascular disease were 2 times more likely to be prescribed these medications than those without these conditions.1 Use of SGAs in patients with comorbid diabetes may put them at higher risk for hyperglycemic events.4
Multiple studies5-10 have documented the risk of new-onset diabetes associated with SGA use. However, the consequence of SGA use on diabetes control in those with preexisting diabetes is not well understood. A limited number of studies in the preexisting diabetes population have reported associations with poor diabetes outcomes4,11-13 and improved diabetes outcomes14,15 in SGA-treated individuals. These studies,4,11-15 which were conducted outside the United States, primarily included patients with schizophrenia, bipolar disorder, or dementia. Two11,12 were restricted to patients over age 64 years, and only 1 study4 focused on SGAs exclusively (the other 5 studies compared first-generation antipsychotic [FGA] and SGA users with nonusers). Studies are needed to examine the US adult population with depression in which FGAs are not a preferred treatment option,16 lower doses of SGAs are typically used,16,17 and SGAs are compared to therapeutic alternatives. The objective of this study was to evaluate the comparative effects of SGAs and alternative depression pharmacotherapies on time to diabetes-related hospitalization or diabetes treatment intensification in US adults aged 18-64 years with unipolar depression and type 2 diabetes mellitus.
METHODS
Data Source
This retrospective cohort study used the 2009-2015 Truven MarketScan Commercial Claims and Encounters Database licensed by and maintained at the University of Illinois at Chicago.18 This administrative claims database reflects real-world treatment patterns for several million individuals who are covered by employer-sponsored private health plans across the United States.18 The de-identified database contains information on patient demographics, medical diagnosis and procedures, and prescription drugs dispensed from outpatient pharmacies. The University of Illinois at Chicago Institutional Review Board determined that this study does meet criteria for human subject research.
Study Population
Adults aged 18-64 years who received a new prescription for an oral nonclozapine SGA (aripiprazole, asenapine, brexpiprazole, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone) or non-SGA depression medication (bupropion, mirtazapine, lithium, tricyclic antidepressants [TCAs], or thyroid hormone) between January 1, 2010, and December 30, 2015, were identified. To be included, patients must have been diagnosed with depression (any inpatient or outpatient International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 296.2x, 296.3x, 300.4, or 311 diagnoses)19 and have received previous treatment with a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor in the 365 days (ie, preindex period) before the date of first SGA or non-SGA prescription (ie, index date). Patients must also have been diagnosed with type 2 diabetes (at least 1 inpatient or 2 outpatient ICD-9-CM 250.x0 or 250.x2 diagnoses or at least 2 prescription fills for a diabetes drug identified by the Healthcare Effectiveness Data and Information Set20) during the preindex period. We required patients to have been continuously insured in the preindex period and for at least 30 days after the index date.
Patients with any previous use of a study medication and those with any prescription fill for clozapine, injectable SGA, or FGA during the preindex period were excluded. Those with any diagnosis for schizophrenia (ICD-9-CM 295.xx), bipolar disorder (ICD-9-CM 296.0x, 296.1x, 296.4x-296.8x),21 dementia (290.xx), delusional disorder (ICD-9-CM 297.xx), nonorganic psychosis (ICD-9-CM 298.xx), autism (ICD-9-CM 299.0), mental retardation (ICD-9-CM 317.0-319.0), cerebral degeneration (ICD-9-CM 331.xx), Parkinson’s disease (ICD-9-CM 332.xx), senility without mention of psychosis (ICD-9-CM 797.x), or thyroid disorder (ICD-9-CM 240.0-246.0)22 during the preindex period were also excluded. Since inpatient medications were unavailable, patients with any hospitalizations in the 30 days prior to the index date were excluded. All ICD-10-CM diagnosis and procedure codes in 2015 (2.7% of all inpatient codes and 3.0% of all outpatient codes) were first mapped to ICD-9-CM codes using the Centers for Medicare and Medicaid Service’s General Equivalence Mappings.23
Exposure Groups
Patients were classified as SGA or non-SGA users on the basis of their first prescription fill. We also conducted 10 pairwise contrasts directly comparing (1) quetiapine versus aripiprazole, (2) quetiapine versus mirtazapine, (3) quetiapine versus TCAs, (4) quetiapine versus bupropion, (5) bupropion versus aripiprazole, (6) bupropion versus mirtazapine, (7) bupropion versus TCAs, (8) mirtazapine versus TCAs, (9) mirtazapine versus aripiprazole, and (10) TCAs versus aripiprazole. These treatment subgroups were selected a priori on the basis of power calculations (80% power to detect a 20% difference).

- Use of second-generation antipsychotics (SGAs) was not associated with an increased risk of diabetes-related hospitalization or diabetes drug intensification compared to non-SGAs in patients with diabetes and depression comorbidities.
- Bupropion was associated with a 15% reduced risk of events compared to tricyclic antidepressants (TCAs).
- Quetiapine was associated with an 18% reduced risk of events compared to mirtazapine, and quetiapine was associated with a 16% reduced risk of events compared to TCAs.
Outcome
The primary outcome was time to first diabetes-related hospitalization (primary hospital discharge for diabetes [ICD–9-CM 250.xx]) or diabetes treatment intensification. Treatment intensification was defined as either an increase in the dose of a noninsulin diabetes medication or the addition of a new diabetes drug class. A baseline diabetes drug regimen was established for every patient on the basis of the diabetes medications patients had in possession during the preindex period. The mean daily dose (drug strength ×— quantity dispensed/day’s supply) was calculated for each noninsulin diabetes drug. Patients were considered to have a dose increase when the mean daily dose during follow-up exceeded the baseline mean daily dose.
Follow-Up
We followed patients from the day after index drug initiation until the first occurrence of the following: (1) outcome, (2) end of continuous insurance coverage, (3) discontinuation of index drug (greater than 60-day gap in therapy), (4) addition or switch to another index drug, or (5) end of study period (December 31, 2015).
Patient and Treatment Characteristics
Baseline clinical and demographic characteristics were assessed during the preindex period, including depression and diabetes history, cardiovascular and psychiatric comorbidities, health care utilization, and provider care team. We used the Massachusetts General Hospital-Antidepressant (MGH-AD)24 score as a proxy for depression severity and the adapted Diabetes Complications Severity Index (aDCSI)25 score as a proxy for diabetes severity. The MGH-AD score has been used in other depression studies22,26,27 wherein higher scores indicate more severe depression. The score is calculated by assigning 1 point for each adequate trial (≥ 2 prescription fills for the same antidepressant) and half a point for extended duration of use (≥ 3 fills of the same antidepressant) or dose titration (prescription fill for the same antidepressant at a higher dose).24 The aDCSI converts diagnostic codes into a single summary score on the basis of 7 categories of diabetes complications: retinopathy; nephropathy; neuropathy; cerebrovascular, cardiovascular, or peripheral vascular disease; and metabolic.25,28 The aDCSI predicts diabetes hospitalizations and is validated against the original DCSI,25 which was developed to model diabetes severity and where higher scores predict increased mortality, hospitalizations, and health care utilization.28
High-dimensional propensity score matching. We used high-dimensional propensity score (HDPS) matching to create similar exposure groups and reduce the potential for selection bias and confounding. The HDPS algorithm is designed to control for observed and unobserved confounders in large database studies29 and has been used in previous antipsychotic safety studies.30,31 The HDPS algorithm empirically selected the top 500 covariates across 6 data dimensions: (1) inpatient diagnoses, (2) inpatient procedures, (3) outpatient diagnoses, (4) outpatient procedures, (5) outpatient medications, and (6) facility and health care provider visit type. These covariates were combined with demographic (age, sex, geographic region, index year) and investigator-predefined variables (MGH-AD score, aDCSI score, provider care team, unique classes of diabetes drugs used, and baseline diabetes regimen) to calculate the final HDPS.
Patients taking SGAs were matched to non-SGA users by their HDPS for the between-class comparison. For the 10 subgroup contrasts, a new HDPS was generated for each pairwise comparison (eg, propensity of aripiprazole versus quetiapine treatment). Patients were matched in a 1:1 ratio without replacement by their HDPS score with the nearest neighbor matching algorithm at 0.2 caliper of the standard deviation of the logit of the HDPS.32,33
Statistical Analysis
Descriptive statistics characterized the patient population. To assess the balance of match, we examined propensity score distributions to ensure that there was sufficient overlap between 2 comparator groups after HDPS matching. Absolute standard differences were used to evaluate balance of potential confounders (absolute standard differences less than 0.1 indicated adequate balance between groups).32,34 Cox proportional hazard models were used to calculate the HDPS-adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the outcome.
We conducted 5 sensitivity analyses to evaluate the impact of study design decisions on the results. Since effects of SGAs on glycemic control may continue after drug discontinuation,35-37 we conducted 2 sensitivity analyses wherein patients who switched, added, or discontinued their index drug were followed for an additional 90 or 180 days to account for carryover effects. In the third sensitivity analysis, patients were not considered at risk for an event until 30 days after the index date. In the primary analysis, patients were considered to be at risk for an event the day after drug initiation since prior research11,38 suggests that the effect of SGAs on glycemic control can occur rapidly, with the highest risk period during early therapy. In the fourth sensitivity analysis, we changed the definition of treatment intensification to include only the addition of a new drug class (excluding dose increases).39-41 We evaluated diabetes-related hospitalization only (excluding treatment intensification) in the fifth sensitivity analysis.
In the post hoc analysis, we examined the mean daily doses and peak daily doses (maximum mean daily dose during follow-up) of aripiprazole, quetiapine, mirtazapine, bupropion, and specific TCAs used. SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) and Stata version 14 (StataCorp LLC, College Station, Texas) were used for data analysis. The SAS HDPS macro and the nearest neighbor matching macro were obtained from the Pharmacoepidemiology Toolbox.42
RESULTS
Before match, 6,625 SGA initiators and 23,921 non-SGA initiators met inclusion criteria (see Appendix 1 for study population selection). The most frequently prescribed SGAs were aripiprazole (52.4%) and quetiapine (29.8%), and the most frequently prescribed non-SGAs were bupropion (64.8%), TCAs (25.0%), and mirtazapine (7.7%). Among TCAs, amitriptyline (60.3%) and nortriptyline (21.3%) were most commonly used. See Appendix 2 for medications included at cohort entry.
Patient characteristics at baseline are shown in Table 1. Doses of aripiprazole, quetiapine, and TCAs used were low. The median mean daily doses for aripiprazole and quetiapine were 5 (interquartile range [IQR] = 2-5) and 50 (IQR = 25-100) mg/d, respectively, and median mean daily doses for specific TCAs were all less than 100 mg/d (dosing is shown in Appendix 3). The median peak mean daily doses used were similar to median mean daily doses.
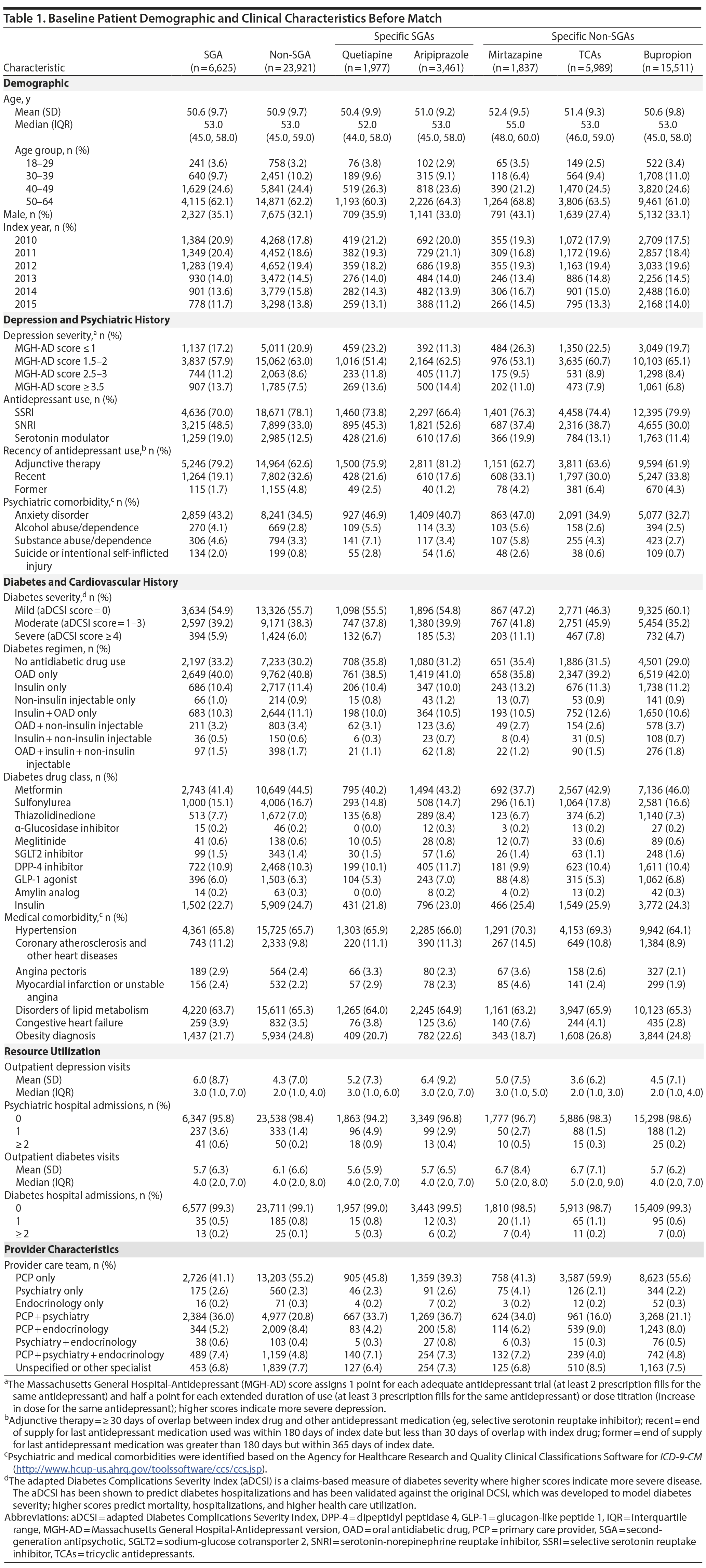
After match, there were 6,465 SGA and 6,465 non-SGA initiators, of whom 1,384 (47.9 per 100 person-years) and 1,461 (43.5 per 100 person-years) developed the event, respectively (Table 2). Patient characteristics and balance of baseline variables after HDPS match are shown in Appendix 4. For subgroup comparisons, the incidence of diabetes hospitalization or drug intensification varied between 40.9 and 57.5 per 100 person-years (Table 2). Diabetes-related hospitalization only was rare and occurred in 39 SGA initiators (10.8 per 1,000 person-years) and 44 non-SGA initiators (10.3 per 1,000 person-years). Appendix 5 provides the incidence of diabetes-related hospitalization for all comparisons.
Risk of diabetes-related hospitalization or drug intensification was not increased with SGA use compared to non-SGA use (aHR = 1.03; 95% CI, 0.96-1.11) (Table 3, Figure 1). The specific breakdown of diabetes-related hospitalization by ICD-9–CM codes is shown in Appendix 6. In the primary analysis, risk was lower comparing quetiapine versus mirtazapine (aHR = 0.82; 95% CI, 0.67-0.99), quetiapine versus TCAs (aHR = 0.84; 95% CI, 0.72-0.98), and bupropion versus TCAs (aHR = 0.85; 95% CI, 0.76-0.86). While risk of the outcome was numerically lower comparing quetiapine versus aripiprazole (aHR = 0.86; 95% CI, 0.73-1.00), results were not statistically significant. For the remaining comparisons, results were not significantly different, and any differences in effect size were small. Alteration of the at-risk window (sensitivity analyses 1 to 3) and the outcome definition to diabetes-related hospitalization or addition of new drug class only (sensitivity analysis 4) did not change our main findings. However, when the outcome was diabetes-related hospitalization only (sensitivity analysis 5), the magnitude or direction of effect did change. In particular, mirtazapine was associated with a 2.7 times higher risk of diabetes-related hospitalization compared to TCAs (aHR = 2.66; 95% CI, 1.11-6.37). For all other comparisons, the CIs were wide, and results were not statistically significant.
DISCUSSION
This study is the first to directly compare SGA and non-SGA depression therapies on the risk of diabetes-related hospitalization or drug intensification in a US insured population with unipolar depression and preexisting diabetes. As a class, SGA use was not associated with an increased risk of events compared to non-SGA use. When comparing specific treatments, bupropion was associated with a small reduction (15%) in risk of the outcome compared to TCAs; quetiapine was associated with an 18% and 16% lower risk of the outcome compared to mirtazapine and TCAs, respectively. Differences between other treatments were small and not statistically significant.
The reduced risk of events comparing bupropion versus TCAs is not unexpected. In randomized studies involving patients with diabetes and depression, bupropion lowered glycosylated hemoglobin (HbA1c) through improvements in depression symptoms and reductions in body weight,43 while nortriptyline did not improve HbA1c compared to placebo.44 Path analysis revealed that nortriptyline directly increased HbA1c but indirectly reduced HbA1c through improvements in depression symptoms, and overall effects on glycemic control were similar to placebo.44 Similarly, SGAs may negatively impact HbA1c through biochemical effects and weight gain3,45,46; however, favorable effects on depression may mitigate these effects. The reduced risk of events observed with quetiapine compared to mirtazapine and TCAs could plausibly be related to improvements in depression symptoms. Quetiapine exhibits antidepressant properties even at the low doses observed in our study; quetiapine 50 mg/d reduces depression symptoms compared to placebo.47 Additionally, previous studies have identified a dose-response effect with quetiapine, where doses > 150 mg are associated with higher diabetes risk48 and > 400 mg are associated with increased HbA1c.49 Also, mirtazapine increases appetite and body weight,50 and TCAs, although effective for depression at low doses (< 100 mg/d as seen in our study),51 may have been used to treat diabetic neuropathy rather than depression. The direct comparative effects between quetiapine, mirtazapine, and TCAs on diabetes and depression at these doses are unknown and require future study.
When we examined the risk of diabetes-related hospitalization only, results deviated from our primary analysis. The small number of events and low incidence rate for the outcome resulted in larger effect sizes with imprecise estimates. As a class, the risk of diabetes-related hospitalization in SGA users was low (10.8 per 1,000 person-years) and comparable to a previous study.4 In Canadian and UK patients aged 18-65 years with preexisting diabetes who used antipsychotics (FGAs or SGAs), risk of hyperglycemic emergencies was 11.5 in 1,000 person-years.4 While we did observe a 2.7 times higher risk of diabetes-related hospitalization with mirtazapine compared to TCAs, and mirtazapine has been associated with a higher risk of weight gain,52 these results may be related to the rareness of the event and multiple testing rather than a true causal effect.
While most studies11-15 in patients with preexisting diabetes have compared antipsychotics (FGAs or SGAs) with nonusers, our results support the need to evaluate specific SGAs individually. In the only other study4 that evaluated within-class comparative effects of antipsychotics on diabetes, risk of hyperglycemic emergencies was not significantly different among quetiapine, risperidone, olanzapine, or FGAs in patients aged 18-65 years with diabetes. We were unable to examine risperidone and olanzapine due to the small sample size, and there is a need for further research on the comparative impact of SGAs.
Limitations
Like other administrative claims studies, limitations include possible misclassification of exposure, diagnosis, outcome, and covariates. While patients have a diagnosis for depression, they may have been using study medications for other indications (eg, bupropion for smoking cessation, TCAs for diabetic neuropathy). There is a potential for confounding by indication wherein patients at higher risk of diabetic complications may be preferentially prescribed non-SGA over SGA therapies due to the anticipated metabolic risks associated with SGAs. In addition, confounding by disease severity may occur wherein patients who have more severe depression may be more likely to be prescribed SGA therapy53,54 and are also at higher risk for diabetic hospitalizations.28 Also, many variables such as HbA1c, weight status, and other laboratory data are unavailable in our database. We attempted to control for unmeasured confounders, disease severity, and diabetes severity at baseline via HDPS matching.
Given that guidelines recommend that patients taking SGAs should be closely monitored for metabolic changes, there could be detection bias wherein patients taking SGAs are more closely monitored for glycemic changes and, therefore, are more likely to have therapeutic escalations compared to those not being as closely monitored. However, given that both cohorts have preexisting diabetes and should be closely monitored on glycemic control regardless of SGA use, we anticipate that this bias would be smaller than in patients without diabetes. Also, we conducted 10 pairwise comparisons but did not adjust for multiple comparisons since we were more interested in the magnitude of effect rather than statistical significance and wanted to explore potential leads that should be further explored in other research settings given the lack of information on this topic.55 In addition, while we achieved 80% power to detect a 20% difference in the composite outcome for all treatment comparisons, diabetes hospitalizations only was a rare event for which analysis should be considered exploratory.
conclusion
In summary, there is a lack of strong evidence for selection of 1 medication over another in terms of risk of diabetes-related hospitalization or diabetes drug intensification. There is strong evidence that SGAs increase the risk of diabetes5-10; however, our understanding on how these medications affect existing diabetes is limited. Whether these drugs should be used in this population will depend not only on their safety profile, but also on their effectiveness for depression and the ability to safely monitor patients in clinical practice. Improvement of depression may indirectly lead to improved glycemic control as patients are better able to manage their diabetes.43,56-58 Importantly, patients with suboptimal glycemic control may not necessarily receive treatment intensification if changes in HbA1c are small, if patients are nonadherent to diabetes therapy, or due to therapeutic inertia or other reasons. Physicians may also choose to discontinue SGA therapy or use low doses in patients who gain weight or have small increases in HbA1c. Therefore, future studies are needed to determine the impact of these therapies on other diabetes outcomes (eg, HbA1c, diabetic complications, mortality). While this study focused on diabetes, SGAs also have various other metabolic (eg, weight gain, hyperlipidemia) and nonmetabolic side effects (eg, extrapyramidal symptoms) that may vary by drug and are also important to study in the diabetes population.
Submitted: September 8, 2017; accepted December 18, 2017.
Published online: May 10, 2018.
Potential conflicts of interest: Dr Touchette has received research funding from Pfizer, Sunovion, and Takeda. Drs Xing, Kim, Schumock, Calip, Leow, and Lee report no conflicts of interest related to the subject of this article.
Funding/support: Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award no. F31MH114465. The University of Illinois at Chicago provided material support for access to the Truven MarketScan Commercial Claims and Encounters Database.
Role of the sponsor: The sponsor provided no input regarding the design or conduct of the study; collection, management, analysis, or interpretation of data; or preparation, review, or approval of the manuscript.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information: Information on the Truven MarketScan Commercial Claims and Encounters Database can be found at http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases.
Supplementary material: See accompanying pages.
REFERENCES
1. Gerhard T, Akincigil A, Correli CU, et al. National trends in second-generation antipsychotic augmentation for nonpsychotic depression. J Clin Psychiatry. 2014;75(5):490-497. PubMed CrossRef
2. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. PubMed CrossRef
3. Ananth J, Venkatesh R, Burgoyne K, et al. Atypical antipsychotic induced weight gain: pathophysiology and management. Ann Clin Psychiatry. 2004;16(2):75-85. PubMed CrossRef
4. Lipscombe LL, Austin PC, Alessi-Severini S, et al. Atypical antipsychotics and hyperglycemic emergencies: multicenter, retrospective cohort study of administrative data. Schizophr Res. 2014;154(1-3):54-60. PubMed CrossRef
5. Smith M, Hopkins D, Peveler RC, et al. First- v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2008;192(6):406-411. PubMed CrossRef
6. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology. 2010;35(9):1997-2004. PubMed CrossRef
7. Perez-Iglesias R, Martinez-Garcia O, Pardo-Garcia G, et al. Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol. 2014;17(1):41-51. PubMed
8. Lipscombe LL, Lévesque LE, Gruneir A, et al. Antipsychotic drugs and the risk of hyperglycemia in older adults without diabetes: a population-based observational study. Am J Geriatr Psychiatry. 2011;19(12):1026-1033. PubMed CrossRef
9. טstbye T, Curtis LH, Masselink LE, et al. Atypical antipsychotic drugs and diabetes mellitus in a large outpatient population: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2005;14(6):407-415. PubMed CrossRef
10. Kessing LV, Thomsen AF, Mogensen UB, et al. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197(4):266-71. PubMed
11. van Keulen K, van der Linden PD, Souverein PC, et al. Risk of hospitalization for hypoglycemia in older patients with diabetes using antipsychotic drugs. Am J Geriatr Psychiatry. 2015;23(11):1144-1153. PubMed CrossRef
12. Lipscombe LL, Lévesque L, Gruneir A, et al. Antipsychotic drugs and hyperglycemia in older patients with diabetes. Arch Intern Med. 2009;169(14):1282-1289. PubMed CrossRef
13. Spoelstra JA, Stolk RP, Cohen D, et al. Antipsychotic drugs may worsen metabolic control in type 2 diabetes mellitus. J Clin Psychiatry. 2004;65(5):674-678. PubMed CrossRef
14. Wake DJ, Broughton P, Perera SM, et al. Altered metabolic parameters in association with antipsychotic medication use in diabetes: a population based case-control study. Psychoneuroendocrinology. 2016;66:214-220. PubMed CrossRef
15. Wu C-S, Gau SS-F. Association between antipsychotic treatment and advanced diabetes complications among schizophrenia patients with type 2 diabetes mellitus. Schizophr Bull. 2016;42(3):703-711. PubMed CrossRef
16. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, Third Edition. APA website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. 2010.Accessed March 19, 2016.
17. Halpern R, Nadkarni A, Kalsekar I, et al. Medical costs and hospitalizations among patients with depression treated with adjunctive atypical antipsychotic therapy: an analysis of health insurance claims data. Ann Pharmacother. 2013;47(7-8):933-945. PubMed CrossRef
18. Hansen LG, Chang S. White Paper. Health research data for the real world: The MarketScan databases. Truven Health Analytics website. http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf. Accessed April 12, 2018.
19. Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14(1):289. PubMed CrossRef
20. Leong A, Dasgupta K, Bernatsky S, et al. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PLoS One. 2013;8(10):e75256. PubMed CrossRef
21. Hartung DM, Middleton L, McFarland BH, et al. Use of administrative data to identify off-label use of second-generation antipsychotics in a Medicaid population. Psychiatr Serv. 2013;64(12):1236-1242. PubMed CrossRef
22. Hassan AK, Farmer KC, Brahm NC, et al. Evaluating antidepressant treatment prior to adding second-line therapies among patients with treatment-resistant depression. Int J Clin Pharm. 2016;38(2):429-437. PubMed CrossRef
23. Centers for Medicare and Medicaid Services (CMS). 2015 ICD-10-CM and General Equivalence Mappings. CMS website. https://www.cms.gov/medicare/coding/icd10/2015-icd-10-cm-and-gems.html. Published 2014. Accessed March 14, 2017.
24. Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370-377. PubMed
25. Chang H-Y, Weiner JP, Richards TM, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721-726. PubMed
26. Hassan AK, Farmer KC, Brahm NC, et al. Does the use of atypical antipsychotics as adjunctive therapy in depression result in cost savings? comparing healthcare costs and utilization between second-line treatment options. J Ment Health. 2016;25(6):486-491. PubMed CrossRef
27. Jing Y, Kalsekar I, Curkendall SM, et al. Intent-to-treat analysis of health care expenditures of patients treated with atypical antipsychotics as adjunctive therapy in depression. Clin Ther. 2011;33(9):1246-1257. PubMed CrossRef
28. Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15-23. PubMed
29. Schneeweiss S, Rassen JA, Glynn RJ, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2011;20(4):512-522. PubMed CrossRef
30. Vigod SN, Gomes T, Wilton AS, et al. Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. BMJ. 2015;350:h2298. PubMed CrossRef
31. Wu C-S, Wang S-C, Yeh I-J, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579. PubMed CrossRef
32. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. PubMed CrossRef
33. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. PubMed CrossRef
34. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. PubMed CrossRef
35. Lean MEJ, Pajonk F-G. Patients on atypical antipsychotic drugs: another high-risk group for type 2 diabetes. Diabetes Care. 2003;26(5):1597-1605. PubMed CrossRef
36. De Hert M, Hanssens L, van Winkel R, et al. Reversibility of antipsychotic treatment-related diabetes in patients with schizophrenia: a case series of switching to aripiprazole. Diabetes Care. 2006;29(10):2329-2330. PubMed CrossRef
37. De Hert M, Hanssens L, van Winkel R, et al. A case series: evaluation of the metabolic safety of aripiprazole. Schizophr Bull. 2007;33(3):823-830. PubMed CrossRef
38. Guenette MD, Hahn M, Cohn TA, et al. Atypical antipsychotics and diabetic ketoacidosis: a review. Psychopharmacology (Berl). 2013;226(1):1-12. PubMed CrossRef
39. Vitry AI, Roughead EE, Preiss AK, et al. Influence of comorbidities on therapeutic progression of diabetes treatment in Australian veterans: a cohort study. PLoS One. 2010;5(11):e14024. PubMed CrossRef
40. Fu AZ, Sheehan J. Treatment intensification for patients with type 2 diabetes and poor glycemic control. Diabetes Obes Metab. 2016;18(9):892-898. PubMed CrossRef
41. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411-3417. PubMed CrossRef
42. Rassen JA, Doherty M, Huang W, et al. Pharmacoepidemiology Toolbox. Division of Pharmacoepidemiology & Pharmacoeconomics website. http://www.drugepi.org/dope-downloads/#Pharmacoepidemiology Toolbox. Accessed December 10, 2016.
43. Lustman PJ, Williams MM, Sayuk GS, et al. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30(3):459-466. PubMed CrossRef
44. Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59(3):241-250. PubMed CrossRef
45. Goncalves P, Araujo JR, Martel F. Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur Neuropsychopharmacol. 2015;25(1):1-16. PubMed CrossRef
46. Kroeze WK, Hufeisen SJ, Popadak BA, et al. HI-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519-526. PubMed CrossRef
47. Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. PubMed CrossRef
48. Ulcickas YM, Delorenze GN, Quesenberry CP Jr, et al. Association between second-generation antipsychotics and newly diagnosed treated diabetes mellitus: does the effect differ by dose? BMC Psychiatry. 2011;11(1):197. PubMed CrossRef
49. Guo Z, L’ Italien GJ, Jing Y, et al. A real-world data analysis of dose effect of second-generation antipsychotic therapy on hemoglobin A1C level. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1326-1332. PubMed CrossRef
50. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder, Section 3: pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. PubMed CrossRef.
51. Furukawa T, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane database Syst Rev. 2003;(3):CD003197. PubMed CrossRef
52. Blumenthal SR, Castro VM, Clements CC, et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry. 2014;71(8):889-896. PubMed CrossRef
53. Gersing KR, Sheehan JJ, Burchett B, et al. Use of augmentation agents for treating depression: analysis of a psychiatric electronic medical record data set. Psychiatr Serv. 2014;65(8):1062-1065. PubMed CrossRef
54. McIntyre RS, Weiller E. Real-world determinants of adjunctive antipsychotic prescribing for patients with major depressive disorder and inadequate response to antidepressants: a case review study. Adv Ther. 2015;32(5):429-444. PubMed
55. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. PubMed CrossRef CrossRef
56. Breum L, Bjerre U, Bak JF, et al. Long-term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance: influence on muscle glycogen synthase and insulin receptor kinase activity. Metabolism. 1995;44(12):1570-1576. PubMed CrossRef
57. Arterburn D, Sofer T, Boudreau D, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5(4):48. PubMed CrossRef
58. Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12(12):CD008381. PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite