aCHU Toulouse, Service de Psychiatrie et Psychologie, Psychiatrie, Toulouse, France
bINSERM U1043, Centre de Physiopathologie de Toulouse Purpan, Université Paul Sabatier, Toulouse, France
cCHU Toulouse, Service de Pharmacologie Médicale et Clinique, Centre d’Evaluation et d’Information sur la Pharmaco-AddictoVigilance, Toulouse, France
dCHU Toulouse, Service de Diabétologie, Maladies Métaboliques et Nutrition, Toulouse, France
*Corresponding author: Juliette Salles, MD, Toulouse University Hospital, 330 Avenue de Grande Bretagne, Toulouse, France 31059 ([email protected]).
Prim Care Companion CNS Disord 2021;23(2):20l02788
To cite: Salles J, Ponté C, Rougier C, et al. Sugar addictive behavior resulting from hypoglycemia provoked by insulin misuse. Prim Care Companion CNS Disord. 2021;23(2):20l02788.
To share: https://doi.org/10.4088/PCC.20l02788
© Copyright 2021 Physicians Postgraduate Press, Inc.
The field of addictive behavior has been the subject of recent research that has underlined the neurobiological mechanisms linked to the reward circuit, enabling concepts of substance-related and non–substance-related addictive disorders to be unified. In the past few years, the literature has considered whether food could also be addictive, describing addictive-like behaviors concerning its consumption. The depiction of these behaviors has since been followed by an elaboration of the food addiction concept. This concept initially arose from a hypothesized connection between bulimia nervosa, binge-eating disorder, and the emergence since the 1980s of growing numbers of new eating conditions due to changes in the food environment. The food addiction concept postulates that food has addictive potential, with foods high in sugar and fat more frequently associated with addictive-like eating. Preclinical studies have confirmed the observation that manipulation of the food environment can lead to binge eating.1 The rationale behind food addiction was bolstered by growing evidence that addiction to food and substance use disorders activate common neurobiological pathways in the brain circuits.2 Similar mechanisms have been identified in neurotransmission, and the recurrent binge eating of food high in fat and sugar is also associated with desensitization of the opioid and dopamine receptors, mimicking the neural underpinning of tolerance and dependence observed in substance use disorders.3
On the basis of these studies, it is now postulated that some eating disorders can be compared to addictive-like behaviors that resemble a food addiction. However, despite such data, the food addiction concept is still controversial and requires further investigation, especially in humans. In addition, the imbrication between food and other addictions must also be explored.
To discuss this issue from a clinical perspective, we present a case of a patient with alcohol use disorders who developed an addictive-like consumption of sugary food associated with the misuse of his insulin.
Case Report
The information for this case report was collected at the Toulouse University Hospital, Toulouse, France, in May 2019 during the patient’s hospitalization in the diabetes unit. According to the relevant legislation, this case was recorded in the French pharmacovigilance database in May 2019.
The patient was a 48-year-old man. His medical history included obesity (body mass index: 36 kg/m2). His weight gain occurred progressively, resulting from overeating and alcohol consumption, but he did not describe a history of eating disorders. The alcohol use disorders began at around age 25 years, first in the form of alcohol binges and then daily consumption with physical dependence. Over the past 10 years, his mean consumption of alcohol has been estimated to be between 180 and 210 g of pure alcohol per day, leading to physical impairment in the form of erosive esophagitis complicated by stenosis and perforation. He has also had 2 episodes of acute hepatitis, but did not meet the criteria for cirrhosis. He was recently hospitalized for alcohol withdrawal and had remained abstinent while the addictive-like behavior surrounding food began. He had also had diabetes type 2 for 12 years and required insulin for the last 5 years. He was first prescribed a combination of oral hypoglycemic agents and basal bolus insulin treatment. The latter was stopped 1 year ago by his physician, but the patient continues to take rapid insulin and tends to overuse it. For about 2 months before his most recent hospitalization, he had administered a systematic injection of 15 UI of rapid insulin at around 7 pm, whether he had eaten or not; he then controlled his capillary glycemia until he raised the hypoglycemia threshold enough to enable him to ingest sugary foods consisting of 1 can of soda, 2 or 3 rusks with marmalade, and candies. He repeated this pattern several times in an evening, and even at night, injecting 15 units each time. He had presented with a first episode of severe hypoglycemia a few weeks before the incident, provoking this report, but was not hospitalized. Despite this, he had continued to induce hypoglycemia.
In May 2019, the patient was hospitalized comatose (with a capillary blood glucose level of 0.36 g/L). During the subsequent medical examination, he had no memory of the episode and presented with cold sweats and shaking. The biological tests, including a standard blood test, complete blood count, and tests of his liver and renal functioning, produced normal results. His glycosylated hemoglobin level (HbA1C) was 6.1%. The patient had gained weight during this 2-month period, which could be due to repeated hypoglycemia. In parallel with this behavior, he had also begun to consume alcohol again.
We considered whether the repeated injection of insulin could be a food addiction, noting compulsive behavior surrounding sugar consumption and an inability to stop the misuse of insulin despite major complications. The Yale Food Addiction Scale4 determined that the patient’s behavior met the food addiction criteria for sugary food (soda, marmalade, and candies). The patient met 7 criteria related to substance abuse/dependence: “Substance taken in larger amount and for longer period than intended,” “Persistent desire or repeated unsuccessful attempts to quit,” “Much time spent using, recovering,” “Use despite knowledge of adverse consequences,” “Characteristic withdrawal symptoms,” Substance taken to relieve withdrawal,” and “Use clinically significant impairment or distress.” Given this score, we concluded that the patient had a food addiction.
Discussion
This case report concerns an adult patient with type 2 diabetes. It is the first to describe addictive eating behavior related to insulin misuse, consisting of sugar intake following provoked hypoglycemia. To our knowledge, insulin overdose was reported in a case report for euphoric experiences.5 In addition, insulin misuse concomitant with addictive eating disorders has only previously been reported in adolescents with type 1 diabetes.6 In those studies, the authors5,6 found that the most common reason for insulin misuse by overdose was the desire to eat more than was appropriate in their diet, followed by self-harming/attention-seeking behaviors and a feeling of euphoria with hypoglycemia.
In this report, the patient’s desired effect in eating sugary foods was the pleasant sensation associated with sugar intake after hypoglycemia. This behavior met the criteria for food addiction and is interesting in relation to the factors that may have led to its development.
The advice concerning sugar administration after hypoglycemia is to choose food with a high glycemic load, which led the patient to experiment with the ingestion of such food after provoked hypoglycemia. High glycemic food is known to have a higher addictive potential,7 even leading to the greater activation of reward circuits. It has also been demonstrated that accentuated fluxes in blood glucose increase the reactivity of the food reward–related circuitry,8 especially in people with a food addiction when fasting.9 It may be that the initial choice of food with addictive potential and the bingeing effect of rapid glycemia elevations played a part in the development of a food addiction in our patient.
The patient also suffered from obesity, which is associated with reduced dopamine binding in the reward circuit regions8 and is likely to reduce sensitivity to natural rewards. Obese individuals who were shown pictures of high calorie food also had increased neural activation of regions that are part of the reward and motivation circuits,10 which could be a cause of vulnerability to a food addiction. These characteristics have been found to be associated with overeating as a way to compensate for hypodopaminergic functioning. We suggest that this state and the need to eat more could have weakened the patient, especially with respect to his dependence on sugar. Moreover, a reduction in the number of dopamine receptors has been linked to the compulsive intake of food in obese rodents,11 while decreased metabolic activity in the orbito-frontal cortex and the anterior cingular cortex, 2 regions that have been implicated in the development of compulsivity, has been identified in obese humans. These factors may have accentuated the patient’s bingeing behavior with food.
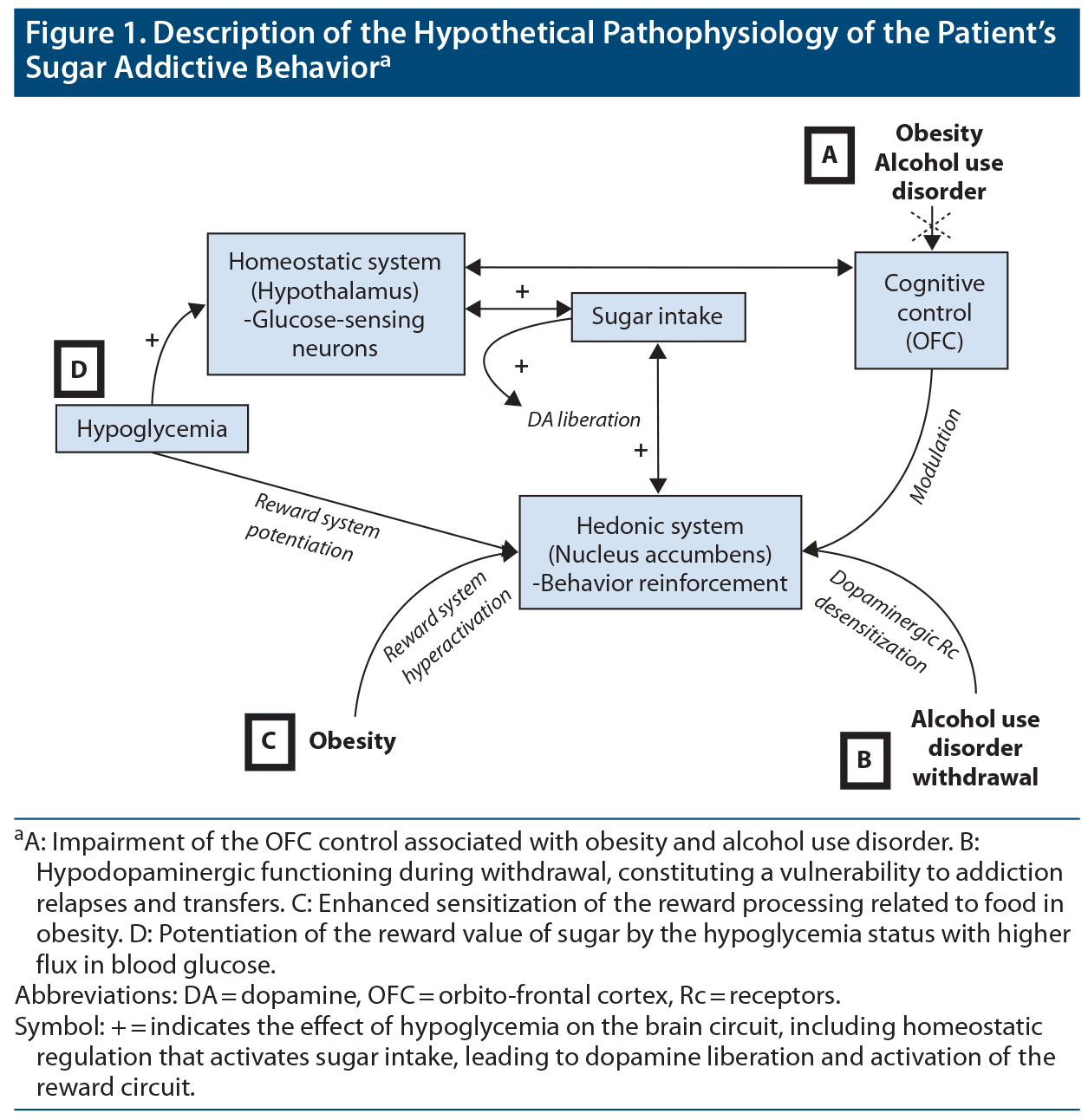
The patient also had an alcohol use disorder, which is known to produce chronic alterations of the reward circuit, with gene modification and neuroplasticity. Indeed, chronic substance abuse induces an enduring liberation of dopamine and has been shown to cause dopamine receptor internalization that mimics a dopamine deficit.12 This hypodopaminergic functioning is significant during withdrawal, which may have been exacerbated given that the patient was abstinent from alcohol at the start of the addictive-like behavior. In addition, it may be that the patient tried to compensate for the hypodopaminergic functioning related to his alcohol withdrawal by inducing a dopamine release due to sugar consumption. Indeed, a study13 revealed that the ingestion of sweet substances is associated with a reduction in alcohol consumption; conversely, a heightened hedonic response to sweet tastes has also been associated with increased alcohol preference and consumption. However, in our patient, alcohol consumption did not have an influence on the evolution of his intake of sugar. Figure 1 summarizes the mechanism implied in the development of addictive-like behavior involving food.
This report has some limitations. First, it is only 1 case study based on an empirical method. Second, we chose to describe the addictive-like behavior as a food addiction, even though the term eating addiction is preferred by other authors.14 However, we chose the term because the patient met the relevant criteria and had a preference for food with a high glycemic load. We also considered the commentary of Schulte et al,7 which suggests that a substance-based food addiction perspective may be more appropriate than regarding eating as a behavioral addiction.7 Indeed, compulsive behavior patterns are also thought to be important for increasing the attraction to high-fat, high-sugar foods, which is relevant because our patient developed a compulsive pattern behavior concerning the injection of insulin every day at the same time. In terms of the behavioral aspect, we believe that patterns of behavior, including engagement with substances, may increase their addictive potential, and the food addiction concept takes behavioral contexts into account.
Conclusion
This is the first case report, to our knowledge, concerning the association between the misuse of insulin and alcohol use disorder and food addiction in a diabetes patient with type 2 diabetes. Current data on the reward circuit have also enabled us to discuss the pathophysiology hypothesis in relation to the development of food addiction. Interestingly, this case is also a clinical illustration of the link between alcohol use disorders and food addiction. As a result, physicians may need to be more attentive to possible addictive behaviors involving insulin misuse in patients who are vulnerable to their development.
Published online: April 15, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Written informed consent was obtained from the patient for the publication of this case report, and the patient was informed that data were collected for an ongoing research project and anonymously collected. Information has been de-identified to protect anonymity.
Compliance with ethical standards: According to French laws (Articles R.5132-113 and R.5132-114), this case was recorded in the French Addictovigilance database in an anonymous way. As defined in Article 2(c) of Directive 2001/20/EC, this study is a noninterventional study that does not require an institutional review board approval.
References (14)

- Wiss DA, Criscitelli K, Gold M, et al. Preclinical evidence for the addiction potential of highly palatable foods: current developments related to maternal influence. Appetite. 2017;115:19–27. PubMed CrossRef
- Gearhardt AN, Yokum S, Orr PT, et al. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68(8):808–816. PubMed CrossRef
- Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–3552. PubMed CrossRef
- Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–436. PubMed CrossRef
- Sun Y-M, Kamel Y, Amin R, et al. Intentional insulin overdose for euphoric experience. Prim Care Companion CNS Disord. 2017;19(6):0–0. PubMed CrossRef
- Snyder LL, Truong YK-N, Law JR. Evaluating Substance Use and Insulin Misuse in Adolescents With Type 1 Diabetes. Diabetes Educ. 2016;42(5):529–537. PubMed CrossRef
- Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? the roles of processing, fat content, and glycemic load. PLoS One. 2015;10(2):e0117959. PubMed CrossRef
- Wang G-J, Volkow ND, Thanos PK, et al. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23(3):39–53. PubMed CrossRef
- Contreras-Rodriguez O, Burrows T, Pursey KM, et al. Food addiction linked to changes in ventral striatum functional connectivity between fasting and satiety. Appetite. 2019;133:18–23. PubMed CrossRef
- Stoeckel LE, Weller RE, Cook EW 3rd, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. PubMed CrossRef
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. PubMed CrossRef
- Ashok AH, Mizuno Y, Volkow ND, et al. Association of stimulants with dopaminergic alterations in users of cocaine, amphetamine, and methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(5):511–519. PubMed CrossRef
- Krahn D, Grossman J, Henk H, et al. Sweet intake, sweet-liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav. 2006;31(4):622–631. PubMed CrossRef
- Hebebrand J, Albayrak Ö, Adan R, et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite