Prim Care Companion CNS Disord 2021;23(6):20cr02838
To cite: Grewal C, Wetzel A, Ravishankar DA, et al. Successful sublingual buprenorphine induction and maintenance for opioid use disorder in a patient with reduced salivary production, aspiration pneumonia, and an enteral tube. Prim Care Companion CNS Disord. 2021;23(6):20cr02838.
To share: https://doi.org/10.4088/PCC.20cr02838
© Copyright 2021 Physicians Postgraduate Press, Inc.
aUniversity of Kansas School of Medicine, Kansas City, Kansas
bDepartment of Psychiatry, University of Kansas Health System, Kansas City, Kansas
*Corresponding author: Roopa Sethi, MD, 3901 Rainbow Blvd, Kansas City, KA 66103 ([email protected]).
Medications for opioid use disorder (OUD) include methadone, buprenorphine/naloxone, and long-acting naltrexone. There are multiple factors to consider while initiating medications for OUD. These factors include medications and their side effect profile, prior response to the medications, patient use of other illicit substances (for example naltrexone and its efficacy in people with alcohol and opioid use disorder), physical dependence on opioids, and patient preference.
Case Report
Mr A is a 66-year-old Caucasian man with a history of OUD, alcohol use disorder, squamous cell carcinoma of the right base of the tongue status post chemoradiation therapy, recurrent episodes of aspiration pneumonia, and chronic back pain. The patient has a 17-year history of opioid use for pain management after completing cancer treatment.
Due to the chemoradiation therapy for the head and neck, Mr A developed dysphagia and decreased saliva production, for which he used artificial saliva. Following his cancer treatment, the patient had multiple episodes of aspiration pneumonia. A swallow study demonstrated aspiration to both thin and thick liquids. Consequently, the patient underwent placement of a percutaneous endoscopic gastrostomy (PEG) tube. Despite the PEG tube and nothing by mouth recommendation, the patient continued to have episodes of aspiration pneumonia requiring hospitalization.
After PEG tube placement, Mr A continued to use opioids to manage his chronic pain, initially taking oxycodone and then later liquid morphine through the PEG tube. Mr A suffered a fall in which he fractured multiple ribs, sustained a pneumothorax, and required chest tube placement. This event escalated the patient’s opioid use, resulting in taking more opioids than was prescribed to him. As a result, Mr A started to run out of prescription opioids early, forcing him to acquire medications from his relatives and friends. He reported cravings for opioids and dreaming of his opiates at night. He gave up some of his daily routine activities, and this resulted in social and interpersonal problems at home with his wife. The patient tried to cut down his use unsuccessfully multiple times, escalating his use further. Over time, he developed criteria for OUD. Additionally, the patient had a multi-year history of alcohol abuse, consuming multiple standard drinks per day through his PEG tube.
The patient’s pulmonology and family medicine physicians recommended he taper off opioids and referred him to the addiction psychiatry clinic for consideration of medication-assisted treatment (MAT) for OUD. Initial considerations were for methadone to be administered through the PEG tube, though it was unknown if the Substance Abuse and Mental Health Services Administration would agree to an exception of using methadone through a PEG tube rather than orally. Administration of buprenorphine/naloxone through a transdermal patch was not an option, as the patient had met criteria for OUD. Alternatively, the initiation of sublingual (SL) buprenorphine/naloxone film was considered. Multidisciplinary discussions among addiction psychiatry, pulmonology, and pharmacy teams helped undertake precautions to limit the risk of aspiration and to achieve effective absorption when initiating buprenorphine/naloxone. He was educated to expectorate the extra residue from his mouth once the buprenorphine/naloxone was absorbed, not using artificial saliva before the administration of buprenorphine/naloxone due to concerns it would hinder absorption. He was educated to rinse his oral mucosa with water before administering buprenorphine/naloxone to ensure adequate oral moisture.
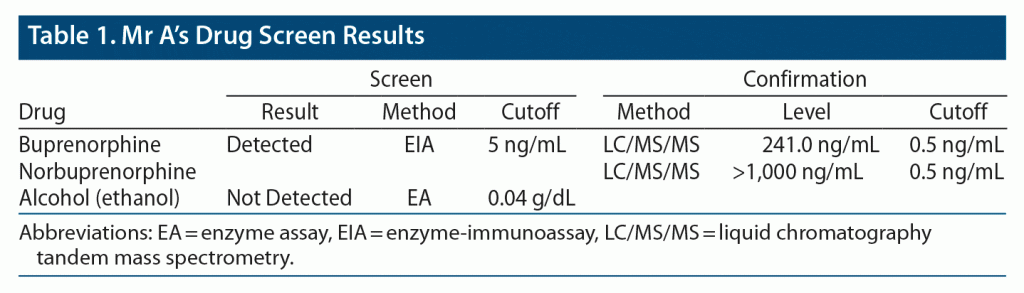
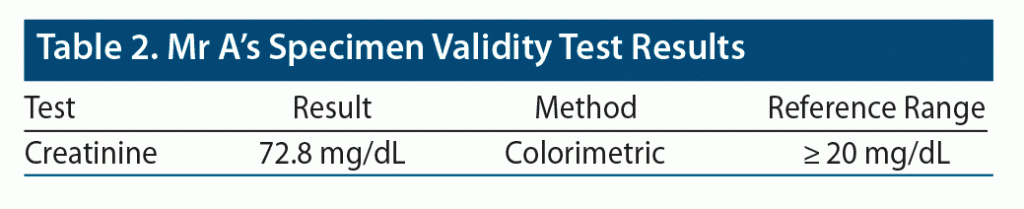
Outpatient induction of buprenorphine/naloxone sublingual film was begun, and the patient tolerated the medication and achieved clinical stability on buprenorphine/naloxone 8/2 mg SL film twice daily. Drug tests collected approximately 2 weeks later revealed appropriate levels of buprenorphine/naloxone and norbuprenorphine. He tested negative for other illicit substances including alcohol, methamphetamine, cocaine, and opiates. Mr A’s drug screen and specimen validity test results are provided in Table 1 and Table 2. He regularly attended the follow-up clinical appointments. His pain and cravings were at baseline after a few months of initiation of treatment.
Discussion
When choosing medications for OUD, physicians should take into consideration multiple patient and clinical factors such as treatment availability, status of physiologic dependence on opioids, medical comorbidities, a patient’s occupation, and a patient’s pregnancy status.1 Owing to his head and neck cancer treatment, Mr A had a unique set of medical comorbidities, including reduced saliva production, nothing by mouth status, the existence of a PEG tube, and recurrent episodes of aspiration pneumonia. Accounting for these factors, the route of administration became a key consideration in deciding among MAT treatment options for Mr A.
The use of MAT for OUD is highly regulated. In particular, the administration of methadone is subject to multiple restrictions when used for detoxification and maintenance in opioid use disorder. The federal opioid treatment standards set forth in the Code of Federal Regulations (CFR) Title 42 Part 8 include the language that “methadone shall be administered or dispensed only in oral form.”2 Interestingly, methadone is considered a practical selection for administration via an enteral feeding tube when given for analgesia.3 However, the CFR language may give pause to an addiction psychiatrist considering the use of methadone for OUD in a patient with a feeding tube in whom oral administration is unsafe.
Alternatively, buprenorphine/naloxone and combination products have been approved by the US Food and Drug Administration for OUD via multiple routes of administration, including SL film and tablet, buccal film, and extended-release injection.1 These last options would have been a good alternative for the patient, but he declined. Naltrexone, a long-acting injectable, was not a good option given that naltrexone requires the patient not to be physiologically dependent on opioids.1 The patient had so many medical complications requiring hospitalizations, making long-acting injectable naltrexone not an option for him.
Thus, with Mr A, buprenorphine/naloxone was felt to be the best option via SL administration. However, there were questions if buprenorphine/naloxone would adequately be absorbed in this patient, given that the literature is scant on the use of these formulations of buprenorphine/naloxone in a patient with reduced saliva production, nothing by mouth status, and the existence of an enteral feeding tube. Despite Mr A’s unique medical comorbidities, the absorption of SL buprenorphine/naloxone did not appear to be significantly affected, as evidenced by the patient having appropriate serum levels of both buprenorphine/naloxone and norbuprenorphine. Another interesting aspect of this case was the patient’s recurrent episodes of aspiration pneumonia. A period of monitoring was done for months to monitor the frequency of his aspiration pneumonia episodes, which were not impacted by his use of SL buprenorphine/naloxone.
Published online: November 24, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Informed consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (3)

- Substance Abuse and Mental Health Services Administration. Tip 63: medications for opioid use disorder. 2019. Accessed September 8, 2021. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document/PEP21-02-01-002
- CFR: Code of Federal Regulations Title 21. 2015. US Food and Drug Administration. Accessed September 8, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm
- Methadone for Analgesia Guidelines. College of Physicians and Surgeons of British Columbia. Accessed September 8, 2021. https://www.cpsbc.ca/files/pdf/DP-Methadone-for-Analgesia-Guidelines.pdf
Please sign in or purchase this PDF for $40.
Save
Cite