ABSTRACT
Background: Side effects of selective serotonin reuptake inhibitors (SSRIs), the most commonly used antidepressants, are usually underreported in clinical trials. Systematic evaluation of side effects associated with SSRIs with structured instruments in a naturalistic setting is an important design to fully understand the side effect profile of various SSRIs. We examined the frequencies of the side effects induced by 3 commonly used SSRIs, sertraline, escitalopram, and fluoxetine, by using a self-rating instrument designed to measure the subjective symptoms of patients in a naturalistic treatment setting.
Methods: The subjects were outpatients recruited from the psychiatry department of a tertiary care hospital. The subjects were aged ≥ 18 years; were diagnosed with depression, anxiety spectrum disorders, adjustment disorder, hypochondriasis, or impulse control disorder according to ICD-10 criteria; and were on SSRI monotherapy. The assessment instrument included 42 items and was devised using drug package insert data on the most commonly observed side effects of antidepressants released by the US Food and Drug Administration.
Results: A total of 100 patients participated in the study. Among them, 70% were women. The most common diagnosis was depression (49%). Of the patients, 53% were taking sertraline, 38% escitalopram, and 8% fluoxetine. The common side effects reported by patients were flatulence (64%), somnolence (59%), memory impairment (51%), decreased concentration (50%), yawning (47%), fatigue (45%), dry mouth (45%), weight gain (45%), light headedness (43%), and sweating (38%). Patients treated with escitalopram had significantly higher incidence of headache, pruritus, memory impairment, decreased concentration, and dizziness. Patients treated with sertraline had significantly decreased appetite.
Conclusions: The study results highlight the prevalence and pattern of side effect profiles of 3 commonly used SSRIs and provide baseline data for comparison with other similar studies.
Prim Care Companion CNS Disord 2021;23(4):20m02747
To cite: Anagha K, Shafeena T, Shihabudheen P, et al. Side effect profiles of selective serotonin reuptake inhibitors: a cross-sectional study in a naturalistic setting. Prim Care Companion CNS Disord. 2021;23(4):20m02747.
To share: https://doi.org/10.4088/PCC.20m02747
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Pharmacy Practice, JDT Islam College of Pharmacy, Vellimadukunnu, Calicut, Kerala, India
bDepartment of Critical Care, Iqraa International Hospital and Research Centre, Calicut, Kerala, India
cDepartment of Psychiatry, Iqraa International Hospital and Research Centre, Calicut, Kerala, India
*Corresponding author: N. A. Uvais, MBBS, DPM, Department of Psychiatry, Iqraa International Hospital and Research Centre, Calicut, Kerala, India ([email protected]).
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed psychotropic agent for the treatment of various psychiatric disorders. Although SSRIs have different chemical structures, they all share the same mechanism of action: selectively inhibiting the reuptake of serotonin. When compared with tricyclic antidepressants (TCAs), SSRIs have a significantly lower side effect profile with comparable efficacy.1 SSRIs also show a significantly lower discontinuation rate compared with other classes of antidepressants and can be used safely in elderly patients, children, and patients with multiple comorbidities.1
Various side effects have been reported with their long-term use. The most commonly reported side effects are sexual dysfunction, gastrointestinal symptoms, neuropsychiatric symptoms, and constitutional symptoms. However, research also indicates a wide discrepancy in the rates of various side effects reported between the original placebo-controlled clinical trials and postmarketing clinical trials.2 Moreover, the side effect profiles of SSRIs provided in the drug manufacturers’ package inserts are derived from company-sponsored studies that have been reviewed by the US Food and Drug Administration (FDA) and usually fail to capture the side effect profiles of SSRIs in the real world.2 The dosages used in early clinical trials may not be sufficient to allow for a full understanding of the side effect profiles of SSRIs.2 Furthermore, trial design, methods for determining adverse effects, and the duration of the clinical trial also can adversely influence the reporting of side effects.2 One large meta-analysis3 and another systematic review4 analyzing clinical trials on antidepressants showed that structured and systematic side effect assessment strategies were used in only 21% of these trials, and only a few trials used an objective side effect rating scale. Because of unknown external validity of side effect data of SSRI use from clinical trials, systematic evaluation of side effects associated with SSRIs with structured instruments in day-to-day clinical practice is an important design to fully understand the side effect profiles of various SSRIs.5
Patients’ subjective feelings and complaints of side effects associated with SSRIs in a naturalistic treatment setting are critical in clinical practice, as they can directly influence treatment continuation and good compliance.6 The purpose of this study was to compare the frequencies of the side effects induced by 3 commonly used SSRIs, sertraline, escitalopram, and fluoxetine, by using a self-rating instrument designed to measure the subjective symptoms of patients in a naturalistic treatment setting.
METHODS
The subjects were outpatients recruited from a psychiatry department in a tertiary care hospital. The subjects were patients (aged ≥ 18 years) diagnosed with any psychiatric disorder according to ICD-10 criteria and who were on SSRI monotherapy. Patients who were on combination therapy, had comorbid physical illness, had poor drug compliance, or were unwilling to provide consent were excluded. The study protocol was approved by the institutional ethics committee. Written informed consent was obtained from the patients prior to the study. Patient demographics, diagnosis, and drug-related details (type of medication, daily dose, and administration duration) were collected from patients using a data collection form. The assessment instrument was devised using drug package insert data on the most commonly observed side effects of antidepressants (those with an incidence rate ≥ 3%) released by the FDA similar to a past study.1 The instrument comprised 42 items designed to measure the antidepressant-induced side effects experienced by the patients within the past month. The data analysis was conducted using t test or χ2 statistics depending on the type of variable being investigated. A probability P value < .05 was considered significant. SPSS 10.0 for Windows (IBM, Armonk, New York) was utilized for the statistical analysis.
RESULTS
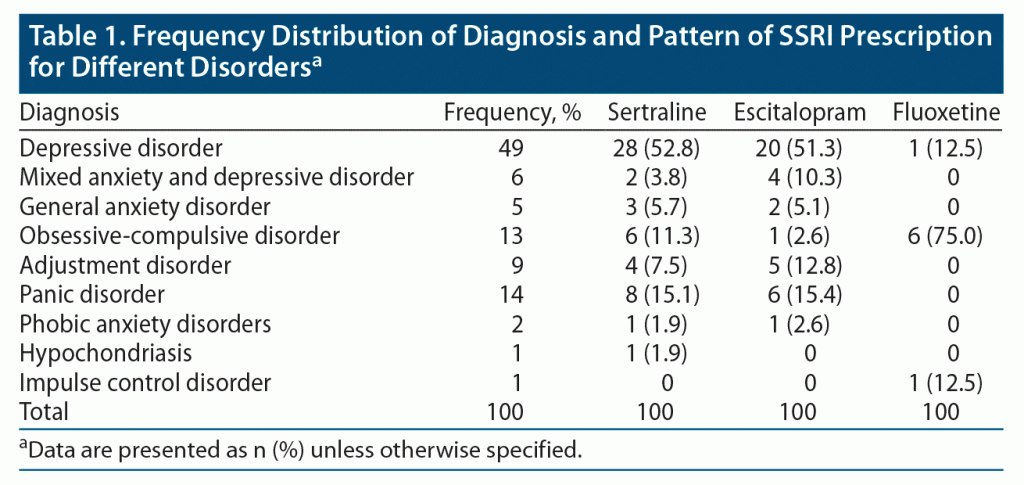
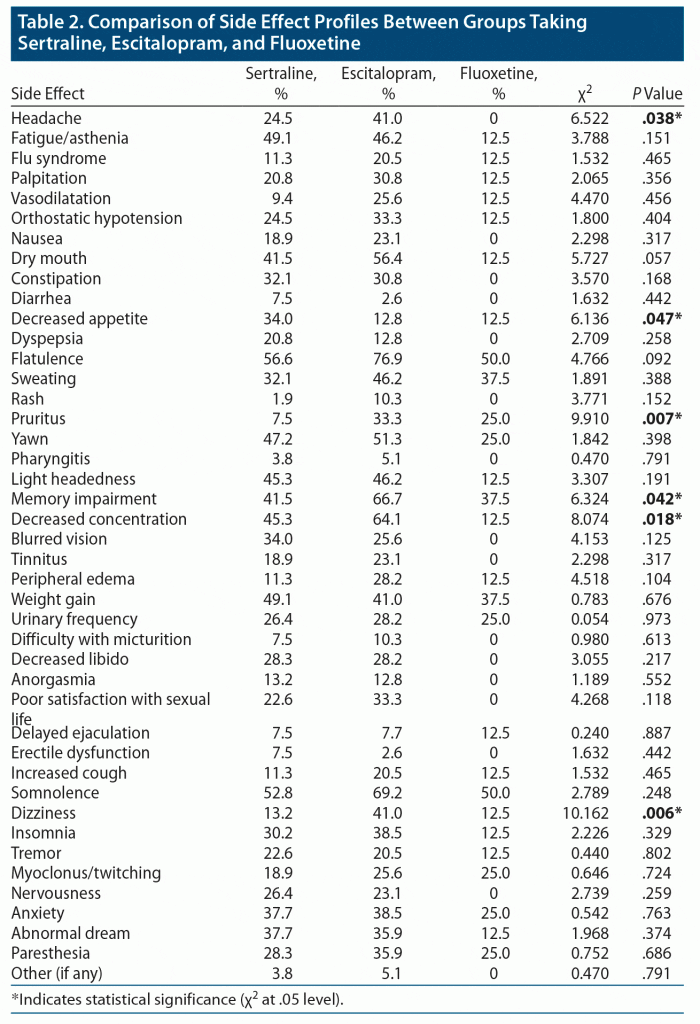
A total of 100 patients participated in the study, and 70% were women. The most common diagnosis was depression (49%), followed by panic disorder (14%) and obsessive-compulsive disorder (13%). The diagnoses of the patients are provided in Table 1. Of the patients, 53% were taking sertraline, 38% escitalopram, and 8% fluoxetine. The pattern of SSRI prescription by various psychiatric diagnoses is also provided in Table 1. There was no significant statistical difference in age, sex, or duration of SSRI administration across the 3 groups of patients taking sertraline, escitalopram, and fluoxetine. The most commonly reported side effects were flatulence (64%), somnolence (59%), memory impairment (51%), decreased concentration (50%), yawning (47%), fatigue (45%), dry mouth (45%), weight gain (45%), light headedness (43%), and sweating (38%). Those patients treated with escitalopram showed significantly higher incidence of headache (χ2 = 6.522, P = .038), pruritus (χ2 = 9.910, P = .007), memory impairment (χ2 = 6.324, P = .042), decreased concentration (χ2 = 8.074, P = .018), and dizziness (χ2 = 10.162, P = .006). Those patients treated with sertraline showed significantly decreased appetite (χ2 = 6.136, P = .047). However, there were no significant differences in the other side effects among the 3 groups. The comparison of side effect profiles between the groups taking sertraline, escitalopram, and fluoxetine is provided in Table 2. Two patients taking sertraline reported other side effects: apathy and hair loss. Two patients taking escitalopram reported shortness of breath.
DISCUSSION
Fluoxetine was introduced in the United States in 1988 as an alternative to TCAs with the same efficacy and favorable side effect profile, especially cardiac conduction abnormalities in overdose and the risk of precipitating seizures.7 However, questions about the safety and tolerability of SSRIs have emerged with their continued use. Very high rates of various side effects such as sexual dysfunction or hyponatremia were reported in postmarketing clinical trials compared to original placebo-controlled clinical trials.2 Various reasons, both patient related and study design related, have been proposed to explain this discrepancy.2 Most of these results were derived from observational studies and case series submitted to regulatory authorities. Thus, the comparative incidence rates of side effects across different antidepressant agents remain incompletely elucidated. Hence, studies conducted in naturalistic settings can only compare side effect profiles of various SSRIs among users, which is the strength of our study.
In this study, we compared the symptoms experienced by patients on SSRI monotherapy with escitalopram, sertraline, and fluoxetine for different psychotic conditions in a naturalistic setting. The most common diagnosis was depressive disorder, and the most common SSRI prescribed was sertraline. There was no significant statistical difference in age, sex, or duration of SSRI administration across the 3 groups taking sertraline, escitalopram, and fluoxetine. The most common side effects reported by the users were gastrointestinal, neuropsychiatric, sexual, and constitutional in nature. In 2009, Cascade et al8 published patient-reported side effects from a cross-section of real-world patients taking 1 of the following SSRI antidepressants: citalopram, escitalopram, fluoxetine, paroxetine, or sertraline. Thirty-eight percent of the approximately 700 patients surveyed reported experiencing 1 or more side effects as a result of taking an SSRI antidepressant. The commonly reported side effects were sexual dysfunction (56%), drowsiness/sleepiness (53%), weight gain (49%), dry mouth (19%), insomnia (16%), fatigue (14%), nausea (14%), dizziness/lightheaded (13%), and tremors (12%).8
The common gastrointestinal symptoms reported in our study were flatulence, dry mouth, nausea, constipation, diarrhea, decreased appetite, and dyspepsia. Flatulence and dry mouth were most frequently reported. We also found a significantly reduced appetite among patients taking sertraline. Serotonin plays a major regulatory role in the motor and sensory regulation of the gastrointestinal (GI) tract affecting gastric motility.9 Moreover, serotonergic agents that act on central 5-HT3 receptors may also lead to nausea and vomiting.10 Other frequently reported GI side effects associated with the use of SSRIs such as GI bleeding and abdominal pain were not reported in our study. A review exploring the side effect profile of SSRIs found GI disturbances to be the most frequently reported side effect of SSRI use, significantly associated with fluvoxamine use, whereas escitalopram was less likely to cause GI side effects.11 Approximately half of all patients started on these agents experienced GI side effects mainly in the first few days/weeks following treatment initiation.12 Some studies12 also have found nausea and vomiting to be one of the most common reasons for treatment discontinuation. The neuropsychiatric symptoms reported in our study were dizziness, lightheadedness, memory impairment, reduced concentration, nervousness, anxiety, somnolence, tremors, myoclonus, paresthesia, apathy, and abnormal dreams. Postmarketing studies11,13 have found that neuropsychiatric symptoms such as anxiety, agitation, and sleep disturbances were most often associated with sertraline and fluoxetine use. However, we found a significantly higher association between memory impairment and dizziness with escitalopram use.
Cognitive dysfunction subsequent to SSRI therapy remains controversial, and studies have reported inconsistent results. In 2016, Sayyah et al14 systematically analyzed cognition of patients with depression or obsessive-compulsive disorder and found that there was a gradual decline in cognitive functions, especially memory impairment, assessed by the Mini-Mental State Examination over the subsequent weeks after taking SSRIs. The memory loss caused by SSRIs has not yet been convincingly explained, although serotonin appears to play an important role in learning and memory. However, a meta-analysis15 explored the effects of antidepressants on cognitive functioning in depressed and nondepressed samples and found that SSRIs have the greatest positive effect on cognition for depressed participants compared to the other classes of antidepressants analyzed.
Sleep disturbances were reported by a large number of participants in our study. Both somnolence and insomnia were reported. Previous research has shown that SSRIs interfere with sleep architecture. Fluoxetine and sertraline delay the onset of rapid eye movement sleep, and fluoxetine increases awakenings and reduces rapid eye movement sleep, slow-wave sleep, total sleep time, and sleep efficiency.2 In a review article16 on the prevalence of treatment-emergent insomnia and somnolence in depressed patients, it was shown that subjective complaints of insomnia or daytime somnolence were frequent in patients with depression or anxiety disorders treated with SSRIs. On the basis of data from the FDA study register,17 the average prevalence of treatment-emergent insomnia in clinical trials with SSRIs was 17%. The average rate of treatment-emergent somnolence in patients being treated with an SSRI amounted to 16% compared to 8% of patients receiving placebo.17
Sexual side effects were reported by a large number of the participants in our study. The common sexual problems reported were reduced libido, anorgasmia, poor satisfaction with sexual life, delayed ejaculation, and erectile dysfunction. These side effects are some of the most underreported adverse effects associated with the use of antidepressants and a major contributor to treatment discontinuation and lack of adherence. An Iranian study18 exploring the effects of SSRIs on the stages of sexual function in patients with major depressive disorder found that a total of 75% of patients reported sexual dysfunction: 66.7% of men and 79.7% of women. A total of 74% of patients on fluvoxamine, 100% on fluoxetine, 75% on sertraline, 71% on citalopram, and 100% on paroxetine reported sexual dysfunction. The most frequent sexual dysfunction was difficulty with orgasm, which affected 41.17% of women and 33.33% of men.18 Another study19 compared sexual side effects of citalopram with fluoxetine in male patients referred to a psychiatric clinic and found that general sexual dysfunction was significantly higher in the fluoxetine group. SSRI-induced sexual side effects probably result from the stimulation of postsynaptic 5-HT2 receptors.2 An incidence of around 55% for sexual dysfunction with SSRI use has been shown in past published studies.20 Although there are minor individual variations in the rate and pattern of sexual side effects with respect to individual SSRIs, a recent network meta-analysis21 found that these differences were not statistically significant.
Constitutional symptoms, especially weight gain, were reported by more than half of the participants. Weight gain was infrequently reported during premarketing clinical trials of SSRIs.2 Although some SSRIs are typically associated with weight loss during initial therapy, weight is often regained after 6 months and can be followed by additional weight gain with long-term use.2 A systematic study from 2010 showed weight increases over 8-month follow-up with antidepressant use.22 Another study23 showed estimates consistent with a 2.1-kg weight gain with fluoxetine treatment and about a 4.8-kg increase with sertraline within 2 years of treatment. A population-based cohort study24 exploring antidepressant utilization and incidence of weight gain during 10-year follow-up found that participants who were prescribed an antidepressant had an increased risk of ≥ 5% weight gain compared with those who had never been prescribed an antidepressant. The study24 also found that the risk of weight gain was substantially increased during the second and third years of treatment. There are multiple interrelated mechanisms contributing to the antidepressant-induced weight gain such as antagonism to histaminergic H1 receptors and serotonin 5-HT2C receptors, reduced physical activity due to sedative effects of certain antidepressants, changes in food preference, and increased intake of caloric beverages due to dryness of mouth associated with SSRI use.12
Our study had certain limitations. It was a small sample size cross-sectional study conducted in an outpatient department setting of a multispecialty hospital using an assessment instrument, and it is possible that we might have missed other rare side effects associated with SSRI use. We did not take diet and other confounding factors including severity of illness into account, which might have influenced subjective experiences of the patients. As this was a cross-sectional study, we could not assess causality for the entire study population, but only for those who completed the assessment. In spite of these limitations, our results highlight the prevalence and patterns of SSRI side effects in South India and provide baseline data for comparison with other similar studies. Our results also have clinical implications. Psychiatrists and other medical professionals prescribing SSRIs should be aware of the side effect profiles of the drugs they prescribe, and patients should be educated regarding the common side effects of SSRI use so that morbidity and treatment discontinuation can be reduced.
Submitted: July 10, 2020; accepted October 23, 2020.
Published online: July 29, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Clinical Points
- Side effects of selective serotonin reuptake inhibitors (SSRIs), the most commonly used antidepressants, are usually underreported in clinical trials.
- The common side effects of SSRIs reported were flatulence, somnolence, memory impairment, decreased concentration, yawning, fatigue, dry mouth, weight gain, light headedness, and sweating.
- Psychiatrists and other medical professionals prescribing SSRIs should be aware of the side effect profiles, and patients should be educated accordingly so that morbidity and treatment discontinuation can be reduced.
References (24)

- Chae JH, Lee KU, Shin YK, et al. Comparison of side effect profiles between mirtazapine and selective serotonin reuptake inhibitors; a naturalistic setting. Clin Psychopharmacol Neurosci. 2004;2:31–35.
- Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22–27. PubMed CrossRef
- Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf. 2009;32(11):1041–1056. PubMed CrossRef
- Gartlehner G, Thieda P, Hansen RA, et al. Comparative risk for harms of second-generation antidepressants: a systematic review and meta-analysis. Drug Saf. 2008;31(10):851–865. PubMed CrossRef
- Bet PM, Hugtenburg JG, Penninx BW, et al. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol. 2013;23(11):1443–1451. PubMed CrossRef
- Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164–172. PubMed CrossRef
- Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry. 1999;60(suppl 4):4–11, discussion 12–13. PubMed
- Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont). 2009;6(2):16–18. PubMed
- Janssen P, Vos R, Tack J. The influence of citalopram on interdigestive gastrointestinal motility in man. Aliment Pharmacol Ther. 2010;32(2):289–295. PubMed CrossRef
- Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci. 2015;9:413. PubMed CrossRef
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20(3):277–287. PubMed CrossRef
- Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–288. PubMed CrossRef
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders, III: tolerability, safety and pharmacoeconomics. J Psychopharmacol. 1998;12(suppl B):S55–S87. PubMed CrossRef
- Sayyah M, Eslami K, AlaiShehni S, et al. Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry J. 2016;2016:5480391. PubMed CrossRef
- Prado CE, Watt S, Crowe SF. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev. 2018;28(1):32–72. PubMed CrossRef
- Wichniak A, Wierzbicka A, Walęcka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63. PubMed CrossRef
- Thompson C. Onset of action of antidepressants: results of different analyses. Hum Psychopharmacol. 2002;17(suppl 1):S27–S32. PubMed CrossRef
- Safa M, Sadr S, Talischi F, et al. Study of effects of selective serotonin reuptake inhibitors on stages of sexual function in Iranian patients with major depressive disorder. Ther Adv Psychopharmacol. 2013;3(6):306–313. PubMed CrossRef
- Herizchi S, Mogaddam A. Comparison of citalopram and fluoxetine sexual side-effects in male patients referred to psychiatric clinic. J Anal Res Clin Med. 2016;4(4):221–227. CrossRef
- Montejo AL, Llorca G, Izquierdo JA. Sexual dysfunction with SSRIs: a comparative analysis. In: New Research Program and Abstracts of the 149th Annual Meeting of the American Psychiatric Association (abstract NR717.266); May 9, 1996; New York, NY.
- Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf. 2014;37(1):19–31. PubMed CrossRef
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. PubMed CrossRef
- Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5(4):E48. PubMed CrossRef
- Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite