Objective: To evaluate the long-term safety, tolerability, and efficacy of 2 strategies for switching from donepezil to rivastigmine transdermal patches in patients with mild to moderate Alzheimer’s disease.
Method: This was a prospective, 25-week, randomized, open-label, parallel-group study to evaluate an immediate or delayed switch (7-day withdrawal) from donepezil (5 to 10 mg/d) to rivastigmine transdermal patches (4.6 mg/24 h). Participants included male and female patients, aged ≥ 50 years, with a DSM-IV-TR diagnosis of mild to moderate dementia of the Alzheimer’s type, defined as a Mini-Mental State Examination score of 10-24, inclusive. Patients were enrolled between February 2007 and February 2008. The study was split into a 5-week core phase and a 20-week extension phase. Safety and efficacy results from the extension phase are presented.
Results: Both switching strategies were well tolerated. Rates of discontinuation for any reason were similar between the groups. Discontinuations due to adverse events were also similar, and the incidence of gastrointestinal adverse events was low. Apart from Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale scores, at the end of the study, there was no statistically significant change from baseline in cognitive, behavioral, or global outcomes. Over half of the patients preferred rivastigmine transdermal patches to a tablet.
Conclusions: This study suggests that the majority of patients receiving donepezil tablets can be safely switched to rivastigmine transdermal patches without significant deterioration in cognition, behavior, and global functioning.
Trial Registration: clinicaltrials.gov Identifier: NCT00305903
Prim Care Companion J Clin Psychiatry 2010;12(5):e1-e8
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: June 11, 2009; accepted December 29, 2009.
Published online: October 28, 2010 (doi:10.4088/PCC.09m00852oli).
Corresponding author: Carl H. Sadowsky, MD, Premiere Research Institute, 4631 N Congress Ave, Ste 200, West Palm Beach, FL 33407 ([email protected]).
Currently, one of the primary approaches for the pharmacologic treatment of Alzheimer’s disease (AD) focuses on reducing the cholinergic deficiency in the central nervous system with cholinesterase inhibitors (ChEIs). Presently available oral ChEIs include rivastigmine, donepezil, and galantamine.1 Memantine, an N-methyl-d-aspartate receptor antagonist, is approved in the United States for treatment of moderate to severe AD.2 While there is consensus that these agents are modestly beneficial,3 treatment adherence is an important challenge facing patients with AD.4 Perceived lack of clinical benefit and the occurrence of adverse events (AEs) may be key reasons for patients with AD to discontinue treatment.4
Clinical Points
- Since many patients will not respond well to initial treatment with cholinesterase inhibitors, switching patients to another drug in this class should be considered.
- Rivastigmine is available in a patch formulation that significantly reduces the incidence of gastrointestinal side effects compared with the rivastigmine capsule; the 10-cm2 rivastigmine patch (delivering 9.5 mg/24 h) also provides similar efficacy to rivastigmine capsules (6 mg twice daily).
- Switching cholinesterase inhibitors from oral donepezil to the rivastigmine patch, either immediately or following a 7-day washout period, is easy to accomplish and well tolerated and should therefore be considered in patients experiencing problems with efficacy and tolerability of oral donepezil.
Some patients with AD may not experience sustained clinical benefit from ChEI treatment owing to a lack of initial efficacy, loss of efficacy during long-term treatment, or tolerability issues.5 Thus, switching to a different ChEI is a common therapeutic strategy. When switching medications, the objectives are to avoid rapid symptomatic worsening resulting from the cessation of the first medication and AE emergence due to initiation of the subsequent treatment.1
Several studies suggest that patients with AD who do not respond to treatment with donepezil or galantamine tablets may be switched to rivastigmine capsules.5-9 Rivastigmine capsules are currently approved in the United States and Europe for the treatment of mild to moderate AD. Clinical studies have shown that rivastigmine is superior to placebo on measures of cognition, activities of daily living, and global functioning.10,11 As with all ChEIs, the most common AEs occurring with the rivastigmine capsule are gastrointestinal (GI) in nature (eg, nausea and vomiting).10,11
In 2007, a transdermal patch formulation of rivastigmine became available in the United States for treatment of mild to moderate AD. The transdermal patch provides drug delivery over 24 hours and reduces fluctuations in plasma concentrations compared with the rivastigmine capsule.12 A 24-week, double-blind, double-dummy, randomized, placebo- and active-controlled study in over 1,100 patients with AD directly compared the rivastigmine capsule with the rivastigmine transdermal patch.13 Results of the study showed that the 9.5-mg 24-hour rivastigmine transdermal patch had similar efficacy to the rivastigmine capsule (12 mg/24 h), with one-third of the incidence of GI side effects.13 This improved tolerability profile could potentially result in greater treatment adherence,4,13 and the patch was preferred to the capsule by the majority of caregivers.14
Results from the core phase of this prospective, randomized, parallel-group, open-label study (Study CENA713DUS38) suggested that both immediate and delayed (following a 7-day withdrawal period) switches from oral donepezil (5-10 mg/d) to rivastigmine patches (4.6 mg/24 h) were safe and well tolerated over 4 weeks of treatment.15 In addition, the mean Clinical Global Impression of Change (CGI-C) score showed no worsening from baseline in either treatment group.15 Here, we report results from the 20-week, open-label extension phase of this study. The primary objective of this extension phase was to provide long-term safety information for patients who were switched from donepezil tablets to the rivastigmine transdermal patch.
METHOD
Patients
Patients enrolled in the study have been described previously.15 In brief, male or female patients, aged ≥ 50 years, with a DSM-IV-TR diagnosis of mild to moderate dementia of the Alzheimer’s type16,17 were included in the study. Patients were enrolled between February 2007 and February 2008. Mild to moderate dementia was defined as a Mini-Mental State Examination (MMSE) score of 10-24, inclusive.18 Patients were required to have a primary caregiver willing to accept responsibility for supervising treatment, assessing the condition of the patient throughout the study and providing input into the efficacy assessments, in accordance with all protocol requirements. Patients were also required to have been receiving donepezil tablets for at least 6 months and taking a stable dose (either 5 or 10 mg/d) for at least the last 3 of these 6 months prior to study entry.
The main exclusion criteria included any primary neurodegenerative disorder other than AD or any other causes of dementia; any disability or unstable disease that may have prevented the patient from completing all study requirements; a current diagnosis of bradycardia (heart rate < 50 bpm), sick sinus syndrome, conduction defects, severe or unstable cardiovascular disease, significant urinary obstruction, peptic ulceration, or GI bleeding; an unstable respiratory condition; any active skin lesion or disorder that would prevent accurate assessment of the adhesion; and patients who discontinued treatment during the core phase of the study owing to skin irritation secondary to the rivastigmine transdermal patch. Patients who were receiving donepezil tablets and concomitant memantine at the beginning of the study were allowed to continue on the same dose of memantine throughout the study; however, not more than 50% of the total study population was to have been receiving combination therapy.
Patients were recruited into the study from 50 clinical and research centers in the United States. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Reporting Practice. The study protocol was approved by an institutional review board, an independent ethics committee, and a research ethics board. Prior to participation in the study, patients were to provide, if mentally competent, written informed consent, along with consent from an appropriately responsible party on the patient’s behalf and from the patient’s caregiver. If the patient was not able to provide written informed consent, this was obtained from the caregiver and the appropriately authorized representative on the patient’s behalf; verbal assent was obtained from the patient if possible and permitted by state, local, and institutional review board regulations. The study is registered in clinicaltrials.gov (Identifier: NCT00305903).
Study Design
Patients who completed the 5-week prospective, randomized, multicenter, parallel-group, open-label core phase of the study (previously reported15) had the option to enter a 20-week, open-label extension phase for further treatment and evaluation. In brief, patients were randomly assigned (1:1) to be switched from donepezil (5-10 mg/d) to 5-cm2 rivastigmine transdermal patches (4.6 mg/24 h) either immediately or following a 7-day withdrawal period. Following the switch, both groups were maintained on rivastigmine patches for 4 weeks before entering into the extension phase. All patients who entered the extension phase were given a dose increase to a 10-cm2 (9.5 mg/24 h) rivastigmine patch and remained on this dose through week 25 unless they experienced dose-limiting AEs. Patients who experienced dose-limiting AEs had their dose reduced back to the 5-cm2 patch and continued on their best-tolerated dose for the remainder of the study. The rivastigmine transdermal patches were applied by the caregiver to clean, dry, and intact skin on the patient’s upper or lower back, upper arm, or chest. Patches were changed every 24 hours in the morning to different sites within these areas, in rotation. Patients in each treatment group were stratified by concomitant memantine use.
Assessments and Outcomes
The primary outcome of the study was the safety and tolerability of the 2 different switching strategies based on the incidence of discontinuation for any reason during the 20-week extension phase. Other safety/tolerability measures included discontinuations due to AEs and the incidence of AEs during the 20-week extension phase. Secondary objectives were to evaluate efficacy outcomes, as well as caregiver preference.
Clinical Outcomes
A CGI-C assessment was conducted during an interview by a rater at weeks 5 and 25,19 and raters received training prior to the start of the study. The scale comprises 13 items in 3 domains: mental/cognitive state (arousal/alertness/attention/concentration), behavior/thought content, and functioning (basic and complex). For patients who withdrew from the study before week 25, the CGI-C at week 5 was carried forward. Other efficacy assessments were obtained via interviews with the caregiver at screening or baseline and at week 25 (or early discontinuation) and included scores for the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale (ADCS-ADL),20 the Neuropsychiatric Inventory (NPI),21 the NPI Caregiver Distress (NPI-D) scale,22 and the modified Alzheimer’s Disease Caregiver Preference Questionnaire (ADCPQ).23 For the NPI and NPI-D, if the neuropsychiatric features for an item were not applicable, the score for that domain was set to missing. If 2 or more items had missing total scores, the NPI total score was set to missing. The MMSE, a brief practical assessment of cognitive dysfunction, was assessed at screening and at week 25. The MMSE consists of 20 questions, divided into 5 sections (orientation, registration, attention-calculation, recall, and language).18 The total MMSE scores range from 0 to 30 with lower scores signifying greater dysfunction. If more than 6 weeks had elapsed between the screening and baseline visits, the MMSE was to be repeated at baseline and the value closest to and prior or equal to the date of randomization was considered the baseline value. For the modified ADCPQ, the primary hypothesis to be tested was that the patch was preferred over the previous pill medication by the majority of caregivers and was tested using item 8 in the follow-up questionnaire, which asked the caregiver which medication he/she preferred.
Statistical Analysis
The safety population consisted of patients who had received at least 1 dose of study medication and who had at least 1 postbaseline safety assessment. The intent-to-treat population consisted of all randomized patients who had received at least 1 dose of study medication and who had at least 1 postbaseline safety/tolerability assessment. The sample size was based on the assumption of a 5% study discontinuation rate for any reason, which resulted in 120 patients for each treatment group to permit an accurate estimation of the discontinuation rate, with a standard error (SE) of 0.02. Discontinuation rates, the corresponding SEs, and the 95% confidence intervals (CIs) for the difference between the 2 treatment groups were calculated. Differences for all-cause discontinuations within treatment groups, based on concomitant memantine usage, were estimated in a similar way. All safety data were summarized according to both treatment groups and for the total population. No adjustment was made for multiple comparisons regarding the secondary and safety outcomes. Descriptive statistics for the CGI-C were calculated. The number and percentage of patients with no decline on the CGI-C (CGI-C score ≤ 4) were presented, together with the 95% CI for the proportion. Changes from baseline/screening to week 25 and end of study on the ADCS-ADL, NPI, NPI-D, and MMSE scores were summarized using descriptive statistics and 95% CIs for the mean change. For the modified ADCPQ, 95% CIs were calculated around the proportion of caregivers choosing the patch over the previous pill medication. If the lower limit of the 95% CI was above 50%, this was hypothesized to confirm caregiver preference for the patch over the previous pill medication.
RESULTS
Study Population and Disposition
The study population and disposition of patients who completed the core phase of the study have been reported previously.15 Of the 240 patients who completed the core phase, 234 entered the extension phase (n = 117 for both the immediate-switch and delayed-switch groups). Overall, the mean age (± SD) of patients was 77.3 ± 8.0 years, 57.9% were female, and the majority (88.1%) were white. The mean ± SD duration of AD and donepezil treatment was 3.9 ± 2.6 years and 29.1 ± 22.9 months, respectively, and the mean ± SD total MMSE score was 18.3 ± 4.0. Both baseline demographics and clinical characteristics were similar for both treatment groups (previously reported).15 Approximately 15% of patients were reported to have experienced AEs or intolerability of donepezil. Prior to study entry, the investigator rated 46.4% of patients to have experienced a decline in activities of daily living, 29.9% to have declined in behavior, 62.8% to have declined in global functioning, and 79.3% to have declined in cognition while on their current therapy.
Treatment Discontinuation
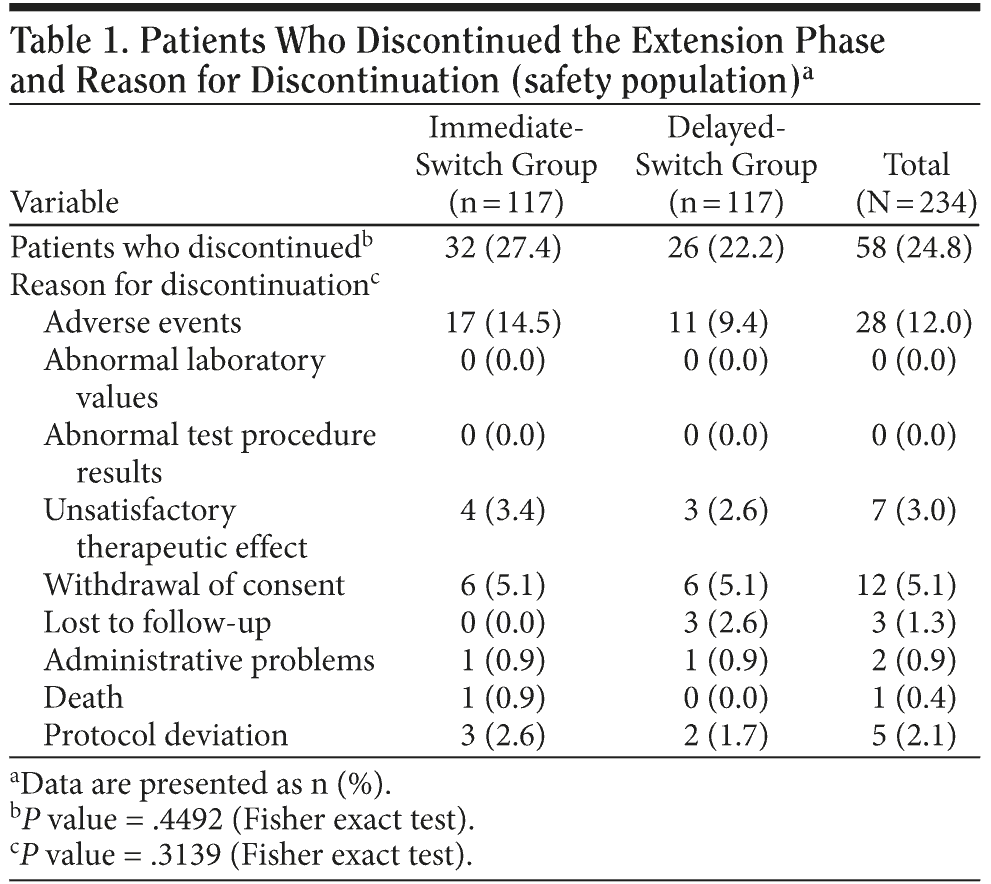
Of the 234 patients who entered the extension study, 176 completed the 20-week phase (n = 85 for the immediate-switch group, n = 91 for the delayed-switch group). Thirty-two patients (27.4%) in the immediate-switch group discontinued the study compared with 26 patients (22.2%) in the delayed-switch group (95% CI for between-regimen difference, -17.0 to 6.8; P = .4492) (Table 1). The primary reason for discontinuations in both treatment groups was AEs. Overall, 28 (12.0%) patients discontinued because of AEs: 17 (14.5%) in the immediate-switch group and 11 (9.4%) in the delayed-switch group (Table 1).
In the immediate-switch group, 6 patients (5.1%) discontinued owing to skin irritation, compared with 5 (4.3%) in the delayed-switch group. In the immediate-switch group, 2 patients (1.7%) discontinued because of vomiting compared with zero patients in the delayed-switch group. In the total population, the AEs that most frequently resulted in treatment discontinuation were application site reaction (11 patients, 4.7%) and disease progression (5 patients, 2.1%) (Table 2). The number of patients who discontinued treatment because of the GI AEs vomiting and diarrhea were low, with only 2 patients (0.9%) for each AE. No patients discontinued owing to nausea. A similar number of patients receiving concomitant memantine discontinued the study in the immediate-switch and delayed-switch groups (18 [30.0%] vs 12 [20.0%], respectively). Overall, the rate of discontinuation was similar for patients who did and did not receive memantine (25.0% vs 24.6%, respectively) (Table 3).
Safety and Tolerability
A total of 184 patients (70.5%) experienced at least 1 AE during the entire 25-week study, including core and extension phases (96 patients in the immediate-switch group [73.3%] and 88 patients in the delayed-switch group [67.7%]), while 139 of 234 patients (59.4%) experienced 1 newly occurring or worsening AE during the extension phase. The most frequently reported AEs in the immediate-switch group were application site reaction (17 patients, 13%) and nausea (9 patients, 6.9%), while in the delayed-switch group, the most frequently reported AEs were application site reactions (23 patients, 17.7%) and agitation (10 patients, 7.7%). Overall, the most frequently reported AEs were application site reaction (40 patients, 15.3%) and agitation (18 patients, 6.9%) (Table 4).
Of those who experienced a newly occurring AE or a worsening of AEs during the extension phase (n = 261), 86 (33.0%) had at least 1 AE that the investigator suspected to be related to the study medication. Of all the AEs reported during the 25-week study, the majority were considered to be mild or moderate in severity. In the immediate-switch group, 16 patients (12.2%) reported severe AEs compared with 11 patients (8.5%) in the delayed-switch group. Serious AEs (SAEs) were reported for 14 (10.7%) and 9 (6.9%) patients in the immediate-switch and delayed-switch groups, respectively (23 patients overall, 8.8%). Two SAEs, 1 case of bradycardia and 1 case of reduced oral intake, were considered by the investigator to be related to the study medication. One patient death due to cardiovascular disease (in the immediate-switch group) was reported during the study. Mean patient body weight remained stable at the end of the 25-week study. Mean ± SD change in body weight from baseline in the immediate-switch group was 0.2 ± 2.2 kg compared with 0.0 ± 2.6 kg in the delayed-switch group. Overall, the mean ± SD change in body weight from baseline was 0.1 ± 2.4 kg.
Efficacy
Global function remained stable and did not change over the course of the study. At week 25, the mean change in CGI-C scores was 4.1 (95% CI, 3.9-4.4) and 4.3 (95% CI, 4.1-4.4) for the immediate-switch and delayed-switch groups, respectively, and 4.2 (95% CI, 4.1-4.3) overall. No statistically significant decline from baseline (ie, improvement or no change in condition [CGI-C rating score ≤ 4]) was seen in 77 patients (65.8%) in the immediate-switch group and 72 patients (62.1%) in the delayed-switch group (149 patients overall, 63.9%).
At the end of the study, there was no statistically significant decline from baseline in cognitive function, as assessed by the MMSE score, in either the immediate-switch (-0.5; 95% CI, -1.2 to 0.1; P = .1248) or the delayed-switch groups (-0.3; 95% CI, -1.1 to 0.4; P = .3604). Overall, the mean change from baseline on the MMSE was -0.4 (95% CI, -0.9 to 0.1; P = .0885). There was a modest reduction on the NPI in the immediate-switch group (−0.8; 95% CI, −2.9 to 1.3; P = .4640) but not in the delayed-switch group (0.5; 95% CI, −1.7 to 2.8; P = .6505). Overall, the NPI scores were maintained over the 25-week period with a mean change from baseline of -0.1 (95% CI, -1.6 to 1.4; P = .8808). Approximately half (50.9%) of the total population exhibited at least a 10% improvement in the total score of item 10 of the NPI.
At the end of the study, there was a statistically significant decline in activities of daily living, as assessed by the ADCS-ADL, for both the immediate-switch (−3.1; 95% CI, −4.9 to −1.4; P = .0007) and the delayed-switch (−4.2; 95% CI, −6.0 to −2.5; P < .0001) groups. Overall, the mean change on the ADCS-ADL from baseline was -3.7 (95% CI, -4.9 to -2.4; P < .0001).
Over half of the patients (55%) preferred the rivastigmine transdermal patch to a pill (modified ADCPQ, item 8); the 95% CI (48.3-61.6) did not exceed the minimum of 50% as hypothesized. For the total population, significant improvements from baseline were reported for the items “administration” (-0.1; 95% CI, -0.18 to -0.02; P = .012), “overall convenience” (-0.5; 95% CI, -0.80 to -0.11; P = .010), and “ease of use” (-0.6; 95% CI, -0.94 to -0.34; P < .001) but not for “skipped/delayed doses” (0.0; 95% CI, -0.16 to 0.09; P = .552), “time required to administer” (0.0; 95% CI, -0.10 to 0.18; P = .564), “overall compliance” (-0.1; 95% CI, -0.32 to 0.13; P = .403), or “satisfaction with medication” (0.0; 95% CI, -0.50 to 0.41; P = .847), which showed no change from baseline. Overall, the pattern of changes was consistent in both groups except for “overall convenience,” which showed improvement (-0.9; 95% CI, -1.34 to -0.46; P < .001) in the delayed-switch group versus no change in the immediate-switch group (0.0; 95% CI, -0.49 to 0.57; P = .875).
DISCUSSION
Results from this 20-week, open-label extension phase demonstrate favorable safety and tolerability for the rivastigmine transdermal patch in patients switching from donepezil tablets. The safety data shown here extend results of the core phase and provide evidence that the majority of patients receiving stable donepezil tablets (or a combination of donepezil and memantine) may be switched safely to the rivastigmine transdermal patch.15
In addition, this study suggests that patients may be maintained on the rivastigmine transdermal patch for up to 25 weeks. Overall, the GI AEs commonly associated with ChEI therapy were low in this long-term study, with only 10 (3.8%) and 11 (4.2%) patients experiencing nausea and vomiting, respectively, over the course of the study. Slightly more patients experienced nausea in the immediate-switch group than in the delayed-switch group (6.9% and 0.8%, respectively), although this did not cause discontinuations during the long-term extension phase.
About 15% of the study population experienced application site reactions. These findings are consistent with earlier results from a 6-month, placebo-controlled trial conducted previously with the 10-cm2 patch.13,14 In that study, skin reactions were not classified as AEs but were instead rated with a special scale at each visit. Overall, 89.6% of patients were rated as having no, slight, or mild skin irritation.
In this trial, less than 5% of patients discontinued from the trial because of application site reactions. This too is similar to the 6-month placebo-controlled trial,13,14 suggesting that skin irritation is not a significant tolerability concern. In clinical practice, there are a number of management strategies that may help to reduce the occurrence of these reactions, such as gentle removal of the patch, use of oil-based soaps and moisturizers to remove excess adhesive and to maintain skin healthiness, and placing patches as far apart from each other as possible.
There was no significant difference in safety outcomes between the 2 switching treatment groups; both switching strategies were associated with similar discontinuation rates for any reason or because of AEs, and the rates of both severe and serious AEs were also similar between the treatment groups. Thus, the results suggest that delaying the treatment switch does not impact the safety of treatment over a 25-week period. These findings support those from previous studies evaluating the switch from donepezil tablets to rivastigmine capsules.6,8
Although the safety of switching from donepezil pills to rivastigmine patches was demonstrated in this analysis, whether or not patients benefit from switching to rivastigmine was not the focus of this investigation—this analysis was not intended to directly compare rivastigmine with donepezil. However, cognition, behavior, and global patient function were maintained over the 25-week period in both treatment groups. The CGI-C scores demonstrate that global function was maintained in the majority of patients, and neither MMSE nor NPI scores deteriorated in this long-term analysis. Activities of daily living, as assessed by ADCS-ADL scores, showed statistically significant decline at week 25. Such deterioration is not unexpected over this period.
Overall, there was a numerical advantage suggesting that more caregivers preferred the rivastigmine patch compared with the previous pill medication (55% vs 45%), although the 95% CI did not exceed a prespecified preference level. The patch was associated with statistically significant improvements in terms of “administration,” “overall convenience,” and “ease of use,” as assessed by the ADCPQ.
A potential limitation of the study is that efficacy variables were not assessed during the core phase; furthermore, since the patient population included patients with both mild and moderate disease, it would have included patients with differing rates of disease progression. Other potential limitations of this study include the open-label nature of the study design and the lack of direct comparisons with rivastigmine capsules or placebo; although the lack of a third study arm may limit the scientific impact of the findings from this study, not having the arm considerably assisted the feasibility of conducting this particular study.
CONCLUSION
The results of this 20-week extension phase suggest that the majority of patients receiving donepezil tablets can be safely switched to rivastigmine transdermal patches without a withdrawal period. In addition, the efficacy data from this study suggest that rivastigmine transdermal patches maintain global and cognitive function and behavioral outcomes, while activities of daily living worsened modestly at week 25. Thus, rivastigmine transdermal patches may provide physicians with a treatment option for patients who require a change in their current oral ChEI therapy owing to safety or tolerability concerns or a lack of therapeutic efficacy.
Drug names: donepezil (Aricept and others), galantamine (Razadyne), memantine (Namenda), rivastigmine (Exelon and others).
Author affiliations: Nova SE University, Fort Lauderdale, and Premiere Research Institute, Palm Beach Neurology, West Palm Beach, Florida (Dr Sadowsky); St Joseph Mercy Health System, Ann Arbor, Michigan (Dr Dengiz); and Novartis Pharmaceuticals Corporation, East Hanover, New Jersey (Drs Meng and Olin).
Potential conflicts of interest: Dr Sadowsky has served as a consultant to, has received grant/research support and honoraria from, and has served on the speakers’ or advisory boards of Forest and Novartis. Dr Dengiz has served as a consultant to and received grant/research support from Novartis and has received honoraria from and served on the speakers’ or advisory boards of Forest and Novartis. Dr Olin was a full-time employee of and stock shareholder in Novartis during the development of this study and manuscript. Dr Meng is a full-time employee of and stock shareholder in Novartis.
Funding/support: This study was funded and sponsored by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
Previous presentations: This work was previously presented in poster format at the Annual Meeting of the American Association of Geriatric Psychiatry; March 5-8, 2009; Honolulu, Hawaii, and at the 162nd Annual Meeting of the American Psychiatric Association; May 16-21, 2009; San Francisco, California.
Acknowledgments: The authors would like to thank Barbara Koumaras, BA, Novartis, East Hanover, New Jersey, for her intellectual contribution and input in the study design, coordination, and data analysis. Ms Koumaras is a full-time employee of and stock shareholder in Novartis. The authors would also like to thank Diya Lahiri, PhD, Medicus International, London, United Kingdom, for her editorial assistance and medical writing. Medicus International activities pertaining to rivastigmine are funded by Novartis. Dr Lahiri reports no other conflicts of interest related to the subject of this article.
List of US38 Investigators: Dr Gus Alva, ATP Clinical Research, Costa Mesa, California; Dr Troy Anderson, Dedicated Clinical Research, Litchfield Park, Arizona; Dr Padmini Atri, International Clinical Research Associates, Richmond, Virginia; Dr Vinod Bhatnagar, Center for Clinical Trials and Research, Venice, Florida; Dr Ronald Brenner, Neurobehavioral Research, Inc, Cedarhurst, New York; Dr Ruben Bravo, Caribbean Education and Research Center, Bayamon, Puerto Rico; Dr Mahan Chehrenama, The Innovative Clinical Research Center, Alexandria, Virginia; Dr Scott Cooper, Medical Association. of North Georgia, Canton; Dr Terry Copeland, Infinity Research Group, Oklahoma City, Oklahoma; Dr Keith Edwards, Neurological Research Center, Inc, Bennington, Vermont; Dr Jay Ellis, Neuroscience Research of the Berkshires, Pittsfield, Massachusetts; Dr Miguel Flores, Berma Research Group, Hialeah, Florida; Dr Bill Fulk, Specpro Research, Inc, Ft. Myers, Florida; Dr Jeffrey Geohas, Radiant Research, Chicago, Illinois; Dr Gary Gerard, Neurology & Neuroscience Center of Ohio, Toledo; Dr Jerome Goldstein, San Francisco Clinical Research Center, California; Dr Phillip Green, Borgess Research Institute, Kalamazoo, Michigan; Dr John Grubbs, Four Rivers Clinical Research, Paducah, Kentucky; Dr Barbara Harris, PsyPharma Clinical Research, Phoenix, Arizona; Dr Sean Hurley, Rowan Research, Spokane, Washington; Dr Arvind Jariwala, Wake Research Associates, Raleigh, North Carolina; Dr Marvin Kalafer, The Clinical Trial Center, Jenkintown, Pennsylvania; Dr Bennett Machanic, Mountain View Clinical Research, Inc, Denver, Colorado; Dr Derek Loewy, PsyPharma Clinical Research, Tucson, Arizona; Dr William Maier, PRO Research, Eugene, Oregon; Dr David Margolin, The Margolin Brain Institute, Fresno, California; Dr Ashok Patel, Bio Behavioral Health Deer Chase Professional Park, Toms River, New Jersey; Dr Meenakshi Patel, Centerville, Ohio; Dr Patricio Reyes, Barrow Neurology Clinic, Phoenix, Arizona; Dr Ralph Richter, Tulsa Clinical Research, Tulsa, Oklahoma; Dr Joel Ross, Memory Enhancement Center of America, Inc, Eatontown, New Jersey; Dr Beth Emmie Safirstein, Hallandale Beach, Florida; Dr Michael Sauter, Westmoreland Neurology, Inc, Greensburg, Pennsylvania; Dr Douglas Scharre, The Ohio State University, Columbus; Dr Harvey Schwartz, Sunrise Clinical Research, Hollywood, Florida; Dr Ram Shrivastava, Eastside Comprehensive Medical Center, New York, New York; Dr Joshua Shua-Haim, Mid Atlantic Geriatric Associates-Ocean Alzheimer’s Research Corporation, Manchester, New Jersey; Dr Alan Siegal, Geriatric and Adult Psychiatry, Hamden, Connecticut; Dr Richard Singer, Neurology Clinical Research, Inc, Sunrise, Florida; Dr Steve Sitar, Orange County Clinical Trials, Anaheim, California; Dr David Spangler, Witham Health Services, Lebanon, Indiana; Dr Mary Stedman, Stedman Clinical Trials, Tampa, Florida; Dr Susan Steen, Axiom Clinical Research of Florida, Tampa; Dr James Sutton, Pacific Neuroscience, Oxnard, California; Dr Todd Swick, Houston, Texas; Dr Thomas Vidic, Elkhart Clinic, Elkhart, Indiana; Dr Jaron Winston, Senior Adults Specialty Research, Austin, Texas.
REFERENCES
1. Doody RS, Stevens JC, Beck C, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management of dementia (an evidence-based review). Neurology. 2001;56(9):1154-1166. PubMed
2. Forest Pharmaceuticals, Inc. Namenda. Prescribing Information. 2009. http://www.frx.com/pi/namenda_pi.pdf. Accessed May 6, 2009.
3. Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121. PubMed doi:10.4088/PCC.v09n0205
4. Small G, Dubois B. A review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23(11):2705-2713. PubMed doi:10.1185/030079907X233403
5. Gauthier S, Emre M, Farlow MR, et al. Strategies for continued successful treatment of Alzheimer’s disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003;19(8):707-714. PubMed doi:10.1185/030079903125002450
6. Auriacombe S, Pere JJ, Loria-Kanza Y, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease who failed to benefit from treatment with donepezil. Curr Med Res Opin. 2002;18(3):129-138. PubMed doi:10.1185/030079902125000471
7. Emre M. Switching cholinesterase inhibitors in patients with Alzheimer’s disease. Int J Clin Pract Suppl. 2002;(127):64-72. PubMed
8. Sadowsky CH, Farlow MR, Atkinson L, et al. Switching from donepezil to rivastigmine is well tolerated: results of an open-label safety and tolerability study. Prim Care Companion J Clin Psychiatry. 2005;7(2):43-48. PubMed doi:10.4088/PCC.v07n0201
9. Bartorelli L, Giraldi C, Saccardo M, et al. Upgrade Study Group. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer’s disease. Curr Med Res Opin. 2005;21(11):1809-1818. PubMed doi:10.1185/030079905X65655
10. Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol. 1998;1:55-65.
11. Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638. PubMed
12. Cummings J, Lefרvre G, Small G, et al. Pharmacokinetic rationale for the rivastigmine patch. Neurology. 2007;69(suppl 1):S10-S13. PubMed doi:10.1212/01.wnl.0000281846.40390.50
13. Winblad B, Grossberg G, Frölich L, et al. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology. 2007;69(suppl 1):S14-S22. PubMed doi:10.1212/01.wnl.0000281847.17519.e0
14. Winblad B, Kawata AK, Beusterien KM, et al. Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(5):485-491. PubMed doi:10.1002/gps.1806
15. Sadowsky CH, Dengiz A, Olin JT, et al. US38 Study Group. Switching from donepezil tablets to rivastigmine transdermal patch in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2009;24(3):267-275. PubMed doi:10.1177/1533317509333037
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Arlington, VA: American Psychiatric Press, Inc; 2000.
17. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. PubMed
18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
19. Ferris SH, Mackell JA, Mohs R, et al. A multicenter evaluation of new treatment efficacy instruments for Alzheimer’s disease clinical trials: overview and general results: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S1-S12. PubMed doi:10.1097/00002093-199700112-00001
20. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. PubMed doi:10.1097/00002093-199700112-00005
21. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. PubMed
22. Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210-215. PubMed
23. Blesa R, Ballard C, Orgogozo JM, et al. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology. 2007;69(4 suppl 1):S23-S28. PubMed doi:10.1212/01.wnl.0000281848.25142.11