Prim Care Companion CNS Disord 2023;25(2):22cr03285
To cite: Debroy K, Yazgi H, Krishnamurthy VB. Sleep state misperception in frontotemporal dementia. Prim Care Companion CNS Disord. 2023;25(2):22cr03285.
To share: https://doi.org/10.4088/PCC.22cr03285
© 2023 Physicians Postgraduate Press, Inc
aDepartment of Psychiatry, Penn State University, Hershey Medical Center, Hershey, Pennsylvania
bDepartment of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania
*Corresponding author: Venkatesh Basappa Krishnamurthy, MD, Department of Pulmonary, Allergy, and Critical Care Medicine, NW 628, 3459 Fifth Ave, Pittsburgh, PA 15232 ([email protected]).
Paradoxical insomnia, in which subjective and objective sleep patterns do not align, can occur in various neuropsychiatric conditions. We report a case of paradoxical insomnia in a patient with frontotemporal dementia (FTD).
Case Report
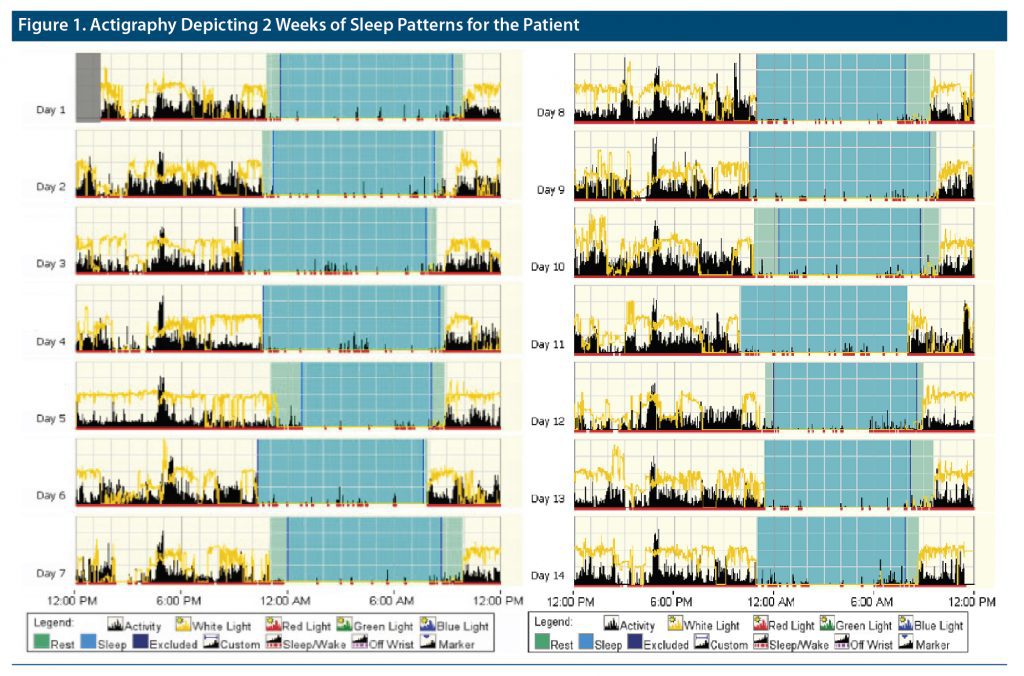
Mr A was a 55-year-old man with cognitive dysfunction and memory impairment secondary to FTD. He presented with complaints of habitual difficulties with falling and staying asleep on most nights for about 2 years. He was a poor historian, unmarried, and living alone and did not have a reliable source to corroborate his complaints. He was recommended to maintain a sleep diary, but he was noncompliant. Given his cognitive issues and poor compliance with sleep diaries, actigraphy, an objective measure of sleep, was recommended for 2 weeks to better estimate his total sleep time (TST). The actigraphy results are shown in Figure 1.
Mr A reported he spent an average of 11 hours in bed, while his actigraphy results showed an average of 10 hours and 21 minutes in bed. His subjective sleep latency was 4 hours, reported TST was 2 hours and 30 minutes, wakefulness after sleep onset (WASO) was 150 minutes, and estimated sleep efficiency (SE) was 22.5%. His objective sleep parameters as measured by actigraphy included a sleep latency of 27.9 minutes, TST of 8 hours and 37 minutes, WASO of 35.9 minutes, and SE of 83.35. Given his subjective-objective sleep discrepancy greater than 30 minutes for sleep latency and over an hour for TST, Mr A’s sleep pattern was suggestive of paradoxical insomnia. He was continued on trazodone 100 mg at night and was recommended to enroll in the behavioral sleep program to address sleep state misperception but did not follow up.
Discussion
FTD is a group of heterogeneous disorders with selective frontal and temporal lobe degeneration and early onset of personality changes with language and cognitive decline. Insomnia is estimated to occur in about 47% of FTD patients,1 with a reported decrease in TST and SE.1,2 Objective polysomnographic (PSG) sleep studies report increased stage N1 and decreased stage N2 and rapid eye movement sleep,3 findings which correlated with reduced working memory.4 FTD-related sleep disturbances are also believed to occur due to disruption of the sleep and circadian rhythm with consequent sleep fragmentation, increased nighttime awakenings, and decreased daytime activity.5,6 This impaired sleep may exacerbate symptoms and decrease efficiency of the glymphatic system that normally clears protein waste products during sleep, making it less likely to clear the phosphorylated tau protein that disrupts neuron function in FTD.7
FTD patients have altered self-assessment, including sleep perception. Sleep perception is highly important in clinical practice, as treatment decisions are often based on self-reported sleep patterns. However, due to altered sleep perception in FTD patients, an accurate objective measure of sleep is important. PSG is considered the gold standard for objective sleep measurements, but it is laboratory-based, expensive, labor intensive, unavailable in certain areas, cannot be conducted in the patient’s habitual environment, and is challenging to perform over several nights.8 Thus, actigraphy, a cost-effective, watch-like wearable technology that measures daily activity levels for longer periods (often 2 weeks) in the patient’s own environment, was used. There is a high degree of correlation between actigraphy and PSG measures of sleep.8
Paradoxical insomnia, a variant of insomnia characterized by extreme discrepancy of subjective-objective sleep measures, is estimated to comprise 5%–9% of all cases of insomnia.9 Previous studies9 report a discrepancy of 60 minutes or more between self-reported and objective TST measured by PSG in patients with paradoxical insomnia. These patients tend to be distressed and anxious due to perceived inadequate sleep, which may eventually lead to objective sleep disturbance.10 Paradoxical insomnia can occur in FTD patients, but the prevalence is not well established. There are no formal guidelines for treatment of paradoxical insomnia, but traditional hypnotic medications appear to have a limited effect on improving the perception of sleep.11 Cognitive-behavioral therapy addressing sleep state misperception can be helpful in the treatment of such patients with paradoxical insomnia.
In our case, Mr A presented with a subjective report of increased sleep latency and decreased TST. However, objective actigraphy showed that his TST was within normal limits. This may have treatment implications, as clinicians can inadvertently increase dosage or try alternate hypnotic medications. Such erroneous medication management can lead to further cognitive decline or precipitate an episode of delirium in at-risk FTD patients.12,13 Hence, clinicians need to evaluate for paradoxical insomnia using an objective measure of sleep like actigraphy in FTD patients, especially when the history is unreliable.
Published online: March 7, 2023.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Informed consent was obtained from the patient for use of the medical record for research, and research authorization is on file at Hershey Medical Center. Information has been de-identified to protect anonymity.
References (13)

- McCarter SJ, St Louis EK, Boeve BF. Sleep disturbances in frontotemporal dementia. Curr Neurol Neurosci Rep. 2016;16(9):85. PubMed CrossRef
- Anderson KN, Hatfield C, Kipps C, et al. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol. 2009;16(3):317–323. PubMed CrossRef
- Bonakis A, Economou NT, Paparrigopoulos T, et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer’s disease. J Alzheimers Dis. 2014;38(1):85–91. PubMed CrossRef
- Kundermann B, Thum A, Rocamora R, et al. Comparison of polysomnographic variables and their relationship to cognitive impairment in patients with Alzheimer’s disease and frontotemporal dementia. J Psychiatr Res. 2011;45(12):1585–1592. PubMed CrossRef
- Sani TP, Bond RL, Marshall CR, et al. Sleep symptoms in syndromes of frontotemporal dementia and Alzheimer’s disease: a proof-of-principle behavioural study. eNeurologicalSci. 2019;17:100212. PubMed CrossRef
- Werdann M, Zhang Y. Circadian rhythm and neurodegenerative disorders. Brain Science Advances. 2020;6(2):71–80. CrossRef
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. PubMed CrossRef
- McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21(1):122–127. PubMed CrossRef
- de Almeida C, Carvalho L, do Prado L, et al. Perception of sound stimuli during sleep in patients with paradoxical insomnia. Neurol Disord Therap. 2018;2(1):1–5. CrossRef
- Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25(5):559–571. PubMed CrossRef
- Wasey W, Saleh S, Abernathy K, et al. Paradoxical insomnia in a frustrated patient treated with hypnotics for ten years. Cureus. 2021;13(7):e16234. PubMed CrossRef
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. PubMed
- Rochon PA, Vozoris N, Gill SS. The harms of benzodiazepines for patients with dementia. CMAJ. 2017;189(14):E517–E518. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite