Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2013;15(1):doi:10.4088/PCC.13alz01507
© Copyright 2013 Physicians Postgraduate Press, Inc.
Received: January 28, 2013; accepted January 28, 2013.
Published online: February 28, 2013.
AUTHORS
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Roy Yaari, MD, MAS, a neurologist, is associate director of the Memory Disorders Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Phoenix.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Phoenix.
Geri R. Hall, PhD, ARNP, GCNS, FAAN, is a gerontology clinical nurse specialist at Banner Alzheimer’s Institute and an adjunct clinical professor at the College of Nursing, University of Arizona, Phoenix.
Jan Dougherty, RN, MS, is director of Family and Community Services at Banner Alzheimer’s Institute.
Helle Brand, PA, is a physician assistant at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Adam S. Fleisher, MD, MAS, is associate director of Brain Imaging at the Banner Alzheimer’s Institute, a neurologist at the Institute’s Memory Disorders Clinic, and an associate professor in the Department of Neurosciences at the University of California, San Diego.
Corresponding author: Anna D. Burke, MD, Banner Alzheimer’s Institute, 901 E. Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Original material is selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME activities on a variety of topics from volume to volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the material and go to PrimaryCareCompanion.com to complete the Posttest and Evaluation online.
CME Objective
After studying this case, you should be able to:
- Diagnose elderly patients who have symptoms of memory impairment and behavioral problems
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through February 29, 2016. The latest review of this material was February 2013.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AstraZeneca, Forest, Janssen, Lundbeck, Merck, Pfizer, and Takeda and has been a member of the speakers/advisory board for Merck. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
HISTORY OF PRESENT ILLNESS
Mr A is a 68-year-old ambidextrous, married man who presented to the Memory Disorders Clinic at Banner Alzheimer’s Institute with his wife for evaluation of cognitive impairment. As per family report, symptoms of short-term memory deficit (such as forgetting to lock a door or to shut off the water) began insidiously approximately 3 years prior to the visit and gradually worsened over the following 3 years. No significant fluctuations of cognition were observed. He continued to be oriented to time and place and was not misplacing items. He had difficulty tracking appointments and was taking more copious notes. Mr A had poor insight into his cognitive changes and stated that the things he forgets are things that he does not want to remember.
Mr A was also noted to have a greater difficulty finding words to express himself and struggled to complete his thoughts. He became increasingly repetitive in conversations. Mr A’s wife reported that he would also become “obsessed” with a thought or event and constantly perseverate on it. For example, he would write multiple letters to his doctors or insurance agents regarding medical articles or billing statements. Mr A also displayed a preoccupation with where the garbage bags were placed in the home. He demanded that his wife leave garbage bags on counters and scattered them throughout the whole house. He became irate when the garbage bags were moved.
Mr A began to display increased irritability as well as verbal aggression toward his spouse. This was most prominent when discussing memory issues. In an attempt to minimize the agitation, Mr A’s primary care physician prescribed lorazepam to which Mr A had a paradoxical response resulting in increased agitation.
Mr A’s impulsivity was also notable in his driving. He was making inappropriate decisions and dangerous maneuvers on the road. As a result, his wife refused to drive with him. It was not until approximately 1 year ago that Mr A actually stopped driving after his primary care physician instructed that he stop. He continued to perseverate about his inability to drive and blamed his wife for his loss of autonomy. Mr A’s wife also reported socially inappropriate and “tactless” behaviors and a seeming loss of empathy toward others.
Gradually progressing visuospatial deficits were also reported. For example, Mr A would walk into a room and seemed unaware of various objects or people standing there. He was also bumping into things such as door jams more often and was tripping or stumbling on curbs more frequently. Gait was noted by family to be more shuffling.
Functionally, Mr A exhibited significant impairments in instrumental activities of daily living. He was no longer able to manage finances and was unable to appropriately use cash or calculate tips. He administered his own medications, but his wife was filling and monitoring his pill box. He would frequently scrutinize his medications and refuse to take them due to concerns about what he had read regarding their side effects on the Internet or perceived side effects that he was experiencing. Mr A continued to participate in regular shopping trips under the supervision of his wife. He was able to use the telephone and television but was no longer able to operate the microwave. He never cooked but continued to help with other household chores when directed. He remained independent in personal hygiene and grooming.
Mr A reported mild difficulty falling asleep, but no nighttime or early morning awakenings were noted. His family observed frequent movements of the limbs and restlessness during sleep. No apnea was noted. Mr A denied daytime somnolence. His appetite was fair with increased appetite for sweets. Mr A denied any symptoms of apathy and depression. Auditory and visual hallucinations were not reported. Mr A was increasingly concerned about others’ intents, but no frank paranoid ideations were observed.
PAST MEDICAL HISTORY
Mr A has a history of glaucoma, osteopenia, hypogonadism, transurethral resection of the prostate, benign prostatic hypertrophy, onychomycosis, and chronic lower back pain.
MEDICATIONS
Current medications included latanoprost, topical testosterone, dutasteride, terazosin, alendronate, terbinafine, tramadol 100 mg once daily, aspirin, fish oil, garlic oil, calcium citrate, fiber, senna, saline enema, multivitamin, and vitamin C.
SOCIAL HISTORY
Mr A has a 16-year educational history. He retired in 1994 as an owner of a retail business. He currently resides with his wife in their home. Mr A admitted to a childhood history of physical and emotional abuse at the hands of his father. He denied any sexual abuse.
Mr A was described by his spouse as always having a very loving though sometimes stubborn character. He had a “type A” personality and was very detail oriented. Mr A’s wife reported that he was always a pessimist and was also very sensitive to criticism. She felt that the negative personality traits were now becoming more pronounced.
SUBSTANCE ABUSE HISTORY
There was no known history of alcohol or illicit drug abuse. Mr A smoked less than a pack of cigarettes per day for approximately 40 years prior to quitting in 2004.
FAMILY HISTORY
As per family report, family history of dementia was unknown.
REVIEW OF SYSTEMS
Review of systems was positive for a more hunched posture, increased irritability and aggression, and difficulty with sleep as well as difficulty with sexual dysfunction.
The DSM-IV defines dementia as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. A diagnosis of dementia should not be made if the cognitive deficits occur exclusively during the course of a delirium (American Psychiatric Association, 2000).
Based on the information so far, do you think a dementia is present?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 80% |
| B. No | 0% |
| C. Not enough information | 0% |
| D. Could be psychiatric | 20% |
The majority of the conference attendees believed that a dementia was present. Multiple domains of cognition were affected as were instrumental activities of daily living. Those who chose “D” felt that Mr A’s affective symptoms, anxiety, and irritability may have been the driving force for his cognitive and functional limitations. Hence, they felt that a psychiatric illness could be primarily responsible for Mr A’s current presentation.
Different dementias may be associated with various physical examination findings. However, most often the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar, or palmomental reflex (Links et al, 2010).
Based on the information so far, what would you expect to see on the neurologic examination?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Normal | 0% |
| B. Objective neurologic findings (including frontal release signs) | 100% |
| C. Nonphysiologic findings (consistent with malingering) | 0% |
PHYSICAL EXAMINATION
The physical examination was unremarkable.
NEUROLOGIC EXAMINATION
Cranial nerve examination revealed broken smooth pursuits as well as bilateral decreased hearing to finger rub with intact gross hearing. A right palmomental sign was noted on examination of frontal release signs. Gait was noted to have been shuffling with stooped posture. Muscle strength was normal; however, muscle tone was mildly rigid in both upper extremities without cogwheeling. There were no abnormal movements or muscle atrophy. The remainder of the neurologic examination was unremarkable.
MENTAL STATUS EXAMINATION
Mr A was a moderately well-groomed elderly white man who appeared to be in no acute distress. He was pleasant and cooperative with the examination, though notably anxious. Affect was noted to be constricted and mildly dysphoric. Thought process was perseverative, and no visual or auditory hallucinations were noted. No suicidal, homicidal, or paranoid ideations were present. Judgment and insight were impaired. Speech was of normal volume and rate and slightly increased in amount. Orientation to time and place was impaired. Recent memory, attention, and concentration were impaired. Fund of knowledge was decreased for age and educational level.
LABORATORIES/RADIOLOGY
Laboratory studies available at the time of the visit were unremarkable. A magnetic resonance image (MRI) of the brain performed approximately 1 year prior revealed generalized cerebral and cerebellar volume loss that was felt to be slightly advanced for Mr A’s age. Mild deep white matter changes were also noted. No acute intracranial pathology was noted.
The origin of white matter changes in dementia is heterogeneous, and their significance in dementia continues to be debated. White matter changes are generally related to a loss of parenchymal integrity, increase in glial cells, vascular changes, atrophy, or necrosis with scarring and cavitation. If mild to moderate in severity, they are believed to be unlikely to significantly impact cognition and should not be interpreted as a “vascular dementia.”
A Mini-Mental State Examination (MMSE) score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and a score > 20 can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff of < 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975).
Based on the information so far, what would you expect the MMSE score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 5% |
| B. 21-25 | 90% |
| C. 16-20 | 5% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
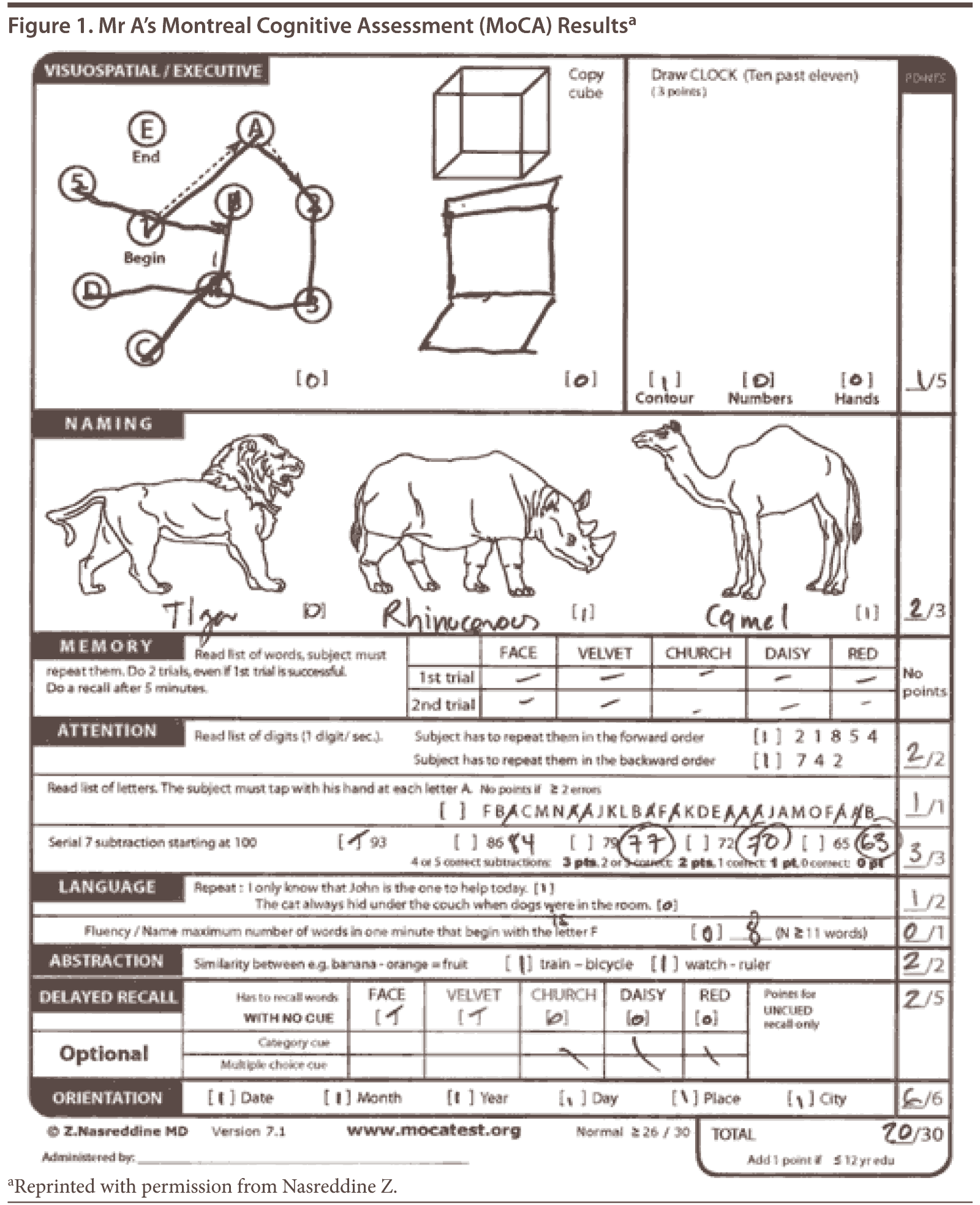
Mr A scored 22 out of 30 on the MMSE, losing 2 points on orientation, 3 points on attention and calculation, 1 point on drawing, and 2 points on delayed recall.
The Montreal Cognitive Assessment is a 30-point test that assesses several cognitive domains. Because it is more challenging than the Mini-Mental State Examination, it has greater sensitivity for mild cognitive impairment and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting mild cognitive impairment (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). This test is available at http://mocatest.org/.
Based on the information so far, what would you expect the MoCA score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 0% |
| B. 21-25 | 10% |
| C. 16-20 | 90% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
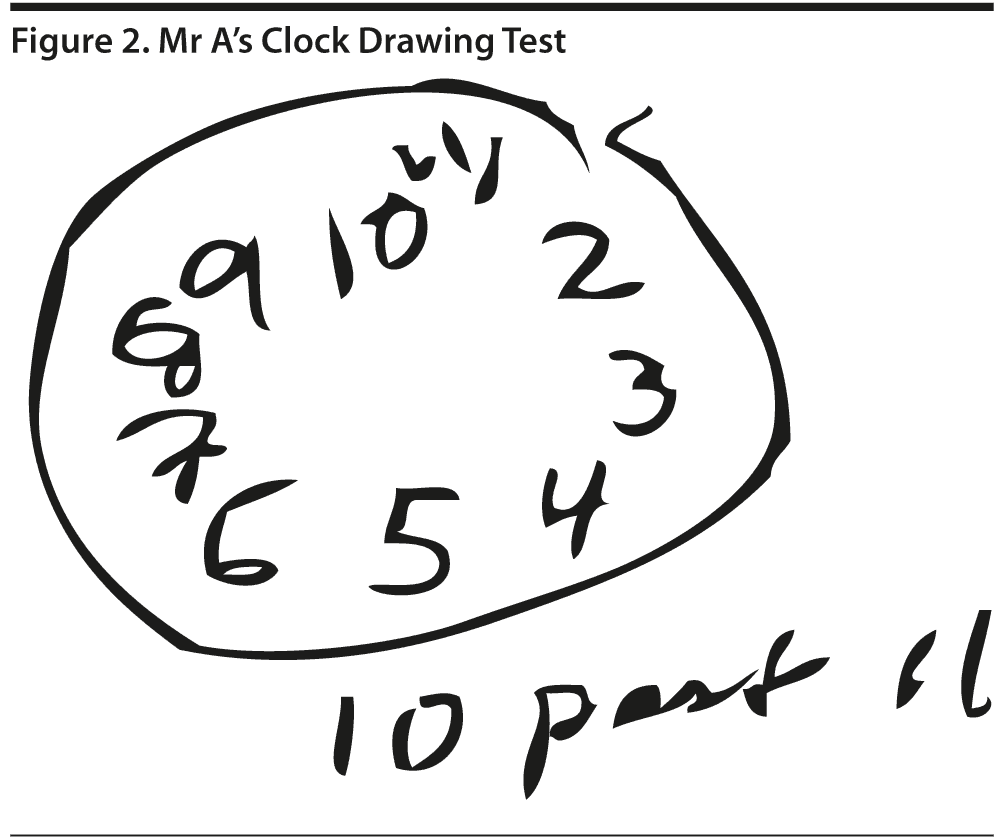
The MoCA revealed a total score of 20 out of 30, with Mr A displaying impairments in areas of visuospatial abilities, executive function, delayed recall, phonemic fluency, language, and naming (Figure 1). His clock drawing was significantly impaired (Figure 2). Previous neuropsychological testing performed approximately 1 year prior indicated a possible frontal lobe pathology, though a vascular etiology could not be ruled out at the time.
In order to better isolate which of the patient’s abilities may have been compromised or affected, a series of tests known as a “neuropsychological battery” may be administered by a neuropsychologist. A typical neuropsychological evaluation might focus on measuring various abilities including general intelligence, attention and concentration, learning and memory, motor and sensory functioning, auditory and visual processing, language functions, thinking, planning and organization, speed of processing, executive functioning, expressive functions, and emotions and personality (Lezak et al, 2004).
A neuropsychological evaluation can achieve the following:
- Provide a baseline for future testing
- Aid in the differentiation of the various dementia-causing presentations
- Identify compensatory strategies
- Assist in helping to judge if deficits are organic or psychiatric in nature
- Aid in earlier detection of “preclinical” dementia, such as mild cognitive impairment
- Describe patterns of cognitive weakness and strengths
- Assist in choosing treatments and preventative/postponing measures
Based on the information provided thus far, what is the patient’s most likely diagnosis?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Alzheimer’s dementia | 20% |
| B. Frontotemporal dementia | 30% |
| C. Dementia with Lewy bodies | 10% |
| D. Vascular dementia | 10% |
| E. Mixed dementia of Alzheimer’s and frontotemporal type | 20% |
| F. Bipolar disorder | 0% |
| G. Obsessive-compulsive disorder | 0% |
| H. Don’ t know | 10% |
Those participants who chose “A” felt that Mr A’s symptoms, which included primarily memory complaints, were most consistent with Alzheimer’s disease. Since Alzheimer’s disease is also the most common form of dementia, it was also the most likely cause of Mr A’s symptoms. Participants who chose “B” believed that Mr A’s impulsivity, personality changes, obsessions, lack of insight, and inappropriate behaviors were more consistent with a behavioral variant of frontotemporal dementia. Those who answered “C” felt that Mr A had prominent visuospatial impairment, a feature of Lewy body dementia. Furthermore, the sensitivity to lorazepam could indicate sensitivity to medications, another feature of dementia with Lewy bodies. Participants who chose “E” felt that both Alzheimer’s disease and frontotemporal dementia might be present, hence resulting in both memory symptoms and behavioral and personality changes.
What should the next step be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Repeat neuropsychological testing | 0% |
| B. Genetic testing for Alzheimer’s dementia | 0% |
| C. FDG-PET scan | 25% |
| D. Amyloid PET imaging | 0% |
| E. A and C | 75% |
| F. A, B, D | 0% |
Those who chose “C” felt that an FDG-PET scan would be best able to provide objective evidence differentiating frontotemporal and Alzheimer’s disease patterns of hypometabolism. The majority of those present believed that while an FDG-PET scan would be a good next step, neuropsychological testing should also be performed. This testing will establish a profile of cognitive strengths and weaknesses that could also help to clarify the diagnosis and be utilized in nonpharmacologic treatment planning. Since previous neuropsychological testing is available, a comparison of current neuropsychological testing will be useful in objectively assessing progression of illness (Pollock et al, 2007).
What should the next pharmacologic management step be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Start a cholinesterase inhibitor | 10% |
| B. Start an NMDA receptor antagonist | 0% |
| C. Start an antidepressant | 20% |
| D. Start an atypical antipsychotic | 10% |
| E. Start a benzodiazepine | 0% |
| F. Hold off on medication management until workup is completed | 60% |
The majority of those present felt that the workup should be completed prior to initiating pharmacotherapy. However, 40% of the participants were concerned about Mr A’s irritability, anxiety, and impulsivity and suggested that an antidepressant would best reduce these behavioral symptoms.
IMPRESSION
Although the physician believed that Mr A’s current symptoms were most likely due to a neurodegenerative process, she felt that the spouse’s reports of impulsivity, decreased judgment and insight, socially inappropriate behaviors, obsessions, and verbally aggressive behaviors were concerning for significant frontal lobe dysfunction. In addition, Mr A displayed short-term memory, visuospatial dysfunction, mild parkinsonian symptoms, rapid-eye-movement sleep disturbances, and a hypersensitivity and paradoxical response to a benzodiazepine. Mr A lacked the hallmark fluctuations of cognition and visual hallucinations commonly present in dementia with Lewy bodies. His parkinsonian symptoms were also felt to be quite mild. Despite a question of a vascular process noted on neuropsychological testing, there was no evidence of vascular pathology on the MRI. Taking into account Mr A’s history and clinical presentation, the physician felt that Mr A has a dementia, but the current diagnosis remained unclear, and further workup was indicated.
PLAN
- An FDG-PET scan of the brain was ordered to differentiate between a frontotemporal dementia and an Alzheimer’s dementia.
- A follow-up appointment was scheduled for Mr A’s wife with the Family and Community Services team to discuss problem behaviors and nonpharmacologic approaches to minimize their impact on the couple’s lives, as well as available resources in the area.
- Due to evidence of emerging paranoid ideations and agitation, quetiapine 25 mg every night at bedtime was started. The off-label nature of the use of this antipsychotic as well as the US Food and Drug Administration (FDA) black box warning were discussed with Mr A and his wife.
Currently, no psychotropic treatments for agitation in dementia have been approved by the US Food and Drug Administration. Consensus guidelines have been set forth by the American Geriatrics Society and American Association for Geriatric Psychiatry in their 2004 statement suggesting use of target symptoms to guide selection of drug class. The utilization of antipsychotics for agitation is an off-label use guided by evidence of their efficacy in agitation related to other psychiatric disorders (Zeller and Rhoades, 2010).
FOLLOW-UP VISIT WITH Family and Community Services TEAM 2 WEEKS LATER
Mr A’s wife met alone with a social worker and advanced practice nurse. At this time, additional information was obtained that Mr A’s spouse felt uncomfortable discussing at the initial evaluation when the patient was present. Mrs A admitted that her husband was verbally aggressive toward her. He was obsessed with her and repeatedly requested sexual relations, which she refused. She stated that he was also obsessed with having a bowel movement and needed to be near a bathroom at all times in case he does have a bowel movement. Mr A remained obsessed with garbage management. He placed plastic garbage bags on counters throughout the house and insisted that every piece of trash must be placed in it or taken outside within 1 minute. Mrs A felt uncomfortable discussing these behaviors at the initial visit due to her fear of triggering his anger.
Mrs A stated that her husband was extremely argumentative about “small things.” She reported that, over the last few nights, she was frightened by his threatening behavior toward her. She stated that she had to run from the room and hide because she was concerned that he might hurt her. Quetiapine 25 mg every night at bedtime was initiated after the initial evaluation with no noticeable effect. The social worker related this information to the treating physician.
What should the next step in management be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Start a cholinesterase inhibitor | 10% |
| B. Start an NMDA receptor antagonist | 0% |
| C. Start an antidepressant | 10% |
| D. Increase the quetiapine dose | 80% |
| E. Start a benzodiazepine | 0% |
| F. Admit to an inpatient psychiatric unit for stabilization | 0% |
The majority of those present felt that Mr A’s behaviors would best be controlled through an increase in the antipsychotic dose or even a change to a different antipsychotic that would be easier to administer and monitor such as risperidone. Although there is evidence that antidepressants and cognitive enhancers such as cholinesterase inhibitors and memantine are helpful in treatment of agitation, Mr A’s symptoms were felt to be severe enough to warrant more aggressive treatment (Fox et al, 2012; Holmes et al, 2004; Howard et al, 2007; Pollock et al, 2007; Wilcock, 2008).
PLAN
As a result of evidence of increased potential for violence, Mr A’s quetiapine dose was increased to 50 mg every night at bedtime with an additional 50 mg/day as needed for agitation. His wife, however, was unable to administer the medications regularly due to Mr A’s suspicions. A dissolvable form of risperidone 0.5 mg twice/day was prescribed to replace the quetiapine.
NONPHARMACOLOGIC MANAGEMENT
The social worker and an advanced practice nurse recommended the following interventions:
- They instructed Mrs A to have her husband complete advanced directives including a durable power of attorney for health care and a durable power of attorney for mental health. The latter affords the family the opportunity to pursue short-term acute psychiatric hospitalization without involving the court should Mr A pose a danger to himself or others.
- Mrs A was encouraged to develop a consistent daily routine for Mr A that, while not timed, follows a consistent pattern. This routine included periods of respite for Mrs A.
- Mrs A was instructed to disable the Internet and hide the postage stamps in order to prevent Mr A from continuing to send hostile letters.
- A male companion was hired to drive and accompany Mr A on outings.
- An adult day center program was suggested for the patient. Mr A felt that he could work there as a volunteer if he was paid. Each morning he attended, his wife would give the staff an envelope with $20 that they would “pay” Mr A at the end of the day. Mrs A would fill his briefcase with file folders, pencils, a spreadsheet, and a calculator. Mr A would wear a coat and tie and after arriving at work would be directed to a desk where he would open his briefcase and “work” off and on during the day.
- Lifeline Medical Alert Service (http://www.lifelinesys.com/content/home) was recommended to summon help in case of an emergency or violent episode. For her own safety, it was suggested that Mrs A sleep in their guest room and install a lock on the door so that Mr A could not enter.
- It was suggested that Mrs A develop an emergency plan such as locking herself in her bedroom or bathroom, summoning help using the Lifeline service, and waiting there until help arrives should she be threatened.
FOLLOW-UP VISIT WITH THE PHYSICIAN
Mrs A returned for follow-up 1 week later. She was growing increasingly fearful of her husband and had not followed through on any of the recommendations made by the social worker or nurse. Additionally, the dissolvable risperidone had not been initiated. She was fearful that that Mr A would be able to detect it in his milk or coffee and would become irate. She did increase his quetiapine to 25 mg in the morning and 50 mg in the evening without incident. Mr A managed his own medications and would not allow his wife to assist. Mrs A reported a mild decrease in agitation with the additional quetiapine dose.
Mr A continued to obsessively write multiple letters to his physicians. Mrs A stated that her husband had always written angry letters to people throughout his adult life. She stated that he had owned a retail business and had good credit and therefore was treated like a “king in his own little kingdom.” Thus, when he wrote angry letters of protest, people in his town paid attention, and he was able to get his way. He now spent a good deal of his day typing out angry letters for all sorts of perceived injustices. This behavior did not appear to respond to quetiapine.
Despite the mild benefit on agitation at home after initiation of quetiapine, Mr A’s ability to tolerate stress remained impaired. When presented with a challenge in public, Mr A would become extraordinarily angry, begin yelling and cursing, and physically threaten his wife. He also physically threatened his wife if he became enraged by something she did or said. Mrs A learned to walk away from such arguments and not engage her husband.
Based on the clinical presentation with the additional clinical history, what is the patient’s most likely diagnosis?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Alzheimer’s dementia | 0% |
| B. Frontotemporal dementia | 70% |
| C. Dementia with Lewy bodies | 0% |
| D. Vascular dementia | 0% |
| E. Mixed dementia of Alzheimer’s and frontotemporal type | 30% |
| F. Bipolar disorder | 0% |
| G. Obsessive-compulsive disorder | 0% |
The majority of the attendees felt that Mr A’s presentation, which was most notable for changes in personality and behaviors, was most consistent with a frontotemporal dementia. However, 30% argued that in addition to the behavioral symptoms, Mr A’s memory impairment indicated that an amnestic process was also present and that the presentation was most consistent with a mixed dementia of Alzheimer’s and frontotemporal type.
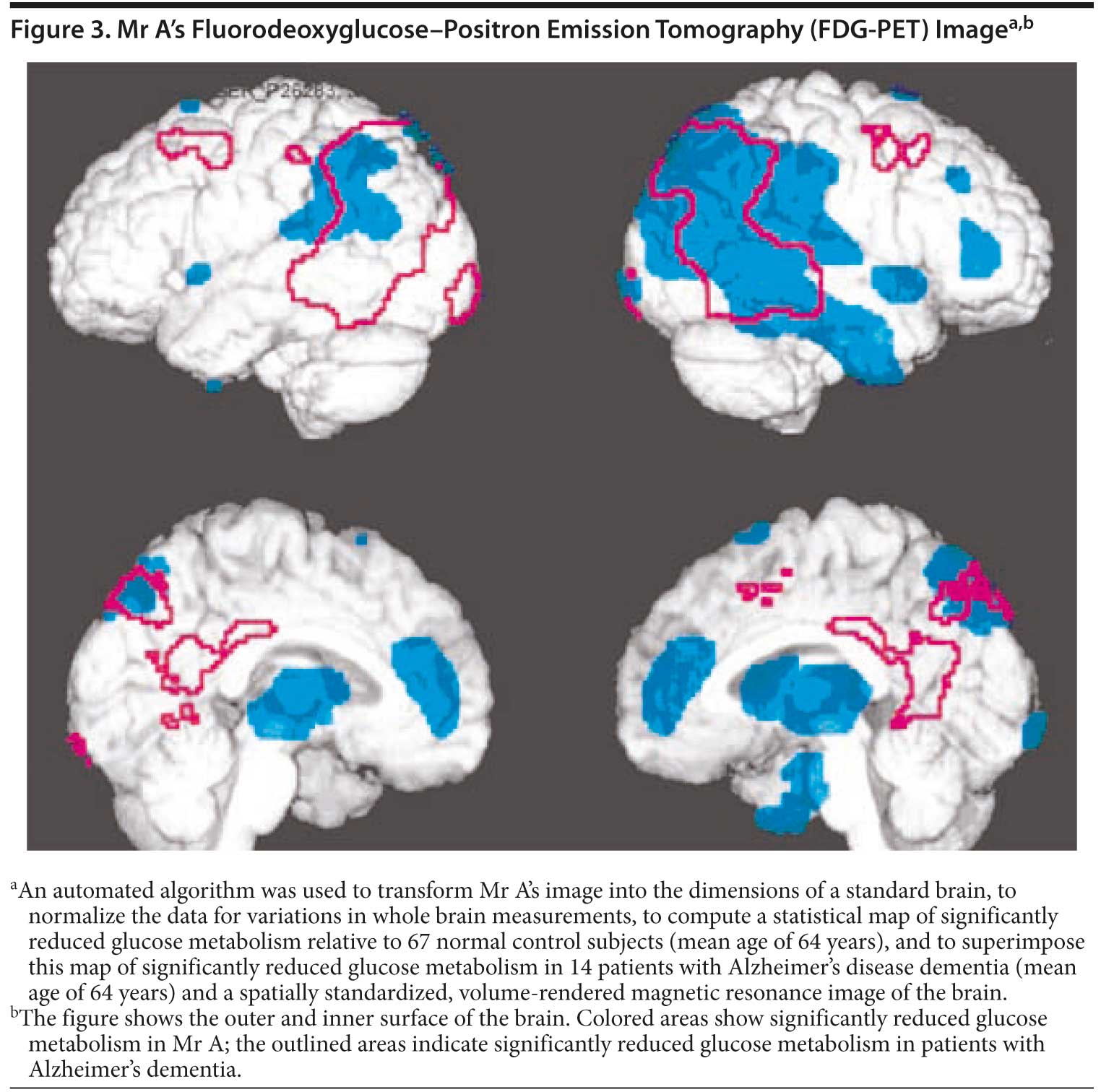
What would you expect to see on FDG-PET imaging?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Normal PET scan | 0% |
| B. Primarily frontal lobe hypometabolism | 40% |
| C. Primarily parietal lobe hypometabolism | 0% |
| D. Both frontal and parietal lobe hypometabolism | 60% |
Due to both prominent behavioral and personality changes as well as the presence of memory impairment, most of those present expected that both frontal lobe and parietal lobe dysfunction would be present on functional neuroimaging. Hypometabolism was present in the bilateral frontal lobes as well as the bilateral parietal lobes. The right temporoparietal region was also affected. The pattern suggested early Alzheimer’s changes (Figure 3). Based on the clinical presentation and PET scan results, the physician felt that Mr A’s presentation was most consistent with Alzheimer’s dementia currently of late mild to early moderate severity with prominent frontotemporal features.
Due to Mr A’s predominance of behavioral symptoms, the physician felt that it would be appropriate to refer the family to the Banner Alzheimer’s Institute Frontotemporal Dementia Support Group and to continue individual family supportive therapy in an attempt to optimize the environment and minimize triggers. More aggressive titration of psychotropics was also felt to be warranted, though due to the family’s reluctance to take over medication administration, this would prove difficult. Mr A’s quetiapine dose was increased to 50 mg twice/day with an additional 25 mg/day as needed for agitation. This resulted in additional mild improvement in agitation. Memantine was also started due to some evidence of its benefit on behavioral disturbances in dementia (Fox et al, 2012; Wilcock et al, 2008). The physician intended to continue titrating up the quetiapine dose and to add donepezil at the next visit. She also considered the addition of an antidepressant if perseverative behaviors remained distressing despite antipsychotic therapy (Pollock et al, 2007).
NONPHARMACOLOGIC MANAGEMENT FOR BEHAVIORAL SYMPTOMS RELATED TO ALZHEIMER’ S DISEASE
A small percentage of patients suffering from Alzheimer’s disease will present with prominent behavioral symptoms. Due to the fact that these symptoms frequently overshadow the short-term memory problems, patients may be misdiagnosed with a frontotemporal dementia. Although the underlying basis of neurodegeneration in Alzheimer’s disease versus frontotemporal dementia varies greatly, the similar clinical presentation prompts clinicians to implement similar nonpharmacologic treatment approaches in addressing problem behaviors. Care of these dementia variants is vastly different from that of a typical Alzheimer’s dementia because of the loss of insight, inability to inhibit, and narcissism. Although memory impairments exist, they tend to cause less distress to family than the changes in personality and behavior. Common symptoms of a frontotemporal variant of Alzheimer’s disease that affect care are listed below.
- Loss of insight
- Reduced capacity for empathy
- Narcissism
- Emotional lability
- Semantic aphasias
- Reduced ability to initiate activity, apathy
- Decreased interpersonal skills
- Obsessions
- Self-absorption
- Consistently poor judgment
- Severe disinhibition
- Able to understand cause and effect but cannot act on it
- Anger with aggression
The first task for families is typically to develop mechanisms to stem damage from disinhibited behavior, usually overspending (shopping, making poor investments, enrolling in scams, gambling, etc). If possible, durable powers of attorney should be obtained; however, because of the lack of insight, patients often refuse. Families may need to pursue guardianship. It is important to provide the family support and encourage contact with an attorney who specializes in elder law while they navigate the legal and financial options.
The second task is to provide for safety. A driving assessment is essential when available, and removal of the car due to tendencies toward impulsive driving, speeding, and vengeful driving patterns may be necessary. Many patients experience a stage of severe anger, heightened by the inability to inhibit responses. Guns, ammunition, power tools, garden machines, and other materials that could be used as weapons must be removed from the house, and care must be taken that they are not replaced.
Some patients may exhibit sexually inappropriate behaviors toward family, strangers, or even children. These behaviors should be monitored and treated with psychiatric follow-up before the patient either harms someone or is arrested.
A third task is to help the family develop activities that support the patient’s obsessions and accommodate shortened attention spans. For example, if the patient obsesses about a particular television show, have the family record it to play repeatedly, as trying to remove the object of an obsession increases patient anger.
PHARMACOLOGIC TREATMENT
Treatment of frontotemporal variants of Alzheimer’s disease requires us to address the underlying neurodegeneration that results in cognitive dysfunction and behavioral symptoms. There is now a mounting body of evidence as to the efficacy of cognitive enhancers such as cholinesterase inhibitors and memantine in the control of behavioral disturbances in Alzheimer’s disease (Fox et al, 2012; Holmes et al, 2004; Howard et al, 2007; Pollock et al, 2007). When these interventions alone fail, it becomes necessary to add therapeutic agents that target the most prominent symptom cluster. For example, if psychotic symptoms or severe agitation predominate, an antipsychotic may be used. If depression, apathy, or irritability are the main symptoms, an antidepressant may be a more appropriate choice.
Use of these pharmacologic agents for neuropsychiatric symptoms of dementia is off-label. There are currently no psychotropic treatments for agitation in dementia that have been approved by the FDA. Consensus guidelines have been set forth by the American Geriatrics Society and American Association for Geriatric Psychiatry in their 2004 statement suggesting use of target symptoms to guide selection of drug class (Table 1). These medications, however, are not without risks. In 2003, the FDA issued a warning titled “Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia” (http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm168933.htm). It referred to cerebrovascular adverse events (eg, stroke, transient ischemic attack), including fatalities, reported in patients in trials of risperidone in elderly patients with dementia-related psychosis and/or agitation. In placebo-controlled trials, a significantly higher incidence of cerebrovascular adverse events was noted in patients treated with risperidone compared to those treated with placebo. Shortly thereafter, a similar warning was applied to olanzapine and aripiprazole. The increased risk for cerebrovascular adverse events was determined from research clinical trials and is based on unbiased estimates. It is important to note that subsequent studies, mainly involving large public databases, have found similar or even higher death rates among seniors receiving conventional antipsychotics, which resulted in a second broader black box warning for all antipsychotics in 2008.
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, citalopram, escitalopram, quetiapine, risperidone, and sertraline are not approved by the US Food and Drug Administration for the treatment of agitation in dementia.
FINANCIAL DISCLOSURE
Dr Yaari is a consultant for Amedisys Home Health. Dr Tariot has served as a consultant for Abbott, AC Immune, Adamas, Avanir, Boehringer-Ingelheim, Chase, Chiesi, Eisai, Elan, MedAvante, Merz, Neuroptix, Otsuka, and Sanofi-Aventis; has received consulting fees and research support from AstraZeneca, Avid, Bristol-Myers Squibb, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, Medivation, Merck, Pfizer, Roche, and Toyama; has received research support only from Baxter, Functional Neuromodulation, GE, and Targacept; has received other research support from Alzheimer’s Association, Arizona Department of Health Services, National Institute of Mental Health, and National Institute on Aging; is a stock shareholder in Adamas; and is listed as a contributor to a patent owned by the University of Rochester (Rochester, New York) for “Biomarkers of Alzheimer’s Disease.” Drs Burke, Hall, and Fleisher and Mss Dougherty and Brand have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
FUNDING/SUPPORT
None reported.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
Clinical Points
- Patients with Alzheimer’s dementia present with both cognitive and behavioral symptoms that can sometimes mimic other forms of dementia or affective disorders.
- Nonpharmacologic interventions aimed at minimizing potential triggers for problem behaviors are key elements in the treatment of agitation and may lessen the need for increasingly aggressive pharmacotherapy.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Arlington, VA: American Psychiatric Association; 2000.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6 PubMed
Fox C, Crugel M, Maidment I, et al. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS ONE. 2012;7(5):e35185. doi:10.1371/journal.pone.0035185 PubMed
Holmes C, Wilkinson D, Dean C, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63(2):214-219. doi:10.1212/01.WNL.0000129990.32253.7B PubMed
Howard RJ, Juszczak EJ, Ballard CG, et al; CALM-AD Trial Group. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med. 2007;357(14):1382-1392. doi:10.1056/NEJMoa066583 PubMed
Lezak MD, Howieson DB, Loring DW, eds. Neuropsychological Assessment, 4th Edition. New York, NY: Oxford University Press; 2004.
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607.PubMed
Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54-58, 63, 66.PubMed
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x PubMed
Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry. 2007;15(11):942-952. doi:10.1097/JGP.0b013e3180cc1ff5 PubMed
Wilcock GK, Ballard CG, Cooper JA, et al. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341-348. doi:10.4088/JCP.v69n0302 PubMed
Zeller SL, Rhoades RW. Systematic reviews of assessment measures and pharmacologic treatments for agitation. Clin Ther. 2010 Mar;32(3):403-425. doi:10.1016/j.clinthera.2010.03.006 PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite