ABSTRACT
Objective: To assess the efficacy, safety, and tolerability of topiramate for the treatment of posttraumatic stress disorder (PTSD) in civilians.
Methods: This 12-week double-blind, randomized, placebo-controlled study enrolled 72 outpatients (aged 19–64 years) with a DSM-IV-TR diagnosis of non–combat-related PTSD and a score ≥ 50 on the Clinician-Administered PTSD Scale (CAPS). The primary efficacy endpoint, percent change in total CAPS score, and secondary efficacy measures were assessed by analysis of covariance. Safety assessments included monitoring of vital signs, physical examinations, clinical laboratory parameters, electrocardiograms, and adverse events (AEs). The study was conducted from October 2001 to March 2004.
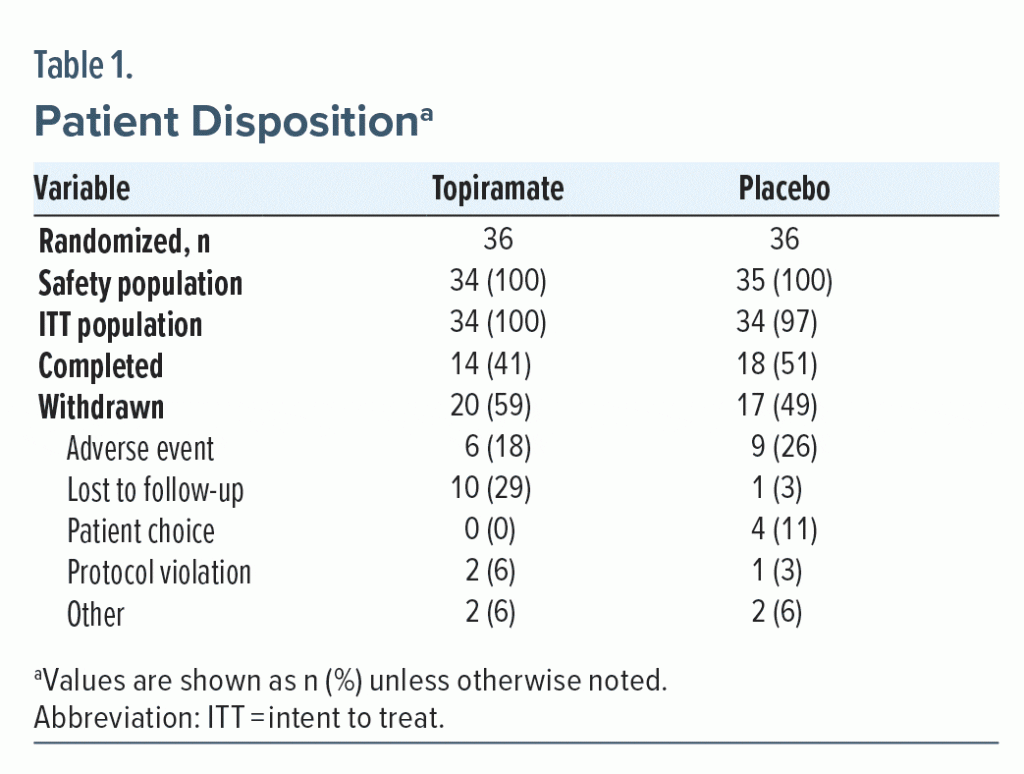
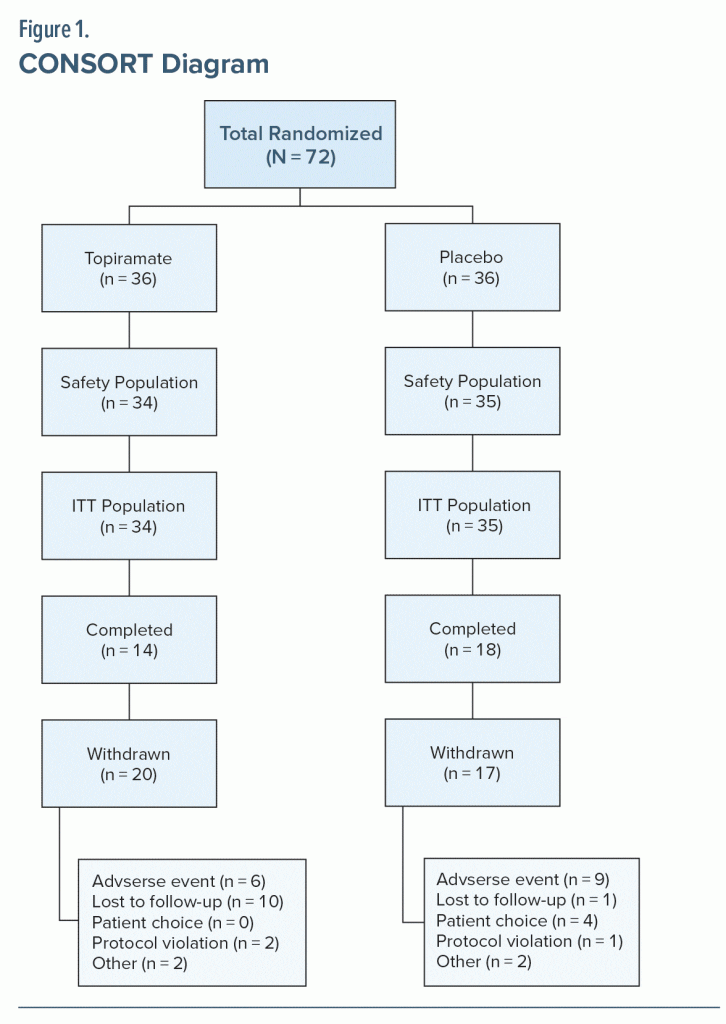
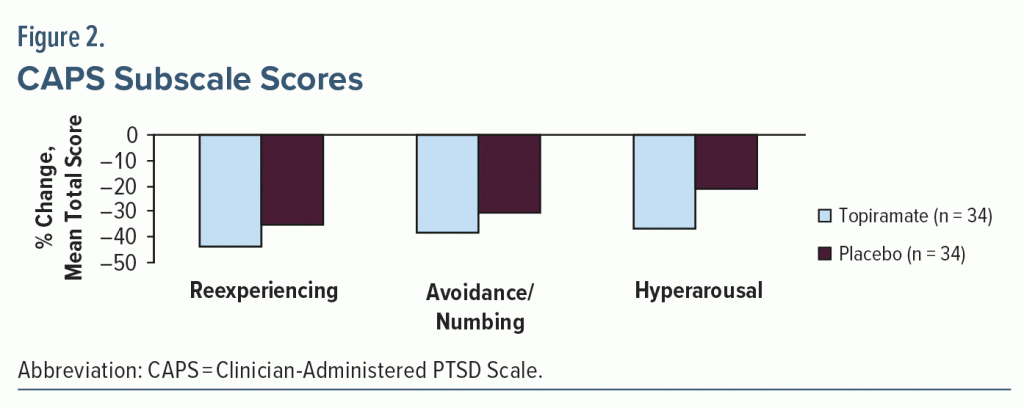
Results: The intent-to-treat (ITT) population (N = 68; mean age = 35 years; 87% women; 74% White) showed greater percent reduction in total CAPS scores with topiramate versus placebo (39.5% vs 29.5%), but the difference was not statistically significant (P = .31). Similarly, higher reductions with topiramate versus placebo were seen in the CAPS subscale scores for symptoms of reexperiencing (43.6% vs 34.8%), avoidance/numbing (38.3% vs 30.6%), and hyperarousal (36.6% vs 21.4%). However, these differences were not statistically significant. Six patients in the topiramate arm had a final CAPS score < 20, whereas only 2 in the placebo arm achieved the result (P = .075). The median final topiramate daily dose was 100 mg/d (range, 25–400 mg/d), and mean ± SD treatment duration was 55 ± 32 days, showing the tolerability of the medication. In topiramate-treated patients, treatment-emergent AEs included paresthesia, headache, fatigue, and insomnia; treatment-limiting AEs included influenza-like symptoms, agitation, cognitive problems not otherwise specified, and somnolence. However, a higher rate of AE-related discontinuation was seen in the placebo group than in the treatment group (26% vs 18%).
Conclusions: In this 12-week civilian PTSD study, topiramate improved the primary and secondary outcome measures at a higher rate than did placebo, but the difference did not reach statistical significance. Further adequately powered studies may be warranted.
Trial Registration: Clinical Trials.gov identifier: NCT00208130
Prim Care Companion CNS Disord 2023;25(5):23m03555
Author affiliations are listed at the end of this article.
Posttraumatic stress disorder (PTSD) is a major public health problem with an estimated lifetime prevalence of 8.3% in the general population in the US.1 A study conducted by Koenen et al2 in 2017 using data from 24 countries with over 68,000 respondents showed that the lifetime prevalence of PTSD was 3.9%. The study revealed that the rates were higher in high-income countries (5.6%) and lower in low- and middle-income countries.2 A broad range of potentially traumatic events common to civilian life such as violent assault, child abuse, accidents, kidnapping, and natural disaster may result in PTSD.3
Classified as an anxiety disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),4 PTSD may cause significant levels of individual distress and functional impairment. PTSD is defined by diagnostic criteria that include a history of exposure to a traumatic event (or events) and symptoms from each of the 3 symptom clusters: intrusive recollections (cluster B), avoidance/numbing (cluster C), and hyperarousal (cluster D). In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), PTSD is classified as a trauma- and stressor-related disorder. Over the past several decades, we have gained greater insight into the diagnosis, treatment, and natural history of PTSD. Standard treatments for PTSD include pharmacotherapy and psychotherapy. Pharmacotherapy targets symptoms such as anxiety, arousal, and depression. Psychotherapy ranges from supportive and skills-building approaches to exposure-based therapies that address dysregulated traumatic memories. Both treatments aim to alleviate symptoms and promote healing.5
The pharmacotherapeutic options for PTSD have been primarily focused on selective serotonin reuptake inhibitors (SSRIs) and are insufficiently developed; most studies are open-label trials and case examples with only a few placebo-controlled trials. Currently, only 2 drugs, sertraline, and paroxetine, are approved by the US Food and Drug Administration (FDA) for the management of PTSD.6,7 Even among the SSRIs, there are mixed results. Whereas sertraline and paroxetine have shown efficacy in management of PTSD, fluoxetine has displayed mixed results.8–12 Tricyclic antidepressants such as amitriptyline and desipramine and the reversible monoamine oxidase inhibitor brofaromine failed to show any significant difference when compared to placebo.13–15 A study comparing imipramine and phenelzine16 found both medications to be effective in treating PTSD; however, both medications had significant side effects such as dry mouth, hypotension, and insomnia. Vilazodone, a newer antidepressant, did not demonstrate any statistically significant improvement in PTSD symptoms.17 While atypical antipsychotics such as olanzapine and risperidone may have a potential role in PTSD, small sample sizes and inconclusive results plague the data.18,19 Furthermore, very few studies have tested atypical antipsychotics as monotherapy for PTSD.20
Anticonvulsants have been found effective in the treatment of a myriad of mental illnesses, and carbamazepine, lamotrigine, valproic acid, and gabapentin have been investigated for the treatment of PTSD. However, the results have been mixed for the treatment of PTSD. A systematic review and meta-analysis performed by Adamou et al21 to determine the efficacy of valproic acid in PTSD found limited evidence for its use. A randomized double-blind placebo-controlled trial of valproic acid in a veteran population22 failed to establish efficacy in PTSD. Other studies using anticonvulsants in PTSD were either inconclusive or had negative results.23 Among antiepileptic drugs, lamotrigine showed some positive response in PTSD; however, the data are limited.24 A retrospective clinical series of 30 patients taking gabapentin25 showed that it was helpful in improving sleep and nightmares in PTSD. Similarly, data on carbamazepine in PTSD are limited and inconclusive.26–28
Topiramate is FDA approved for the treatment of epilepsy and migraine prophylaxis and has several broad-spectrum pharmacologic modes of action. It has been investigated for its potential efficacy in treating PTSD. Topiramate enhances γ-aminobutyric acid A (GABAA) receptor function, blocks the voltage-sensitive sodium and calcium ion channels, acts as an antagonist at the kainite/α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) subtype glutamate receptors, inhibits the action of carbonic anhydrase, and enhances the conductance of potassium channels.29–31 Because of its broad-spectrum mechanisms of action, it was hypothesized that topiramate will be effective in treating PTSD symptoms. The primary objective of our study was to investigate the efficacy and safety of topiramate for the treatment of civilian PTSD.
METHODS
Study Design
This study was a 12-week, multicenter, randomized, double-blind, placebo-controlled trial in outpatient clinics with a 1:1 parallel-group investigation. The study spanned a period from October 2001 to March 2004, during which data were collected, analyzed, and evaluated. Written informed consent was obtained from all participants, and approval was received for this study by the institutional review boards at participating sites. The study was conducted in compliance with the Declaration of Helsinki, and it was registered at ClinicalTrials.gov (identifier: NCT00208130).
The study consisted of 4 phases: screening/washout (up to 30 days before randomization), double-blind (titration ≤ 8 weeks, maintenance ≥ 4 weeks), taper (~1 week), and open-label extension (12 weeks; not reported here). Patients were sequentially randomized upon study qualification for enrollment to receive either topiramate or placebo in a 1:1 ratio according to computer-generated coding. Randomization was balanced by use of permuted blocks. Topiramate dosing was initiated at 25 mg. Dosage was titrated weekly by 25- to 50-mg increments based on efficacy and tolerability, and topiramate was given twice daily with a maximum daily dose of 400 mg.
Patient Population, Inclusion Criteria, and Exclusion Criteria
Inclusion criteria were being a man or woman aged 19 to 64 years, diagnosis of civilian, non–combat-related PTSD according to DSM-IV-TR for more than 6 months, and a score ≥ 50 on the Clinician-Administered PTSD Scale32,33 on day 1. Patients with major organic psychiatric disease, current substance dependence or abuse (excluding nicotine or caffeine), serious or unstable concurrent illness, medical conditions potentially affecting drug absorption, history of nephrolithiasis or seizures, current enrollment in a cognitive-behavioral therapy program, or known hypersensitivity to or a prior adverse event (AE) with topiramate, as well as pregnant and nursing women, were excluded from the study.
Study Procedure
Interventions. Following a designated washout period for protocol-prohibited medications (carbonic anhydrase inhibitors, SSRIs [including fluoxetine], tricyclic antidepressants, benzodiazepines), study medication was started at 25 mg/d and was titrated weekly over a maximum 8-week period to the maximum tolerated dose until complete or near-complete efficacy was achieved or until the maximum dose allowed (400 mg/d) was reached. This dose was then maintained over an additional 4 weeks.
Outcome assessments. The primary efficacy variable was the change in the 17-item total severity score of the CAPS32,33 from baseline to the last visit of the double-blind phase. Changes from baseline in symptom severity for the 3 CAPS subscales (measuring symptoms of reexperiencing, avoidance/numbing, and hyperarousal) were also assessed. Secondary efficacy variables consisted of changes from baseline to last visit of the double-blind phase in scores on the Hamilton Anxiety Rating Scale (HARS),34 the Hamilton Depression Rating Scale (HDRS),35 the Treatment Outcome PTSD Scale (TOP-8),36 and the Clinical Global Impressions scale (CGI).37 Outcomes were assessed according to the following schedule: (a) days 1, 28, 56, and 84: CAPS, HDRS, and HARS; (b) days 1, 14, 42, 70, and 84: TOP-8; (c) days 1, 7, 14, 28, 42, 56, 70, and 84: CGI.
Safety assessments including monitoring of vital signs, clinical laboratory (blood chemistry and complete blood count) parameters, and AEs were performed at each visit. AEs were coded according to the World Health Organization Adverse Reaction Terms (WHOART) dictionary.
Statistical analysis. Efficacy analysis was conducted on the intent-to-treat (ITT) population that included all randomized patients who had taken ≥ 1 dose of study medication and had ≥ 1 post-baseline efficacy evaluation. SAS (version 6.12) was used for all statistical analyses. The primary efficacy analysis for the change in CAPS score (comparison between treatment groups) was based on an analysis of covariance (ANCOVA) model using treatment as qualitative factor and baseline CAPS score as covariate. Secondary efficacy analyses of TOP-8, HARS, and HDRS scores were analyzed by ANCOVA using treatment as qualitative factor and its baseline score as covariate. ANCOVA was also performed for CGI score using treatment as qualitative factor and baseline CAPS score as covariate. All statistical tests were 2-sided at a significance level of .05. The temporal course of response to treatment was examined by summarizing the efficacy data by treatment over the time points when the assessments were conducted. An a priori power analysis was not performed for this study because it was a proof-of-concept study and was exploratory in nature.
RESULTS
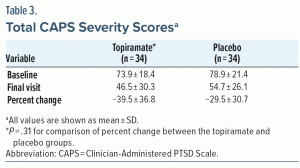
A total of 72 patients were randomized, and 68 patients constituted the ITT population. One patient in the placebo group discontinued study participation because of an AE and was considered evaluable for safety but was not included in the ITT population. Two patients in the topiramate group and 1 in the placebo group were not included in either evaluation. Attrition rates in both treatment arms of this study were high (topiramate, 58.8%; placebo, 48.6%). A summary of patient disposition and reasons for withdrawal is provided in Table 1 and Figure 1. Demographic characteristics were similar between treatment groups (Table 2). Mean baseline CAPS total severity scores were 73.9 ± 18.4 (topiramate, n = 25) and 78.9 ± 21.4 (placebo, n = 30); PTSD scores measured with this instrument can vary between 0 (no symptoms) and a maximum of 136 (all symptoms most severe). Median final daily topiramate dose was 100 mg/d (range, 25–400 mg/d). Mean ± SD topiramate treatment duration was 55.3 ± 31.6 days.
The total CAPS severity score was decreased from baseline (topiramate, −39.5% ± 36.8%; placebo, −29.5% ± 30.7%), but this difference did not reach statistical significance (P = .31) (Table 3). Responder analysis revealed final CAPS scores were < 20 in 6 topiramate patients compared with 2 placebo patients (P = .075). Mean percent change in CAPS subscale scores for symptoms of reexperiencing (topiramate, 43.6 ± 44.9 vs placebo, 34.8 ± 41.2; P = .45), avoidance/numbing (38.3 ± 41.5 vs 30.6 ± 36.9; P = .56), and hyperarousal (36.6 ± 42.3 vs 21.4 ± 39.3; P = .16) were lower, but not significantly, in topiramate versus placebo patients (Figure 2). Mean percent change at final visit scores of secondary efficacy measures are as follows: HARS: topiramate, −22.2 ± 62.6; placebo, −20.4 ± 62.9 (P = .82); HDRS: topiramate, −21.3 ± 55.5; placebo, −13.1 ± 75.4 (P = .49); TOP-8: topiramate, −46.0 ± 35.3; placebo, −31.7 ± 41.3 (P = .11); and CGI-Severity: topiramate, −15.9 ± 22.2; placebo, −21.2 ± 23.9 (P = .51). Mean total CGI-Improvement scores were similar between treatment groups (topiramate, 3.0 ± 1.3; placebo, 3.0 ± 1.6; P = .94).
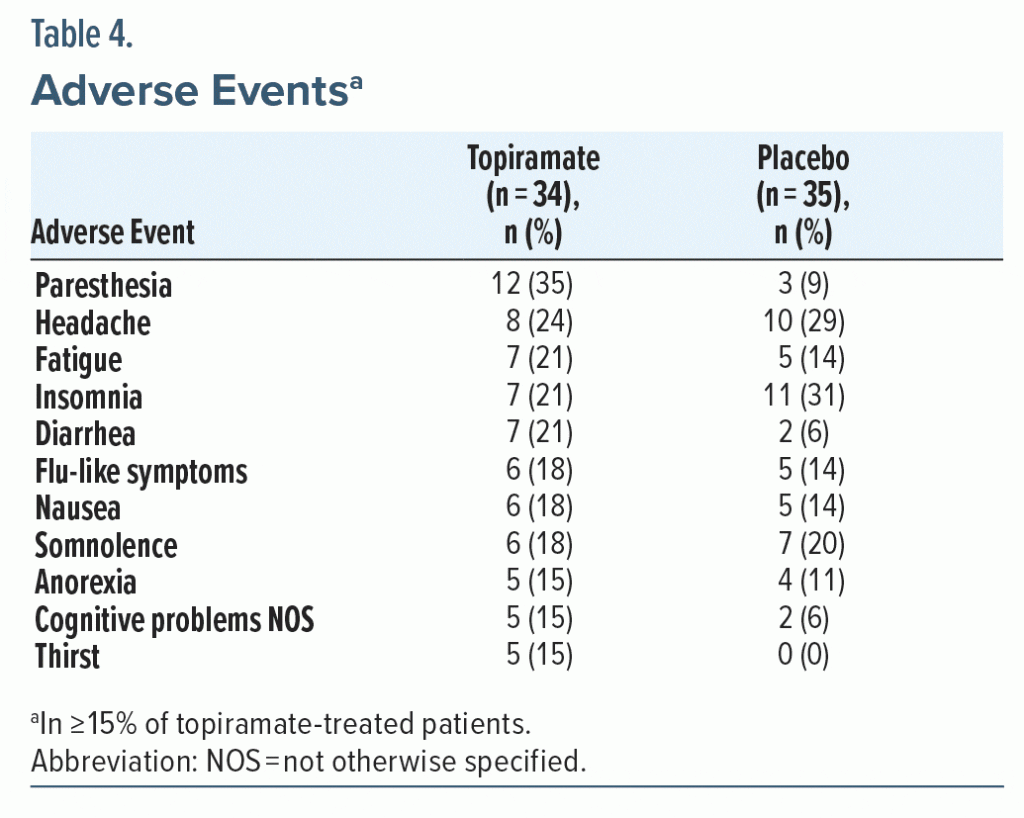
AEs occurring in ≥ 15% of topiramate-treated patients are summarized in Table 4. The most common treatment-emergent AEs occurring in the topiramate group included paresthesia, headache, fatigue, and insomnia (Table 4). The numbers of discontinuations attributed to AEs for each group are listed in Table 1. Treatment-limiting AEs occurring in the topiramate group included influenza-like symptoms, agitation, cognitive problems not otherwise specified, and somnolence. No meaningful differences in any laboratory test or vital sign parameter were documented in the treatment groups.
DISCUSSION
This double-blind, placebo-controlled study investigated topiramate for the treatment of civilian, non–combat-related PTSD. The mean CAPS scores in this study represent a patient population that is moderately to severely affected by the disease. Topiramate was safe to use in this population. Although the topiramate group showed a decrease in CAPS score, it was not statistically significant compared to placebo. Even without a statistically significant improvement in the active arm, it is noteworthy that an almost 40-point decrease in the CAPS scores with topiramate is considered a clinically meaningful improvement in any setting. While there is no standard threshold to define clinically significant change in CAPS score, the Institute of Medicine (IOM) advises common methods to define clinically significant change in CAPS score as a 10-point decrease, a 30% decrease, or 2 standard deviations below pretreatment level.38–40 In this study, participants in the active arm had a 39.5% decrease in CAPS score, certainly a clinically significant change per the IOM recommendation. However, participants in the placebo arm improved by 29.5% as well. Our a priori data analytic plan included the analysis of CAPS data as a continuous variable, but future studies might consider using more recently available cutoffs for clinical response/remission. Similarly, the secondary efficacy measures HARS, HDRS, TOP-8, and CGI-S could not determine any statistically significant difference compared to placebo.
The results of the current study are consistent with the literature. Tucker et al41 conducted a similar study in 38 patients. A greater decrease in total CAPS score was noted with topiramate (−52.7% vs –42.0% with placebo), but this difference was not statistically significant (P = .232). Twice as many patients receiving topiramate as receiving placebo achieved remission. Topiramate-treated patients exhibited a significant percent reduction in reexperiencing symptoms (CAPS cluster B; topiramate, 74.9%; placebo, 50.2%; P = .038) and score on the TOP-8 overall severity scale (topiramate, 68.0%; placebo, 41.6%; P = .025). Mean ± SD total CGI-Improvement scores (topiramate, 1.9 ± 1.2; placebo, 2.6 ± 1.1; P = .055) also demonstrated marginally significant reductions favoring topiramate. Another study conducted at Federal University of Sao Paulo Hospital in 35 patients42 showed that topiramate was effective in improving reexperiencing and avoidance/numbing symptom clusters in patients with PTSD, with 82.35% of patients in the topiramate group exhibiting improvement in PTSD symptoms. Topiramate was well tolerated, and the mean dose was 102.94 mg/d.42
Other data investigating the role of topiramate in PTSD are limited to civilian, non–combat-related PTSD. A 4-week study of prospective open-label monotherapy or adjunctive therapy43 was conducted in civilians with chronic PTSD (N = 33). At week 4, total symptoms as measured with the PTSD Checklist–Civilian Version decreased by 49% (paired t test, P < .001), with subscale score reductions that were similar for reexperiencing, avoidance/numbing, and hyperarousal symptoms. A 77% response rate was documented at week 4.43
A naturalistic data review of medical records examining open-label topiramate as monotherapy or adjunctive therapy in civilians with chronic PTSD44 was conducted (N = 35); mean duration of treatment was 33 weeks. The study found that a decrease in nightmares and flashbacks was noted in 79% and 86% of patients, respectively, with full suppression in 50% and 54% of patients with these symptoms.44 Another double-blind, placebo-controlled study examined topiramate as augmentation therapy in combat veterans with chronic PTSD (N = 40) for 7 weeks.45 Primary treatment outcomes reflected in CAPS scores did not reveal significant treatment effects versus placebo, which may relate to the specific population investigated. Treatment response analysis was limited by a high discontinuation rate (topiramate, 55%; placebo, 25%).45
Experience with other antiepileptic agents (carbamazepine, valproic acid, gabapentin, and lamotrigine) in the treatment of PTSD is also limited, and even randomized double-blind placebo-controlled trials have failed to show any significant efficacy.20–27
Several measures were taken to maintain the blinding during the study. For the participants, we used look-alike placebo pills for the study. For the investigators, the randomization was balanced by use of permuted blocks. It is possible that the investigators might have guessed the arm allocation due to potential AEs reported during the titration phase. However, the AEs, vital signs, and laboratory results were not markedly different between the groups. Furthermore, the rate of discontinuation due to AEs was higher in the placebo group than in the active group (26% vs 18%). There was no instance of accidental unblinding during the study. Given these factors, we feel confident about maintaining the blinding throughout the study. Future research may consider asking the investigators and participants about the perceived group assignment at the end of the intervention to analyze the impact of expectation on results.
Strengths and Limitations
Strengths of this study include the study design, use of well-validated primary and secondary outcome measures, and pragmatic inclusion and exclusion criteria to enroll real-life patients.
However, there are several limitations, including small sample size, lack of prior power analysis due to the pilot nature of the study, high attrition rates, and the heterogeneity of the study population. The potential impact of differences in the degree and duration of symptomatology, the characterization of trauma type, and the presence and duration of comorbidities on outcome have not been evaluated. It is unclear if a longer-duration study would have shown separation in the 2 arms.
CONCLUSIONS
In this pilot civilian PTSD study, topiramate was safe to use and resulted in clinically meaningful reduction in PTSD symptomatology. However, the improvement in CAPS score in the active arm did not significantly separate from that seen in the placebo arm. Given that the mean difference in all primary and secondary outcomes assessed demonstrated a tendency in favor of topiramate, it is possible that further adequately powered studies may find topiramate to be efficacious.
Article Information
Published Online: October 19, 2023. https://doi.org/10.4088/PCC.23m03555
© 2023 Physicians Postgraduate Press, Inc.
Submitted: May 3, 2023; accepted June 28, 2023.
To Cite: Monga V, Petty F, Padala K, et al. Topiramate monotherapy for civilian posttraumatic stress disorder: a controlled pilot study. Prim Care Companion CNS Disord. 2023;25(5):23m03555.
Author Affiliations: Banner Thunderbird Medical Center, Glendale, Arizona (Monga); Orlando VA Medical Center, Orlando, Florida (Petty); The University of Central Florida College of Medicine, Orlando, Florida (Petty); Central Arkansas Veterans Healthcare System, Little Rock, Arkansas (K. Padala, P. R. Padala); University of Arkansas for Medical Sciences, Little Rock, Arkansas (K. Padala, P. R. Padala); Baptist Health-UAMS Graduate Medical Education, North Little Rock, Arkansas (P. R. Padala).
Corresponding Author: Varun Monga, MD, Banner Medical Group, Banner Thunderbird Medical Center, 5555 W Thunderbird Rd, Glendale, AZ 85306 ([email protected]).
Relevant Financial Relationships: Dr Petty has received grant funding from Ortho-McNeil Janssen Scientific affairs, LLC. Drs Monga, K. Padala, and P. R. Padala report no relevant financial relationships.
Funding/Support: The study was supported by grants from Ortho-McNeil Janssen Scientific Affairs, LLC.
Role of the Funders/Sponsors: Ortho-McNeil Janssen Scientific Affairs, LLC, provided funding for the study and supply of medication/placebo. The funder had no oversight on study data analysis and interpretation.
ORCID: Varun Monga: https://orcid.org/0000-0002-2994-0085; Prasad R Padala: https://orcid.org/0000-00031921-9806; Kalpana Padala: https://orcid.org/0000-0001-6021-0593
Clinical Points
- Only a few pharmacologic options for the treatment of posttraumatic stress disorder (PTSD) have been approved by the US Food and Drug Administration.
- In this pilot study on civilian PTSD, the use of topiramate was found to be safe and demonstrated a clinically significant reduction in PTSD symptoms.
- Further research is needed on newer pharmacologic treatments for the management of PTSD.
References (45)

- Kilpatrick DG, Resnick HS, Milanak ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537–547. PubMed CrossRef
- Koenen KC, Ratanatharathorn A, Ng L, et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med. 2017;47(13):2260–2274. PubMed CrossRef
- Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2–9. PubMed
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
- Ressler KJ, Berretta S, Bolshakov VY, et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol. 2022;18(5):273–288. PubMed CrossRef
- Zoloft (sertraline HCl) Tablets and Oral Concentrate, prescribing information. New York: Roerig/Pfizer; 2011. https://labeling.pfizer.com/ShowLabeling.aspx?id=517.
- Paxil (paroxetine HCl) Tablets and Oral Suspension, prescribing information. Research Triangle Park, N.C: GlaxoSmithKline; 2011. http://us.gsk.com/products/assets/us_paxil.pdf.
- Davidson JR, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58(5):485–492. PubMed CrossRef
- Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837–1844. PubMed CrossRef
- Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62(11):860–868. PubMed CrossRef
- Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in post-traumatic stress disorder: randomised, double-blind study. Br J Psychiatry. 1999;175(1):17–22. PubMed CrossRef
- Martenyi F, Brown EB, Caldwell CD. Failed efficacy of fluoxetine in the treatment of posttraumatic stress disorder: results of a fixed-dose, placebo-controlled study. J Clin Psychopharmacol. 2007;27(2):166–170. PubMed CrossRef
- Davidson J, Kudler H, Smith R, et al. Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry. 1990;47(3):259–266. PubMed CrossRef
- Reist C, Kauffmann CD, Haier RJ, et al. A controlled trial of desipramine in 18 men with posttraumatic stress disorder. Am J Psychiatry. 1989;146(4):513–516. PubMed CrossRef
- Baker DG, Diamond BI, Gillette G, et al. A double-blind, randomized, placebo-controlled, multi-center study of brofaromine in the treatment of post-traumatic stress disorder. Psychopharmacology (Berl). 1995;122(4):386–389. PubMed CrossRef
- Frank JB, Kosten TR, Giller EL Jr, et al. A randomized clinical trial of phenelzine and imipramine for posttraumatic stress disorder. Am J Psychiatry. 1988;145(10):1289–1291. PubMed CrossRef
- Ramaswamy S, Driscoll D, Reist C, et al. A double-blind, placebo-controlled randomized trial of vilazodone in the treatment of posttraumatic stress disorder and comorbid depression. Prim Care Companion CNS Disord. 2017;19(4):17m02138. PubMed CrossRef
- Krystal JH, Rosenheck RA, Cramer JA, et al; Veterans Affairs Cooperative Study No. 504 Group. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306(5):493–502. PubMed CrossRef
- Carey P, Suliman S, Ganesan K, et al. Olanzapine monotherapy in posttraumatic stress disorder: efficacy in a randomized, double-blind, placebo-controlled study. Hum Psychopharmacol. 2012;27(4):386–391. PubMed CrossRef
- Padala PR, Madison J, Monnahan M, et al. Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. Int Clin Psychopharmacol. 2006;21(5):275–280. PubMed CrossRef
- Adamou M, Puchalska S, Plummer W, et al. Valproate in the treatment of PTSD: systematic review and meta analysis. Curr Med Res Opin. 2007;23(6):1285–1291. PubMed CrossRef
- Davis LL, Davidson JR, Ward LC, et al. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J Clin Psychopharmacol. 2008;28(1):84–88. PubMed CrossRef
- Wang HR, Woo YS, Bahk WM. Anticonvulsants to treat post-traumatic stress disorder. Hum Psychopharmacol. 2014;29(5):427–433. PubMed CrossRef
- Hertzberg MA, Butterfield MI, Feldman ME, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45(9):1226–1229. PubMed CrossRef
- Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141–146. PubMed CrossRef
- Keck PE Jr, McElroy SL, Friedman LM. Valproate and carbamazepine in the treatment of panic and posttraumatic stress disorders, withdrawal states, and behavioral dyscontrol syndromes. J Clin Psychopharmacol. 1992;12(Suppl):36S–41S. PubMed CrossRef
- Lipper S, Davidson JR, Grady TA, et al. Preliminary study of carbamazepine in post-traumatic stress disorder. Psychosomatics. 1986;27(12):849–854. PubMed CrossRef
- Looff D, Grimley P, Kuller F, et al. Carbamazepine for PTSD. J Am Acad Child Adolesc Psychiatry. 1995;34(6):703–704. PubMed CrossRef
- Latini G, Verrotti A, Manco R, et al. Topiramate: its pharmacological properties and therapeutic efficacy in epilepsy. Mini Rev Med Chem. 2008;8(1):10–23. PubMed CrossRef
- Bai YF, Zeng C, Jia M, et al. Molecular mechanisms of topiramate and its clinical value in epilepsy. Seizure. 2022;98:51–56. PubMed CrossRef
- Fariba KA, Saadabadi A. Topiramate. In: StatPearls. Treasure Island, FL: StatPearls Publishing; January 31, 2023.
- Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75–90. PubMed CrossRef
- Blake D, Weathers F, Nagy L, et al. Clinician-Administered PTSD Scale (CAPS). Instruction Manual; 2000:1–149.
- Hamilton M. The assessment of anxiety states by rating. Br J Psychol. 1959;32(1):50–55. CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Davidson JR, Colket JT. The eight-item treatment-outcome post-traumatic stress disorder scale: a brief measure to assess treatment outcome in post-traumatic stress disorder. Int Clin Psychopharmacol. 1997;12(1):41–46. PubMed CrossRef
- Guy W. Clinical Global Improvement Scale. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare; Rockville, MD: 1976:218-222
- Leon AC, Davis LL. Enhancing clinical trial design of interventions for posttraumatic stress disorder. J Trauma Stress. 2009;22(6):603–611. PubMed CrossRef
- Halvorsen JØ. Defining clinically significant change. Br J Psychiatry. 2016;209(1):85. PubMed CrossRef
- Institute of Medicine. Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. National Academies Press; 2008:224.
- Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(2):201–206. PubMed CrossRef
- Yeh MS, Mari JJ, Costa MC, et al. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNS Neurosci Ther. 2011;17(5):305–310. PubMed CrossRef
- Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4(24):24. PubMed CrossRef
- Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15–20. PubMed CrossRef
- Lindley SE, Carlson EB, Hill K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677–681. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite