Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss the diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(3):23f03644
Author affiliations are listed at the end of this article.
Have you been uncertain about how you can help your patients cut down or stop drinking alcohol? Have you been wary about prescribing psychopharmacologic agents because you had little confidence in your knowledge about their side effects, drug-drug interactions, or toxicity? If you have, the following brief vignette and discussion should prove useful.
CASE VIGNETTE
Ms D, a 35-year-old woman, presented to a substance use disorder (SUD) clinic saying that she wanted to stop drinking, as it was beginning to interfere with her relationships. She reported that she consumed 8–10 shots of vodka daily on weekends. Her body mass index was 32 kg/m2, and her medical history was notable for poorly controlled type I diabetes mellitus (with insulin doses administered on a sliding scale); her complications of diabetes included peripheral neuropathy and chronic kidney insufficiency. She also endorsed a history of an opioid use disorder (OUD) in the context of chronic pain, which was well controlled following a 30-day addiction treatment program admission and initiation of buprenorphine/naloxone (8 mg/2 mg sublingual twice a day) and gabapentin (400 mg oral administration 3 times a day) for her diabetic neuropathy, which was being managed by her primary care physician.
Ms D’s laboratory data were significant for an aspartate aminotransferase/alanine aminotransferase of 84/46 U/L, consistent with mild transaminitis, with a creatinine clearance (CrCl) of 42 mL/min. She said that she had never seen a therapist/counselor or attended a 12-step program.
DISCUSSION
How Does Drinking Alcohol Excessively Differ From Having an Alcohol Use Disorder?
Alcohol is a legal and socially acceptable substance; however, it can be misused and cause physiological dependence, addiction, and significant health-related complications. According to a recent Gallup poll, almost two-thirds (63%) of US adults (aged 18 years and older) report that they drink alcohol.1 Alcohol use may be light and intermittent, involve binge drinking, or be heavy and/or chronic. Although “excessive drinking” of alcohol is not a diagnosis, it does have specific parameters and adverse health consequences. Excessive drinking often overlaps with binge drinking and alcohol misuse, and it is usually a subclassification of an alcohol use disorder (AUD), which can be mild, moderate, or severe.
Which Complications Can Arise From Excessive Alcohol Use?
Excessive alcohol consumption is a major public health concern that contributes to more than 5 million emergency department (ED) visits annually. Moreover, it is a leading cause of preventable deaths in the United States, and it is associated with approximately 140,000 deaths each year. Medical complications of excessive alcohol use affect multiple organ systems (eg, gastrointestinal, hepatic [such as cirrhosis and alcoholic hepatitis], immune, endocrine, pulmonary, cardiovascular, hematologic, and neurological), and they can often lead to certain cancers, as well as to significant morbidity and mortality.2,3 Furthermore, excessive alcohol use is associated with thoughts of suicide, suicide attempts, motor vehicle accidents, violence, congenital abnormalities, chronic pain, and perioperative complications.2
Who Is at Risk for Alcohol-Related Complications?
Unfortunately, both genetic and environmental factors (eg, lower levels of education and income) contribute to AUDs.3 In addition, there is a notable link between AUDs and coexisting mental illnesses.3
In recent years, the COVID-19 pandemic increased alcohol consumption. This contributed to a 25% increase in alcohol-related deaths in 2020, a fact that underscored the potential for alcohol-related harm during periods of isolation and stress.4
Individuals at high risk for alcohol-related complications include those who drink heavily and chronically, those with a genetic predisposition (related to at least 7 known genetic mutations that may predispose individuals to alcohol having a higher impact on the body’s organ systems), and those with lower levels of education and income.3,4 Targeted interventions and comprehensive strategies are crucial to mitigate these risks and prevent their associated morbidity and mortality.
Which Types of Interventions Are Most Often Used to Help People Cut Down or Stop Drinking Alcohol?
A range of nonpharmacologic interventions have proved effective in helping individuals to reduce or stop their alcohol use. Many of these interventions have been developed or adapted to treat individuals across different levels of care (eg, inpatient, residential, sober homes, intensive outpatient programs, and outpatient) and at different stages of readiness to change their alcohol consumption. While nonpharmacologic treatments have historically focused on abstinence-based models (eg, 12-step programs), harm-reduction models of care are increasingly available. Harm-reduction approaches to alcohol treatment encompass an array of practices and policies that aim to minimize the negative physical, social, and/or functional impacts of alcohol use, and they typically embrace the notion that there are multiple pathways to recovery from a SUD. Accordingly, strict adherence to maintaining abstinence is not a requirement for participation. Interventions that follow a harm reduction philosophy can offer considerable benefits to individuals who are neither interested in, nor feel capable of, fully stopping their alcohol use.5
Several psychotherapeutic approaches are available for individuals with either abstinence or reduction goals. Motivational interviewing (MI) is a frequently employed and extensively researched therapeutic approach that can be used as a brief, stand-alone intervention or alongside other pharmacologic or nonpharmacologic psychotherapeutic options. MI involves a collaborative, patient-centered method of communication that explores ambivalence, enhances change-oriented self-talk, and bolsters intrinsic motivation for behavioral change. Motivational enhancement therapy (MET) encompasses MI principles and further incorporates the use of patient reported assessment data and empirically informed feedback to assist in goal setting. Cognitive-behavioral therapy (CBT) for SUD (CBT-SUD) is another common intervention that helps individuals build awareness of the antecedents and consequences of their alcohol use and learn alternative coping skills to interrupt their use patterns. Individuals often learn and practice skills to challenge unhelpful thought processes, cope with cravings, decline offers to drink, and establish personal and interpersonal boundaries. Numerous CBT derivatives have been developed. Recent versions of CBT, referred to as “Third Wave” approaches, often include a mindfulness component to assist individuals in slowing down, observing their cravings nonjudgmentally, and responding in ways that are more consistent with their values and goals. These options include acceptance and commitment therapy6 and mindfulness-based relapse prevention.7 MI and CBT-based interventions have demonstrated efficacy in reducing alcohol consumption in both individual and group therapy formats.8,9 CBT and related interventions can be particularly useful for individuals who suffer from a co-occurring mental health condition (eg, anxiety, depression, and posttraumatic stress).
Incorporating social supports is also considered a key component of initiating and sustaining positive changes around alcohol use. Accordingly, evidence supports the use of couples therapy approaches, like behavioral couples therapy,10 in which both partners develop daily routines and learn behavioral skills to reinforce changes in alcohol use. For individuals with an AUD who are unwilling to engage in treatment, but who have a loved one who is trying to assist them, community reinforcement and family training11 is an empirically supported approach that teaches caregivers skills around communication, strategies for healthy boundary setting, and effective self-care practices to use while assisting with a loved one’s recovery.
Alternatively, peer-based interventions have been efficacious in providing a supportive and sustainable recovery community. These options are not considered clinical interventions; instead, they are comprised of, and led by, others in recovery from an AUD or another SUD. In addition to longstanding 12-step approaches12 (eg, Alcoholics Anonymous), there are nonsecular options, such as SMART Recovery as well as Women for Sobriety,13 and groups that are based on Buddhist philosophies, such as Recovery Dharma and Refuge Recovery. Many hospitals, outpatient clinics, and community centers also involve individuals with lived experience into their treatment teams, referred to as recovery coaches or peer support specialists. Recovery coaches frequently provide valuable support and assistance by helping individuals navigate options for levels of care and by addressing stigma and other psychosocial factors that impede recovery. Additionally, recovery community centers are a growing nonclinical resource and are based on the philosophy that there are many pathways to recovery. These centers are peer-operated and provide an array of local psychosocial supports, which may include social activities, peer-facilitated groups (eg, nondenominational All Recovery meetings), and connection to housing, education, volunteer, and employment opportunities. A recent cross-sectional survey study found regular recovery community center participation to be associated with improved quality of life and decreased psychological distress, particularly for individuals with high levels of pathology and limited psychosocial resources.14
Finally, in severe cases of AUD, legal options are available for health care providers and/or family members to consider that compel the individual to seek treatment for their AUD. The processes and limitations for involuntary commitment or civil commitment vary by country and state. Unfortunately, evidence on the efficacy of involuntary treatment is limited. Studies in the United States and internationally suggest that involuntary treatment may offer some benefits in severe cases of AUD, in which an individual is considered at high risk to themselves or others and would not have otherwise received treatment. For instance, a recent retrospective cohort study15 conducted in New South Wales, Australia, found that patients with severe AUD who underwent a mandated 28-day hospital admission followed by voluntary aftercare support for up to 6 months showed reductions in ED and unplanned hospital admissions in the 12 months following treatment. These outcomes were comparable to those of a matched control group of patients who had participated in voluntary care.15 However, given the potential to infringe upon an individual’s rights and the resource-intensive nature of involuntary treatment, it is widely considered an option of last resort; therefore, the potential harms must be considered carefully.
AUD PHARMACOTHERAPY TOOLKIT
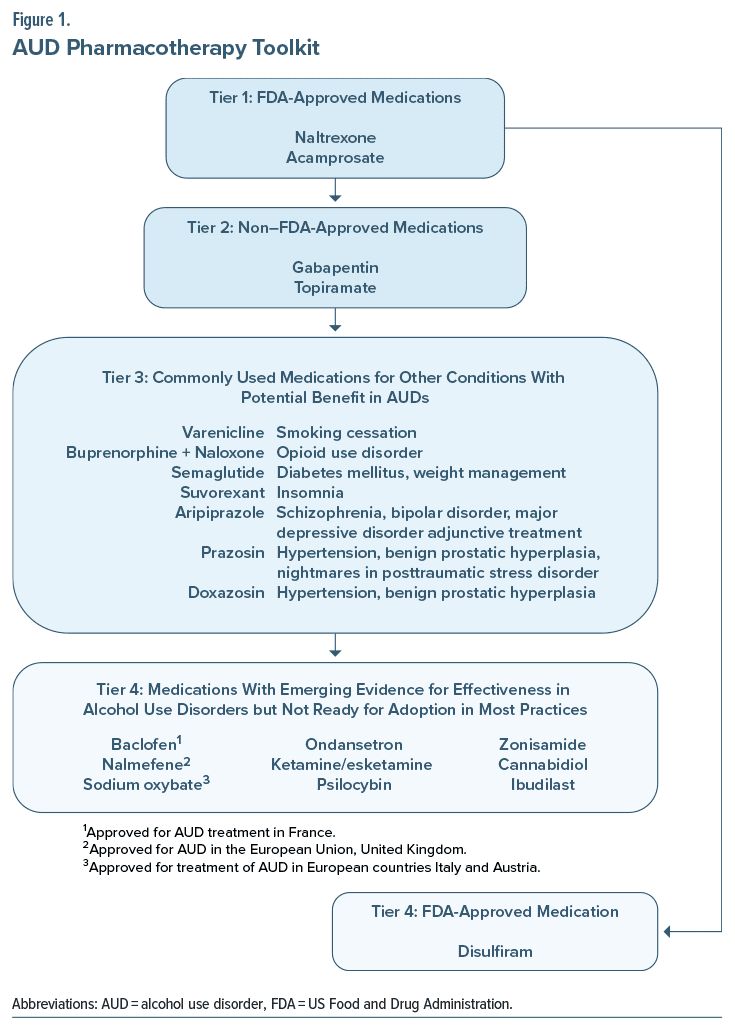
As detailed below, we discuss US Food and Drug Administration (FDA)–approved and off-label medications utilized for the treatment of AUD (Figure 1). In general, psychopharmacologic agents useful in AUD act on reward circuitry and lower arousal through modulation of γ-aminobutyric acid (GABA) and/or glutaminergic systems. In the following sections, we review FDA- and non–FDA approved medications for AUD. They are separated into tiers based on the level of evidence for the medications. Details regarding individual medications (including dosing, side effects, and relative contraindications) are provided. Tier 1 includes the first-line FDA-approved medications (ie, naltrexone and acamprosate).16,17 Tier 2 includes the 2 non–FDA-approved medications (gabapentin and topiramate), which have shown benefits in the treatment of AUD and can be used alone or in combination with FDA-approved agents and psychosocial interventions.18 Overall, however, the combination of medications for AUD, due to the synergistic effect of medications with different mechanisms of action and cautions/ contraindications/drug interactions, is hampered by a lack of evidence in the literature. Where such information is known, it is provided here. Tier 3 includes commonly used medications that are prescribed for other conditions but have potential benefits in AUD. Tier 4 includes medications with emerging evidence for effectiveness in AUD, but they are not yet ready for adoption in most practices. Tier 4 also includes disulfiram, which is FDA approved but considered a second-line agent due to its side effect profile. It has fallen out of favor due to the potential for severe reactions, including death if ingested with alcohol.
Tier 1: Which FDA-Approved Medications Can Help People Cut Down or Stop Drinking Alcohol
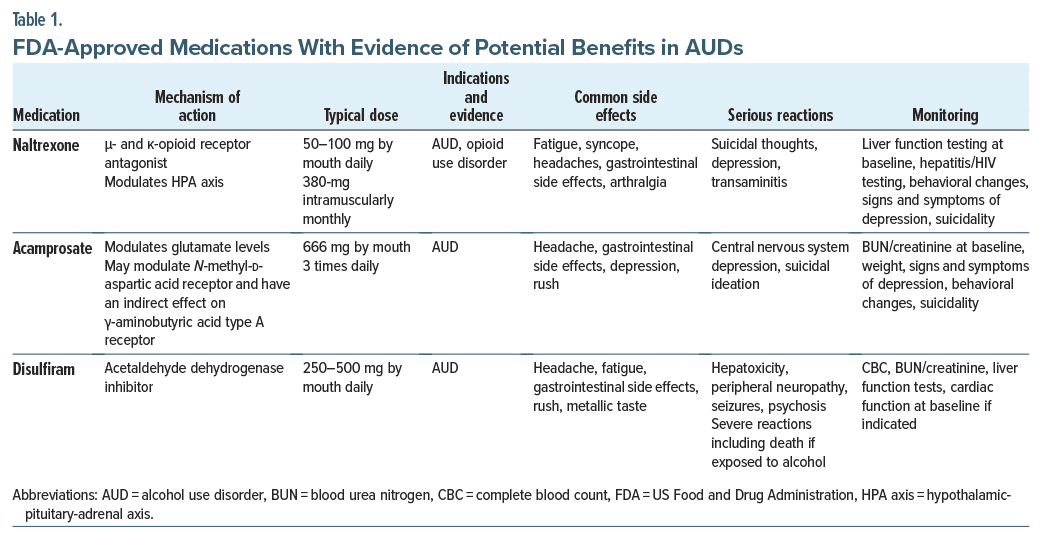
Naltrexone. Naltrexone is a μ-opioid receptor antagonist that was approved for the treatment of AUD in 1994 (Table 1). Its mechanism of action involves blocking opioid receptors; this leads to a reduction in the pleasurable effects of alcohol and to the extinction of the behavioral response to alcohol. Sinclair’s method describes the administration of naltrexone while the patient is actively drinking.19 Sinclair reviewed trials that tested naltrexone in combination with supportive therapy in support of complete abstinence and in combination with therapy that accepted potential relapses and how to cope with them.19 Although naltrexone with coping therapy had benefits, none were significant when combined with support for abstinence. However, additional studies have shown that naltrexone also reduces alcohol cravings, so that it can be used during abstinence.20,21 Recent data show that beneficial clinical responses to naltrexone might involve antagonism of κ-opioid receptors by reducing dysphoria, anxiety, and drug-seeking behaviors.22,23 In addition, naltrexone affects alcohol consumption through modulation of the hypothalamic-pituitary-adrenal axis.24 Naltrexone might be more effective for individuals who consume alcohol for pleasurable/rewarding effects rather than to relieve negative states.25
Naltrexone is rapidly absorbed following oral administration; however, it undergoes significant first pass metabolism, and its bioavailability ranges from 5% to 40%. Naltrexone’s half-life is 3.9–10.3 hours.26
Typical oral daily dosing for adults is 50 mg. It is also available in a depot formulation, Vivitrol, a 380-mg monthly intramuscular suspension. Vivitrol tends to be more effective than oral naltrexone when the risk of poor adherence is high.27 Given its opioid receptor antagonism, emergency pain management of patients on the depot formulation can be challenging, and patients receiving Vivitrol are instructed to carry a card indicating that they are taking it. For emergent pain management in patients on Vivitrol, use of regional anesthesia and non–opioid pain medications (such as nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentin or pregabalin, ketamine, lidocaine, and duloxetine) and nonpharmacologic management is recommended. Postoperative pain management may be complicated by Vivitrol, so that future surgical procedures might be timed to correspond to a Vivitrol washout period and augmented with oral naltrexone. Discontinuation of Vivitrol may lead to a vulnerability for relapse. Current recommendations are to discontinue oral naltrexone at least 72 hours before surgery to allow 5 half-lives to pass, resulting in elimination of nearly 98% of the naltrexone.
Recent evidence also shows that naltrexone is safe in patients with low-level underlying liver disease and compensated cirrhosis.28 However, naltrexone should be avoided in those with acute liver failure or decompensated cirrhosis. Naltrexone’s association with anhedonia29 can interfere with adherence to naltrexone.
Naltrexone’s most crucial drug-drug interaction is with full opioid agonists, as it can precipitate opiate withdrawal. Overall, naltrexone is well tolerated and widely used as a first-line treatment of AUD.
Acamprosate. Acamprosate was FDA approved for the treatment of AUD in 2004. Its presumed mechanism of action is through the glutamatergic system.30 Chronic heavy alcohol use leads to a compensatory increase in glutamate concentration, which is associated with hyperexcitation (and to symptoms of anxiety, insomnia, and restlessness). By lowering glutamate levels, acamprosate reduces arousal, anxiety, and insomnia.31 Acamprosate has been especially helpful for individuals who consume alcohol to alleviate negative symptoms, such as those of early withdrawal.32 Moreover, data suggest that acamprosate is more effective for supporting abstinence than is naltrexone.33,34
With oral administration, acamprosate’s bioavailability is 11%; with 3 times/day administration, it reaches a steady state in 5 days. Since it is primarily renally excreted, its dosing needs to be reduced in those with moderately severe renal disease (ie, with a CrCl of 30–50 mL/min), and its use is contraindicated in those with severe renal disease (ie, with a CrCl<30 mL/min). After a steady-state concentration is achieved, the half life increases to 20–33 hours.30,31 The typical oral dose is 666 mg (administered as two 333-mg delayed-release tablets), administered 3 times a day. The need for frequent dosing often leads to poor adherence.
Acamprosate is usually well tolerated, and it has a relatively mild side effect profile. Since it is not metabolized by the liver, it is safe to administer acamprosate to patients with liver disease. Fortunately, it lacks significant drug-drug interactions. Along with naltrexone, it is a first-line treatment for AUD.
A recent systematic review by McPheeters and colleagues17 revealed that the number needed to treat to prevent one person from returning to alcohol use was 11 for acamprosate and 18 for oral naltrexone at 50 mg daily.17 Their findings supported the use of oral naltrexone and acamprosate as first-line pharmacotherapies for AUD.17
Tier 4: Disulfiram
The negative interaction between disulfiram and alcohol was first noticed among rubber factory workers who drank alcohol. Following similar observations during trials of disulfiram as a vermicide, it was studied as a treatment for AUD and was approved by the FDA in 1951.35
Disulfiram’s mechanism of action results from its inhibition of a key enzyme in ethanol metabolism (acetaldehyde dehydrogenase) that breaks down acetaldehyde to acetate. When disulfiram blocks acetaldehyde dehydrogenase, acetaldehyde accumulates, causing nausea, vomiting, headache, flushing, hypotension, and tachycardia. In cases of severe acetaldehyde toxicity, death may ensue. Given disulfiram’s adverse reaction with alcohol, abstinence from alcohol use is facilitated by operative conditioning.36 When disulfiram is administered orally, its bioavailability is high (80%–90%); moreover, it is lipophilic and is metabolized by the liver. It has a half-life of 7.5 hours; however, adverse reactions can occur up to 2 weeks after administration. Unfortunately, its plasma levels are highly variable, and in some patients, it fails to induce a noticeable interaction with alcohol even at therapeutic doses, while in others it can induce severe toxicity at low therapeutic doses.15 Initially, disulfiram was prescribed at higher doses (eg, 1,500–3,000 mg/d); however, due to reports of severe reactions and deaths, the recommended daily dose was lowered to 250–500 mg.
To improve adherence, depot formulations of disulfiram were introduced in the 1960s. However, due to a combination of health and ethical concerns, its use has not been adopted in the United States.37
Before disulfiram is initiated, the patient needs to be abstinent from alcohol for at least 12 hours. Since an adverse reaction can arise up to several weeks following disulfiram’s discontinuation, ongoing caution (and abstinence) is warranted. In addition, since adverse interactions with products for oral use that contain alcohol (eg, mouthwashes) and even topical products (eg, hand sanitizers) have been reported, patients should be educated about these adverse interactions.38,39 Despite being the first to be approved by the FDA for AUD, disulfiram is not recommended as a first-line treatment for AUD, and it should be avoided in individuals with serious medical comorbidities, especially with cardiovascular disease, prompting it to be placed as Tier 4. Table 1 provides an overview of FDA-approved medications with evidence of potential benefits in AUD.
Which Other Medications Have Been Used (but have not been FDA approved) to Help People Cut Down or Stop Drinking Alcohol?
FDA-approved medications for AUD focus on decreasing the positive or rewarding effects of alcohol use.18 However, many of those who drink alcohol care more about avoiding the negative effects associated with acute alcohol withdrawal than reducing the rewarding effects of alcohol.18 Decisions regarding these medications should factor in other comorbid conditions that have FDA indications or evidence of potential benefits in AUD that may guide the choice of treatment, as detailed in Figure 1 and Tables 2 and 3.
Tier 2: Non–FDA-Approved Medications With Evidence of Potential Benefits in AUD
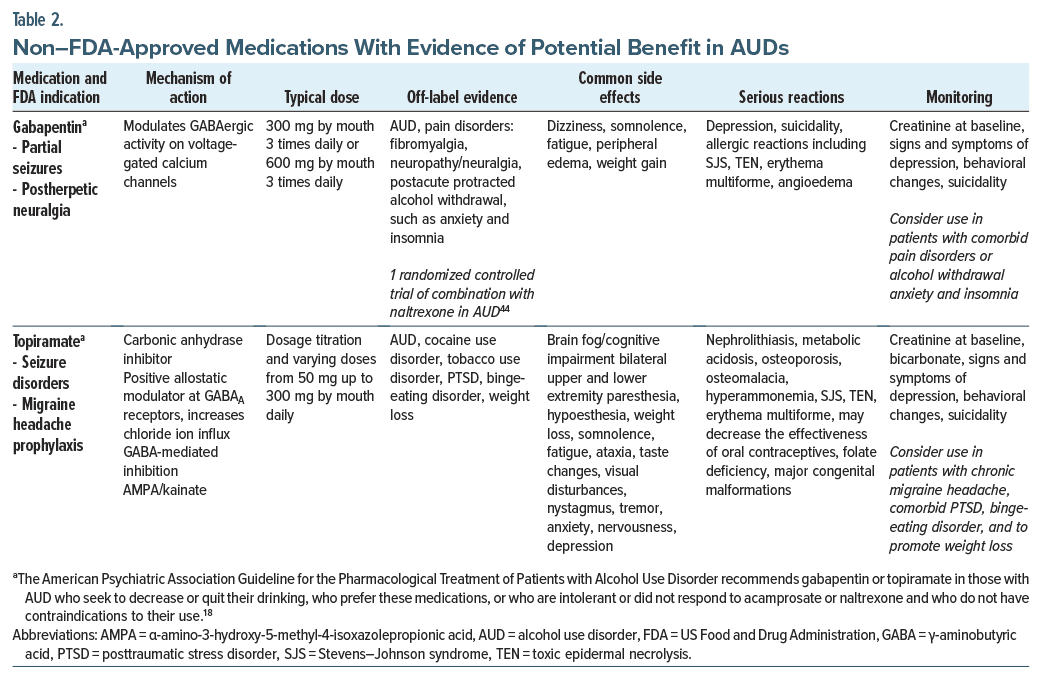
Gabapentin. Gabapentin, which is thought to act on voltage-gated calcium channels,40 has been FDA approved for the treatment of partial-onset seizures, postherpetic neuralgia, and restless legs syndrome.41 However, it has also been trialed in AUDs. In a meta-analysis of
7 randomized controlled trials (RCTs), gabapentin (with a dose range from 600 to 3,600 mg/d) outperformed placebo; the only significant difference was in the percentage of heavy drinking days.41 Since gabapentin is not significantly metabolized by the liver and instead is excreted by the kidneys, it can be used in those with acute or chronic liver disease. Gabapentin should be used with caution if a patient is receiving either a benzodiazepine or phenobarbital (eg, as part of a withdrawal protocol), as its effects are synergistic with these medications. Those who received 1,800-mg doses had significantly fewer relapses to drinking and had higher rates of abstinence when compared with placebo, while those treated with a 900-mg dose had an intermediate effect.18 Moreover, gabapentin was well tolerated18; however, at higher doses, it may result in dizziness, somnolence, ataxia, gait disturbances, and peripheral edema.40,41 In addition, gabapentin can be misused and abused.40,41 Given their structural similarities and adverse effects, prescribing gabapentin and pregabalin together is relatively contraindicated.
Topiramate. Topiramate has been used to treat seizure disorders and prevent migraines and, along with phentermine, contributes to weight loss.18,41,42 It has also shown benefits in cocaine use disorder, tobacco use disorder, posttraumatic stress disorder (PTSD), and binge-eating disorder.42
Topiramate is thought to act on the prefrontal cortex18 and to exert its effects on the dopaminergic pathways from the ventral tegmental area to the nucleus accumbens, by enhancing GABAergic neurotransmission and antagonizing glutamatergic transmission, thereby leading to a decrease in dopamine in the nucleus accumbens.42 Topiramate works by inhibiting carbonic anhydrase through non–benzodiazepine-binding sites on GABAA receptors.40,42 In addition, topiramate may counteract excitability that may result from elevation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/ kainite receptor subunits in the hippocampus, orbital frontal cortex, and anterior cingulate cortex.43 Topiramate may cause a myriad of side effects and rare but significant adverse events that are detailed in Table 2. Topiramate can induce cytochrome P450 (CYP) 3A4 and inhibit CYP2C19; however, interactions with other medications, including anticonvulsants and psychotropics, are minimal, except in combination with valproic acid.42 Topiramate may decrease the efficacy of oral contraceptives and is associated with the risk of major congenital malformations (including cleft palate), and it is classified as a category D drug in pregnancy.42 Due to its side effect profile, lower-dose regimens (from 50 mg daily) have been studied, up to 200 or 300 mg, with a dosage titration schedule over 4–6 weeks. A starting dose of 50 mg daily in divided doses and a dose increase by 50 mg/d per week over 6–8 weeks may help to minimize adverse effects.42 Dosing at bedtime, instead of following a twice-daily schedule, may help to decrease cognitive impairment. Topiramate has been used in heavy drinkers even when their goal is not abstinence.41,42 The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder (2017) recommended gabapentin or topiramate in those with an AUD who seek to decrease or quit their drinking, who prefer these medications, who are intolerant or did not respond to acamprosate or naltrexone, and who do not have contraindications to the use of gabapentin or topiramate.18
Combined treatment. Given that naltrexone works by reducing cravings and blunting the pleasurable effects of alcohol, while acamprosate restores balance in the glutaminergic system, different mechanisms of action can function in a complementary fashion. Some evidence shows that this combined treatment is more effective and well tolerated.34 Additionally, clinical experience regarding the mechanism of action of gabapentin and topiramate within AUD treatment suggests that a combination of these 2 medications along with naltrexone may be relatively well tolerated despite lack of significant literature. There is 1 RCT by Anton and colleagues of 150 subjects with AUD evaluating naltrexone versus combination of naltrexone and gabapentin that showed during the first 6 weeks that the naltrexone-gabapentin group improved drinking outcomes over naltrexone alone with a longer interval to heavy drinking that did not endure after gabapentin discontinuation.44
Tier 3: Commonly Used Medications for Other Conditions With Potential Benefits in AUD
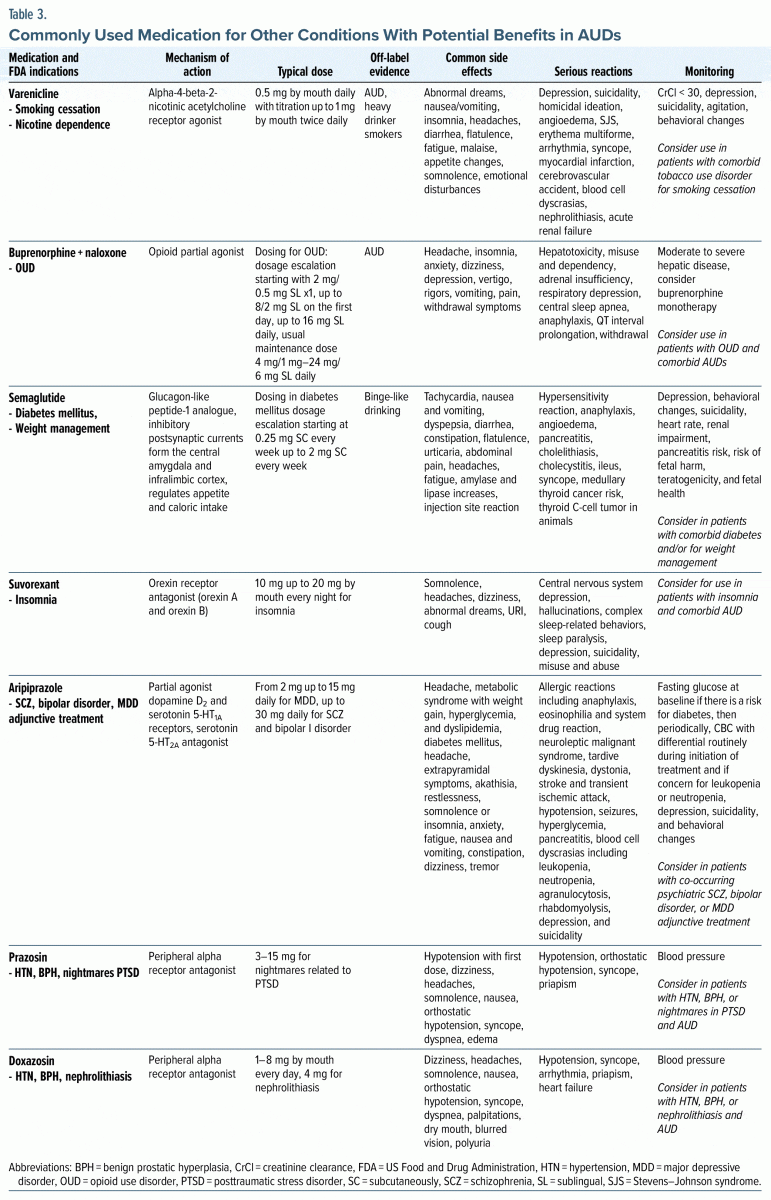
Varenicline. Varenicline is an α-4-β-2-nicotinic acetylcholine receptor agonist medication that is FDA approved for smoking cessation. The typical dosage used for smoking cessation is 0.5 mg/d with titration up to 1 mg by mouth twice daily. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinical Investigations Group found that it reduced alcohol consumption and craving among patients with AUD.18,45 Varenicline may work by partially stimulating nicotinic acetylcholine receptors, a promising molecular target implicated in patients with both nicotine disorders and AUDs.18,45 Overall, varenicline has been well tolerated.18,45
Buprenorphine-naloxone. Buprenorphine, in combination with naloxone, is FDA approved for the treatment of OUDs. Buprenorphine acts as a partial agonist at the μ-opioid and nociceptin/orphanin (NOP) receptors.46 Buprenorphine at higher doses decreases alcohol intake by activating NOP receptors.46 When extended-release naltrexone versus buprenorphine in combination with naloxone was studied for relapse prevention in OUD,47 treatment with the buprenorphine naloxone or long-acting injectable antagonist naltrexone (XR-NTX) produced a similar decreased risk of relapse in those with an OUD who inject opioids. XR-NTX decreases the risk of relapse and binge drinking in those with AUD through its action in the brain reward pathway in the basal ganglia.18,47 Secondary analysis examined whether patients with an OUD also experienced a reduction in drinking or heavy drinking. They found that both treatment groups reduced drinking from baseline to posttreatment with no differences between groups. There was an overall reduction in drinking in both XR-NTX and buprenorphine/naloxone groups. The results of a study provided the first clinical evidence of the potential efficacy of high-dose buprenorphine for the suppression of alcohol intake in patients with OUD and a comorbid AUD. Rare but serious effects include hepatotoxicity, misuse, dependency, adrenal insufficiency, respiratory depression, central sleep apnea, QTc interval prolongation, anaphylaxis, and withdrawal. Monitoring liver function should be performed in those with moderate to severe hepatic disease.48
Semaglutide. Semaglutide is a glucagon-like peptide-1 (GLP-1) analogue that is FDA approved for the treatment of diabetes mellitus and weight loss for those with obesity or those who are overweight and have comorbidities (such as high blood pressure and cardiovascular disease). It acts on inhibitory postsynaptic currents from the central amygdala and infralimbic cortex, regulating appetite and caloric intake. There is growing evidence that the GLP-1 system is involved in addictive behaviors, with anecdotal reports of unexpected decreases in urges for alcohol, cigarettes, and nail biting. A recent National Institutes of Health and joint National Institute on Drug Abuse/NIAAA preclinical trial examined the effect of semaglutide in binge-like drinking and dependence-induced alcohol drinking49 and found that semaglutide reduced binge-like alcohol use in a dose dependent fashion. Side effects may be common and may include serious reactions that are detailed in Table 3. Monitoring is recommended for depression, behavioral changes, and suicidality, as well as alterations in heart rate, renal impairment, and pancreatitis. There is also a risk of fetal harm, teratogenicity, and pregnancy loss.
Suvorexant. Suvorexant is a dual orexin receptor antagonist at orexin A and orexin B used for the treatment of insomnia with a dose of 10–20 mg at bedtime.40,50,51 The orexin/hypocretin system is involved in sleep-wake regulation, and more recently, it has been implicated in the treatment of AUD.40,50,51 Orexin neuropeptides are concentrated in the lateral hypothalamus.51 Orexins A and B bind to G-protein–coupled orexin receptors. Dense orexin projections are found from the lateral hypothalamus to the ventral tegmental area as part of the brain reward pathway, providing support that orexins may impact the rewarding effects of alcohol.40 The orexin system also regulates stress and has been identified as a potential brain circuit in the treatment of AUD, especially given the sleep cycle disruptions seen in patients with AUD.50,51 Most current treatments of AUD focus on the brain reward pathway in relapse prevention, rather than on sleep disruption.51
Aripiprazole. Aripiprazole is an atypical antipsychotic that acts as a partial agonist at dopamine D2 and 5- hydroxytryptamine (5-HT) 1A receptors and an antagonist at the 5-HT2A receptor.40 It is FDA approved for the treatment of schizophrenia and bipolar I disorder and as an adjunctive treatment for major depressive disorder. The modulation of the dopaminergic brain reward pathway involving the ventral tegmental area and nucleus accumbens is central in the management of AUD and SUDs. As a partial agonist at dopamine D2 receptors, aripiprazole has a unique pharmacodynamic profile. Aripiprazole is thought to promote abstinence and alcohol-seeking behavior in patients with AUD, potentially through dopaminergic and serotoninergic changes in the brain reward pathway, as well as through decreased alcohol-related anxiety, low mood, and anhedonia.52 A small human laboratory study of healthy participants found that aripiprazole affected subjective response to alcohol consumption, including reduced euphoric effects of alcohol and increased sedative effects.40 An RCT of patients with AUD showed that aripiprazole attenuated cue-induced neural activation in the ventral striatum, part of the brain reward pathway.40 A recent clinical laboratory study of 99 participants with AUD found that aripiprazole reduced the number of drinks consumed in a bar lab setting, especially among those with low self-control.40 It also prolonged the latency to drink in those with high impulsivity.40 Overall, the results from clinical trials of aripiprazole in AUD were mixed.40 In review of the available studies, Burnette and colleagues40 suggested that aripiprazole may be more effective at lower doses and in patients with more impulsive drinking.
Prazosin and doxazosin. Prazosin and doxazosin are α-1 adrenergic receptor antagonists that are FDA approved for the treatment of hypertension, while prazosin is used off label for the treatment of nightmares associated with PTSD and benign prostatic hypertrophy (BPH). Doxazosin is FDA approved for the treatment of BPH and is used off-label for the treatment of kidney stones. Prazosin and doxazosin have similar structures and readily cross the blood-brain barrier where they block noradrenergic excitation of the mesolimbic dopaminergic system.40 Alpha-adrenergic receptors regulate the sympathetic nervous system, the fight or flight response pathway, through activation of the neurotransmitter norepinephrine.40 Chronic alcohol use disrupts stress physiology particularly early on during abstinence.40 Individuals with AUD in early abstinence may experience more emotional dysregulation, stress, and alcohol cravings, which increases the risk of relapse.40 Prazosin and doxazosin may help to normalize these stress response changes seen in patients with AUD. A small 6-week pilot study in individuals with AUD showed that prazosin treatment was associated with fewer drinking days per week and fewer drinks per week compared to placebo.40 A larger double-blind study of prazosin in individuals with AUD showed that prazosin participants had greater reductions in heavy drinking and rates of drinking over time, although with a modest effect size.40
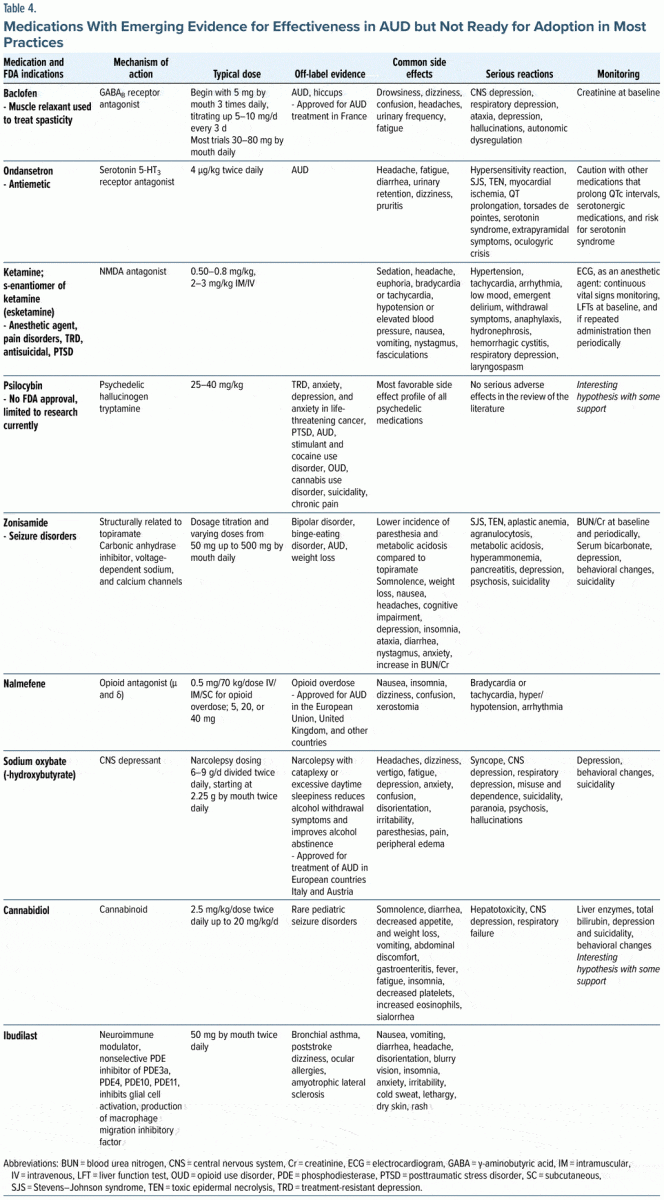
Tier 4: Medications With Emerging Evidence for Effectiveness in AUD but Not Ready for Adoption in Most Practices
Baclofen. Baclofen is a GABAB agonist that is used as a muscle relaxant and to relieve spasticity related to multiple sclerosis and spinal cord injuries.40,53,54 Baclofen exerts its effects on GABAB receptors on presynaptic and postsynaptic neurons in the central and peripheral nervous system, resulting in inhibition of transmission of mono- and polysynaptic reflexes at the spinal cord, easing muscle spasticity.54 It has been used off-label for the management of musculoskeletal pain, hiccups, gastroesophageal reflux disease, and other disorders. Baclofen use may encourage abstinence, has relative safety in those with advanced liver disease, and has been approved for AUD treatment in France.53–55 Its use may be associated with tolerance and with withdrawal phenomenon.40,54 Numerous RCTs investigating the use of baclofen in AUD have had mixed results.53–55 Baclofen has shown increased rates of abstinence, time to first relapse, and possibly reduced heavy drinking days.40,53,54 The Cagliari Expert Consensus Group recommended starting with an oral baclofen dose of 5 mg by mouth 3 times daily with a gradual titration by 5–10 mg every 3 days to the fixed dose of 30–80 mg daily.54,55 Baclofen has minimal hepatic metabolism, and it is considered safe in those with liver disease.40,54,55
Ondansetron. Ondansetron is a serotonin 5-HT3 antagonist used to treat nausea and vomiting; it has shown promise in those with AUDs, especially in patients with early-onset AUD.40,56 Although the exact mechanism of its action in AUD is not fully elucidated, it may address serotonergic dysfunction that is commonly seen in early onset AUD.40,56 Serotonin 5-HT3 projections to dopaminergic connections in the midbrain may lead to decreased alcohol cravings and a decreased dopaminergic response in the brain’s reward pathway in the nucleus accumbens.40 Ondansetron was well tolerated with only mild side effects.56 Caution should be used when combining ondansetron with other medications that prolong the QTc interval, or with co-prescription of serotonergic medications due to the risk for serotonin syndrome.
Ketamine. Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist. Ketamine, and its s-enantiomer, esketamine, are used as anesthetics, as well as for pain disorders, treatment-resistant depression (TRD) with antisuicidal properties, and PTSD.57–59 Depressive symptoms are common in individuals with AUD and those who present with alcohol withdrawal symptoms and may be a risk for a return to drinking and alcohol relapse in those who enter treatment.57 Ketamine may temporarily relieve depressive symptoms during the acute withdrawal phase where negative effect may be pronounced.18,57
Psilocybin. Psilocybin is a schedule I substance with high abuse potential. It is a classic psychedelic substance categorized as a tryptamine hallucinogen derivative of N,N dimethyltryptamine with serotonergic and dopaminergic properties.60 Psilocybin has been used for TRD, anxiety, depression, and anxiety in life-threatening cancer, PTSD, AUD, stimulant and cocaine use disorder, OUD, and cannabis use disorder, as well as suicidality and chronic pain.60 Psilocybin is found naturally in psilocybin-containing mushrooms. A recent double-blind RCT compared 2 administrations of high-dose psilocybin versus diphenhydramine in combination with manualized psychotherapy using MET and CBT in patients with AUD and at least 4 heavy drinking days during the 30 days prior to screening.61 The active arm of the study received psilocybin 25 mg/70 kg for the first session and psilocybin 25–40 mg/70 kg for the second session. The percentage of heavy drinking days during the 32-week double-blind period was significantly lower (P = .01) for the psilocybin group. The psilocybin group also had a lower mean level of daily alcohol consumption. There were no serious adverse events in those who received psilocybin, consistent with a recent review on psilocybin’s therapeutic potential.60,61
Zonisamide. Zonisamide is FDA approved for the treatment of seizure disorders, and it is used off-label for a bevy of psychiatric disorders (eg, bipolar disorder and AUDs).62 Typical doses of zonisamide range from 200 to 600 mg daily. Zonisamide shares structural features with topiramate and has a similar mechanism of action to inhibition of carbonic anhydrase inhibition, with potential benefits in migraine prevention, weight loss, and decreased alcohol use.43 Zonisamide may have a lower incidence of paresthesia and metabolic acidosis than topiramate.43,62 In a study comparing zonisamide, topiramate, and placebo, treatment with zonisamide and topiramate each produced significant reductions in alcohol consumed per day, percent of days drinking, and percent of days with heavy drinking.62 Zonisamide may have efficacy in the treatment of AUD, with effect sizes similar to topiramate. Both zonisamide and topiramate may cause cognitive impairment and modest reductions of verbal fluency and working memory.62
Nalmefene. Nalmefene is approved for the treatment of AUD in the European Union, the United Kingdom, and other countries.18,40 Like naltrexone, it acts as an antagonist at the μ- and δ-opioid receptors. It is also a κ-opioid receptor partial agonist.18,40 Nalmefene interrupts the binge intoxication phase of relapse and appears to exert its effect in the basal ganglia, nucleus accumbens, and brain reward pathway through its κ-agonist activity, thereby diminishing the rewarding effects of alcohol.18 Through its κ-agonist activity, it may decrease anxiety and dysphoria that is associated with not drinking in those with AUD.18 Nalmefene was approved by the European Medications Agency ([EMA], the FDA equivalent in Europe) in 2013 for the reduction of alcohol consumption among patients with AUD, following 3 clinical trials where nalmefene reduced the number of heavy drinking days and the total alcohol consumed.40 Adverse events were frequent and are detailed in Table 4.63
Whereas most medications for AUD are taken daily, nalmefene is only taken as needed when there is a risk that they would start drinking (with only 1 tablet taken daily, preferably 1–2 hours before they are likely to start drinking).18 The typical dosage used in research trials varied from 5, 20, to 40 mg; however, the dosage prescribed is 18 mg daily as needed.53
Sodium oxybate. Sodium oxybate (SMO), better known as γ-hydroxybutyric acid (GHB), has a history of recreational use and misuse with distinctive intoxication and withdrawal phenomena. SMO has received approval for the treatment of AUD in European countries, but not from the FDA or the EMA.40 It is widely employed as a treatment for alcohol withdrawal and for maintenance of abstinence in Italy and Austria. The structure of SMO is like that of GABA. It exerts a similar effect to alcohol and binds as a partial agonist to GABAB receptors and indirectly through GHB-derived GABA.40 Severe intoxication and death with GHB can be seen when used recreationally; however, its clinical use is felt to be safe.40 A Cochrane meta-analysis of 13 RCTs found that SMO was effective in treating alcohol withdrawal syndrome and preventing relapses in patients who were previously detoxified.40 In addition, SMO was found to be more effective than naltrexone or disulfiram in maintaining abstinence.40 A recent review examined the role of SMO in AUD treatment and reduction of alcohol consumption.64 A large double-blind, randomized, placebo-controlled trial in detoxified patients with AUD from 11 sites in 4 European countries was performed (randomized to 6 months of treatment with SMO at 3.3–3.9 g/d vs placebo, followed by a 6-month medication-free period) showed efficacy in cumulative abstinence duration during the 6-month treatment period with a sustained effect during the 6-month medication-free period.65
Cannabidiol. Cannabidiol (CBD) is a nonpsychoactive cannabinoid found in Cannabis sativa that acts as a negative allosteric modulator of CB1 and CB2 receptors.40 CBD blocks anandamide uptake and inhibits its enzymatic hydrolysis.40,66 It also acts as an agonist primarily at 5-HT1a serotonin receptors and to a lesser extent at 5-HT2a receptors.66 CBD has also been found to be an allosteric modulator at μ- and δ-opioid receptors.67 CBD is being touted as a panacea for numerous health conditions; however, only 1 CBD product has been approved by the FDA for the treatment of rare pediatric seizure disorders.68 CBD is generally well tolerated and does not interact with the subjective effects of alcohol.40 It exhibits no effects indicative of abuse or dependence.40 A recent systematic review evaluated the potential therapeutic benefit of CBD as a possible treatment of AUD and noted preclinical and clinical evidence in other SUDs and active clinical trials in AUD, AUD with comorbid PTSD, and alcohol withdrawal in AUD.40,69
Ibudilast. Ibudilast is an inhibitor of phosphodiesterase-3, -4, -10, and -11 and a macrophage migration inhibitory factor, which shows promise as a novel treatment for AUD.40,70 Ibudilast received approval for the treatment of asthma in Japan more than 20 years ago. In addition, it is being studied in poststroke dizziness, ocular allergies, amyotrophic lateral sclerosis, progressive multiple sclerosis, and pain disorders. A recent study examined the efficacy of ibudilast to improve negative mood, reduce heavy drinking, and attenuate neural reward signals in individuals with AUD.70 Although ibudilast did not significantly impact negative mood, it did reduce the odds of heavy drinking by 45% and attenuated alcohol cue-elicited activation in the ventral striatum (which is the brain region most closely associated with reward).70 The anti inflammatory and neuroprotective effects of ibudilast are thought to be part of the underlying molecular mechanism of action of ibudilast in treating AUD.40,70
N-acetylcysteine. The main central nervous system (CNS) excitatory neurotransmitter, glutamate, works in balance with GABA, the main CNS inhibitory transmitter. Chronic alcohol use and binge drinking disrupt the GABA glutamate balance, where cessation of drinking leads to excess glutamate excitation at NMDA receptors, leading to increased glutamate in the alcohol withdrawal syndrome.40 N-acetylcysteine (NAC) is an over-the-counter supplement marketed for use in numerous health conditions without significant scientific evidence. It is the treatment of choice to prevent or limit liver injury in acetaminophen toxicity/ overdose by providing cysteine for glutathione synthesis. NAC is a cysteine prodrug that works to restore glutamatergic homeostasis in the brain reward pathway by improving the expression and function of the cysteine glutamate exchanger and normalizing glial glutamate transporters.40 A recent meta-analysis of NAC compared to placebo showed reduced craving across a number of SUDs.40,71 In individuals with cannabis use disorder, NAC was shown to increase abstinence rates and to reduce drinks per week.40 The recommended dosage of NAC ranges from 600 mg twice daily up to 4 times daily.
Which Medications Have Been Used (but have not been FDA approved) to Help People Cut Down or Stop Drinking Alcohol?
For a summary of non–FDA-approved agents that have been used to help people cut down or stop their use of alcohol (along with their mechanisms of action, adverse effects, and evidence for efficacy), see Table 2.
What Happened to Ms D?
Given that Ms D was being treated with buprenorphine/ naloxone for the management of pain and an OUD, naltrexone was contraindicated. Moreover, since a severe reaction to alcohol (eg, hospitalization and death) may develop when disulfiram is used, it should be avoided in patients (like Ms D) with extensive medical comorbidities. However, acamprosate, at a reduced dose, was considered (with careful monitoring of her) renal function.
Following a careful discussion of the risks and benefits of pharmacologic treatment, Ms D decided not to take additional medication for her AUD. Instead, she met with a recovery coach and began to attend meetings of Alcoholics Anonymous. Now, 6 months later, Ms D remains abstinent from alcohol.
CONCLUSION
Excessive alcohol consumption is a major public health concern that contributes to more than 5 million ED visits annually and approximately 140,000 deaths each year. Fortunately, targeted interventions and comprehensive strategies are available to mitigate these risks and prevent their associated morbidity and mortality. While nonpharmacologic treatments have historically focused on abstinence-based models (eg, 12- step programs), harm-reduction models of care are increasingly available. MI is a frequently employed and extensively researched therapeutic approach that can be used as a brief, stand-alone intervention or alongside other pharmacologic or nonpharmacologic psychotherapeutic options. MI involves a collaborative, patient-centered method of communication that explores ambivalence, enhances change-oriented self talk, and bolsters intrinsic motivation for behavioral change. CBT for SUDs is another common intervention that helps individuals build awareness of the antecedents and consequences of their alcohol use and learn alternative coping skills to interrupt their use patterns. In severe cases of AUDs, legal options are available for health care providers and/or family members to compel the individual to seek treatment for their AUD. Since the processes and limitations for involuntary commitment or civil commitment vary by county and state, practitioners should learn the local options and regulations. In addition, there is a bevy of FDA-approved and non–FDA-approved agents that have helped people cut down and stop their alcohol use.40,72 Knowledge of their mechanisms of action, side effects (eg, common, rare, and serious), and drug-drug interactions will facilitate their timely and appropriate use in those who would stand to benefit from them.
Although there is significant evidence to support the use of pharmacologic and nonpharmacologic treatments for patients with AUD, only a small fraction of those with AUD receive appropriate treatment. Only 7%–8% of individuals with AUD received any treatment in 2019, while less than 2% were prescribed an FDA-approved medication for the treatment of AUD.18 Effective treatments are available but can only help if they are used.18 Recommendations for FDA-approved medications, such as naltrexone and acamprosate, are appropriate as first-line treatments based on their relative contraindications and benefits. Naltrexone as a once daily medication may offer benefits for those who have problems with adherence to medications that require 3 times daily dosing. Further, long-acting naltrexone may provide the benefit of monthly injections over medications that are dosed frequently. In addition, the American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder (2017) recommended gabapentin or topiramate for those with an AUD who seek to decrease or quit their drinking, who prefer these medications, who are intolerant or did not respond to acamprosate or naltrexone, and who do not have contraindications to the use of gabapentin or topiramate.18
Knowledge of nonpharmacologic approaches as well as medications’ mechanisms of action, side effects (eg, common, rare, and serious), and drug-drug interactions can facilitate the timely and appropriate use of psychotropics to help individuals cut down or stop their use of alcohol.
Article Information
Published Online: May 28, 2024. https://doi.org/10.4088/PCC.23f03644
© 2024 Physicians Postgraduate Press, Inc.
Submitted: September 14, 2023; accepted January 16, 2024.
To Cite: Matta SE, Terechin O, Podesta A, et al. Strategies for cutting down and stopping alcohol use. Prim Care Companion CNS Disord. 2024;26(3):23f03644.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Matta, Lento, Stern); Department of Psychiatry, Mass General Brigham Salem Hospital, Salem, Massachusetts (Terechin); Department of Psychiatry, Tulane University School of Medicine, New Orleans, Louisiana (Podesta).
Corresponding Author: Sofia E. Matta, MD, Home Base, a Red Sox Foundation and Massachusetts General Hospital Program, One Constitution Road, Suite 140, Charlestown, MA 02129 ([email protected])
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- Excessive alcohol consumption is a major public health concern that contributes to more than 5 million emergency department visits annually and approximately 140,000 deaths each year.
- Targeted interventions (both nonpharmacologic [eg, motivational interviewing, 12-step programs, and cognitive-behavioral therapy] and pharmacologic [eg, disulfiram, naltrexone, acamprosate, topiramate, and gabapentin]) are available to mitigate the risks of excessive alcohol use and prevent their associated morbidity and mortality.
- Knowledge of the mechanisms of action, side effects (eg, common, rare, and serious), and drug-drug interactions of psychotropics that can help individuals cut down or stop drinking will facilitate their timely and appropriate use in those who would stand to benefit from them.
References (72)

- What percentage of Americans drink alcohol? Gallup. December 29, 2022. Accessed December 8, 2024. https://news.gallup.com/poll/467507/percentageamericans-drink-alcohol.aspx.
- NIAAA Core Resource on Alcohol. Medical complications: common alcohol related concerns. [Last Revised April 10, 2023]. Accessed December 8, 2024. https://www.niaaa.nih.gov/health-professionals-communities/coreresource-on-alcohol/medical-complications-common-alcohol-relatedconcerns#pub-toc1
- Nehring SM, Chen RJ, Freeman AM. Alcohol use disorder. In: StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed December 8, 2024. https://www.ncbi.nlm.nih.gov/books/NBK436003
- NIH COVID-19 research: News and Stories. Alcohol use during the COVID-19 pandemic. April 3, 2023. Accessed December 8, 2024. https://covid19.nih.gov/news-and-stories/alcohol-use-during-covid-19-pandemic
- Witkiewitz K, Heather N, Falk DE, et al. World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom. Addiction. 2020;115(9):1668–1680. PubMed CrossRef
- Lee EB, An W, Levin ME, et al. An initial meta-analysis of Acceptance and Commitment Therapy for treating substance use disorders. Drug Alcohol Depend. 2015;155:1–7. PubMed CrossRef
- Ramadas E, Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133. PubMed CrossRef
- Santa Ana EJ, LaRowe SD, Gebregziabher M, et al. Randomized controlled trial of group motivational interviewing for veterans with substance use disorders. Drug Alcohol Depend. 2021;223:108716. PubMed CrossRef
- Magill M, Ray L, Kiluk B, et al. A meta-analysis of cognitive-behavioral therapy for alcohol or other drug use disorders: treatment efficacy by contrast condition. J Consult Clin Psychol. 2019;87(12):1093–1105. PubMed CrossRef
- O’Farrell TJ, Fals-Stewart W. Behavioral couples therapy for alcoholism and drug abuse. J Subst Abuse Treat. 2000;18(1):51–54. PubMed
- Meyers RJ, Roozen HG, Smith JE. The community reinforcement approach: an update of the evidence. Alcohol Res Health. 2011;33(4):380–388. PubMed
- Kelly JF, Humphreys K, Ferri M. Alcoholics Anonymous and other 12-step programs for alcohol use disorder. Cochrane Database Syst Rev. 2020;3(3):CD012880. PubMed CrossRef
- Zemore SE, Lui C, Mericle A, et al. A longitudinal study of the comparative efficacy of Women for Sobriety, LifeRing, SMART Recovery, and 12-step groups for those with AUD. J Subst Abuse Treat. 2018;88:18–26. PubMed CrossRef
- Kelly JF, Stout RL, Jason LA, et al. One-stop shopping for recovery: an investigation of participant characteristics and benefits derived from U.S. Recovery Community Centers. Alcohol Clin Exp Res. 2020;44(3):711–721. PubMed CrossRef
- Vuong T, Gillies M, Larney S, et al. The association between involuntary alcohol treatment and subsequent emergency department visits and hospitalizations: a Bayesian analysis of treated patients and matched controls. Addiction. 2022;117(6):1589–1597. PubMed CrossRef
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889–1900. PubMed CrossRef
- McPheeters M, O’Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023;330(17):1653–1665. PubMed CrossRef
- Mason BJ. Looking back, looking forward: current medications and innovative potential medications to treat alcohol use disorder. Alcohol Res. 2022;42(1):11. PubMed CrossRef
- Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001;36(1):2–10. PubMed CrossRef
- O’Malley SS, Krishnan-Sarin S, Farren C, et al. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160:19–29. PubMed
- Helstrom AW, Blow FC, Slaymaker V, et al. Reductions in alcohol craving following naltrexone treatment for heavy drinking. Alcohol Alcohol. 2016;51(5):562–566. PubMed CrossRef
- de Laat B, Nabulsi N, Huang Y, et al. Occupancy of the kappa opioid receptor by naltrexone predicts reduction in drinking and craving. Mol Psychiatry. 2021;26(9):5053–5060. PubMed CrossRef
- Chavkin C. Kappa-opioid antagonists as stress resilience medications for the treatment of alcohol use disorders. Neuropsychopharmacology. 2018;43(9):1803–1804. PubMed CrossRef
- Williams KL, Broadbear JH, Woods JH. Noncontingent and response-contingent intravenous ethanol attenuates the effect of naltrexone on hypothalamic-pituitary adrenal activity in rhesus monkeys. Alcohol Clin Exp Res. 2004;28(4):566–571. PubMed CrossRef
- Witkiewitz K, Roos CR, Mann K, et al. Advancing precision medicine for alcohol use disorder: replication and extension of reward drinking as a predictor of naltrexone response. Alcohol Clin Exp Res. 2019;43(11):2395–2405. PubMed CrossRef
- Crabtree BL. Review of naltrexone, a long-acting opiate antagonist. Clin Pharm. 1984;3(3):273–280. PubMed
- Malone M, McDonald R, Vittitow A, et al. Extended-release vs. oral naltrexone for alcohol dependence treatment in primary care (XON). Contemp Clin Trials. 2019;81:102–109. PubMed CrossRef
- Ayyala D, Bottyan T, Tien C, et al. Naltrexone for alcohol use disorder: hepatic safety in patients with and without liver disease. Hepatol Commun. 2022;6(12):3433–3442. PubMed CrossRef
- Mallik A, Chanda ML, Levitin DJ. Anhedonia to music and mu-opioids: evidence from the administration of naltrexone. Sci Rep. 2017;7(1):1–8. PubMed CrossRef
- Wright TM, Myrick H. Acamprosate: a new tool in the battle against alcohol dependence. Neuropsychiatr Dis Treat. 2006;2(4):445–453. PubMed CrossRef
- Kalk NJ, Lingford-Hughes AR. The clinical pharmacology of acamprosate. Br J Clin Pharmacol. 2014;77(2):315–323. PubMed CrossRef
- Roos CR, Mann K, Witkiewitz K. Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addict Biol. 2017;22(6):1528–1539. PubMed CrossRef
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–293. PubMed CrossRef
- Kiefer F, Wiedemann K. Combined therapy: what does acamprosate and naltrexone combination tell us? Alcohol Alcohol. 2004;39(6):542–547. PubMed CrossRef
- Lanz J, Biniaz-Harris N, Kuvaldina M, et al. Disulfiram: mechanisms, applications, and challenges. Antibiotics (Basel). 2023;12(3):524. PubMed
- Hegde A, Singh SM, Sarkar S. Long-acting preparations in substance abuse management: a review and update. Indian J Psychol Med. 2013;35(1):10–18. PubMed
- Wilkins JN. Traditional pharmacotherapy of alcohol dependence. J Clin Psychiatry. 2006;67(14):14–22.
- Ghosh A, Mahintamani T, Balhara YPS, et al. Disulfiram ethanol reaction with alcohol-based hand sanitizer: an exploratory study. Alcohol Alcohol. 2021;56(1):42–46. PubMed
- Stokes M, Abdijadid S. Disulfiram. In: StatPearls [Internet]. StatPearls Publishing; 2023. Updated October 24, 2022. Accessed December 8, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459340/
- Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251–274. PubMed CrossRef
- Kranzler HR. Overview of alcohol use disorder. Am J Psychiatry. 2023;180(8):565–572. PubMed CrossRef
- Manhapra A, Chakraborty A, Arias AJ. Topiramate pharmacotherapy for alcohol use disorder and other addictions: a narrative review. J Addict Med. 2019;13(1):7–22. PubMedPubMed
- Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34–42. PubMed CrossRef
- Anton RF, Myrick H, Wright T.M, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709–717. PubMed CrossRef
- Litten RZ, Ryan ML, Fertig JB, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7(4):277–286. PubMed CrossRef
- Ciccocioppo R, Economidou D, Rimondini R, et al. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61(1):4–12. PubMed
- Roache JD, Pavlicova M, Campbell A, et al. Is extended release naltrexone superior to buprenorphine-naloxone to reduce drinking among outpatients receiving treatment for opioid use disorder? A secondary analysis of the CTN X: BOT trial. Alcohol Clin Exp Res. 2021;45(12):2569–2578. PubMed CrossRef
- Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867–1872. PubMed CrossRef
- Chuong V, Farokhnia M, Khom S, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8(12):e170671. PubMed CrossRef
- Flores-Ramirez FJ, Illenberger JM, Pascasio GE, et al. Alternative use of suvorexant (Belsomra®) for the prevention of alcohol drinking and seeking in rats with a history of alcohol dependence. Front Behav Neurosci. 2022;16:1085882. PubMed CrossRef
- Campbell EJ, Norman A, Bonomo Y, et al. Suvorexant to treat alcohol use disorder and comorbid insomnia: plan for a phase II trial. Brain Res. 2020;1728:146597. PubMed CrossRef
- Martinotti G, Orsolini L, Fornaro M, et al. Aripiprazole for relapse prevention and craving in alcohol use disorder: current evidence and future perspectives. Expert Opin Investig Drugs. 2016;25(6):719–728. PubMed CrossRef
- Leggio L, Litten RZ. The GABA-B receptor agonist baclofen helps patients with alcohol use disorder: why these findings matter. Neuropsychopharmacology. 2021;46(13):2228–2229. PubMed CrossRef
- Romito JW, Turner ER, Rosener JA, et al. Baclofen therapeutics, toxicity, and withdrawal: a narrative review. SAGE Open Med. 2021;9:20503121211022197. PubMed CrossRef
- de Beaurepaire R, Sinclair JMA, Heydtmann M, et al. The use of baclofen as a treatment for alcohol use disorder: a clinical practice perspective. Front Psychiatry. 2019;9:708. PubMed
- Johnson BA, Seneviratne C, Wang XQ, et al. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT(3) antagonist ondansetron. Am J Psychiatry. 2013;170(9):1020–1031. PubMed CrossRef
- Grabski M, McAndrew A, Lawn W, et al. Adjunctive ketamine with relapse prevention-based psychological therapy in the treatment of alcohol use disorder. Am J Psychiatry. 2022;179(2):152–162. PubMed CrossRef
- Kelson M, Burnett JM, Matthews A, et al. Ketamine treatment for alcohol use disorder: a systematic review. Cureus. 2023;15(5):e38498. PubMed CrossRef
- Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193–202. PubMed CrossRef
- Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. PubMed
- Bogenschutz MP, Ross S, Bhatt S, et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial [published correction appears in JAMA Psychiatry. 2022 Sep 14]. JAMA Psychiatry. 2022;79(10):953–962. PubMed CrossRef
- Buoli M, Grassi S, Ciappolino V, et al. The use of zonisamide for the treatment of psychiatric disorders: a systematic review. Clin Neuropharmacol. 2017;40(2):85–92. PubMed
- Paille F, Martini H. Nalmefene: a new approach to the treatment of alcohol dependence [published correction appears in Subst Abuse Rehabil. 2015;6:81]. Subst Abuse Rehabil. 2014;5:87–94. PubMed CrossRef
- Caputo F, Vignoli T, Tarli C, et al. A brief up-date of the use of sodium oxybate for the treatment of alcohol use disorder. Int J Environ Res Public Health. 2016;13(3):290. PubMed
- Guiraud J, Addolorato G, Antonelli M, et al. Sodium oxybate for the maintenance of abstinence in alcohol-dependent patients: an international, multicenter, randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2022;36(10):1136–1145. PubMed CrossRef
- Turna J, Syan SK, Frey BN, et al. Cannabidiol as a novel candidate alcohol use disorder pharmacotherapy: a systematic review. Alcohol Clin Exp Res. 2019;43(4):550–563. PubMed CrossRef
- Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–1043. PubMed CrossRef
- Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(5):354–361. PubMed CrossRef
- Sholler DJ, Schoene L, Spindle TR. Therapeutic efficacy of cannabidiol (CBD): a review of the evidence from clinical trials and human laboratory studies. Curr Addict Rep. 2020;7(3):405–412. PubMed CrossRef
- Grodin EN, Bujarski S, Towns B, et al. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl Psychiatry. 2021;11(1):355. PubMed CrossRef
- Duailibi MS, Cordeiro Q, Brietzke E, et al. N-acetylcysteine in the treatment of craving in substance use disorders: systematic review and meta-analysis. Am J Addict. 2017;26(7):660–666. PubMed CrossRef
- 72. Fischler PV, Soyka M, Seifritz E, et al. Off-label and investigational drugs in the treatment of alcohol use disorder: a critical review. Front Pharmacol. 2022;13:927703. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite