Treatment of Generalized Anxiety Disorder: A Comprehensive Review of the Literature for Psychopharmacologic Alternatives to Newer Antidepressants and Benzodiazepines
Objective: Generalized anxiety disorder (GAD) is common, chronic, and debilitating. Treatment with benzodiazepines and newer antidepressants is often inadequate. This article reviews the effectiveness of alternative and augmenting medications, such as older antidepressants, antipsychotics, anticonvulsants, and β-blockers.
Data Sources: A search using MEDLINE (1980 to week 4 of May 2010) with the key words generalized anxiety disorder or GAD and therapeutics or treatment was conducted. Articles included adult patients with a GAD diagnosis that established chronicity of illness. These included a small number of studies that used DSM-III criteria but added a chronicity of symptoms and included all studies that used DSM-III-R and DSM-IV criteria. Articles that did not include medications or that exclusively focused on newer antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, bupropion, and mirtazapine), buspirone, benzodiazepines, or herbal or investigational medications were excluded. Review articles and non-English-language articles were also excluded.
Results: Thirty-six studies were reviewed. All of the references were then analyzed, and key portions were extracted. Many studies were open trials. Double-blind, placebo-controlled studies with imipramine, risperidone, olanzapine, hydroxyzine, ondansetron, tiagabine, valproate, and pregabalin had been conducted. Imipramine, hydroxyzine, valproate, and pregabalin were the most effective, although risperidone, olanzapine, ziprasidone, and aripiprazole may also reduce symptoms.
Conclusions: Several medication strategies can be considered as promising alternatives or augmenting to antidepressant or benzodiazepine therapy in GAD.
Prim Care Companion CNS Disord 2011;13(2):e1-e9
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: August 28, 2008; accepted June 24, 2010.
Published online: March 24, 2011 (doi:10.4088/PCC.08r00709).
Corresponding author: John Huh, MD, Department of Psychiatry, University of Hawaii, 1356 Lusitana St, 4th Fl, Honolulu, HI 96813 ([email protected]).
Anxiety disorders are the most common type of psychiatric illness, with a 12-month prevalence approaching 1 in 5 adults (18.1%).1 Generalized anxiety disorder (GAD) is the most frequent anxiety disorder, affecting about 5% of adults in the primary care setting.2 Often suffering since childhood or adolescence,2 individuals with GAD experience a constant state of worry and anxiety on most days that is out of proportion with their life stressors.3 The natural course of GAD is characterized as a chronic condition with few remissions, waxing and waning course, and the occurrence of substantial comorbidity including, but not limited to, depression, alcohol abuse, and other anxiety disorders.3-7 The probability of remission of GAD is only 38% at 5 years, and the probability of relapse after remission is 27% by 3 years.8 There is a growing appreciation of disability and impaired quality of life associated with anxiety disorders, including GAD.4 Anxiety disorders not only create a significant economic burden by decreasing work productivity but also strain health care services, accounting for one-third of the costs of treating psychiatric disorders.5-7
Clinical Points
- Standard benzodiazepine and antidepressant treatment for generalized anxiety disorder has been inadequate.
- Current evidence favors hydroxyzine and pregabalin as safe and effective second-line alternatives for generalized anxiety disorder, and there are limited but promising data to support the use of antipsychotics, anticonvulsants, and β-blockers.
Primary care physicians often underdiagnose GAD but usually recognize clinically significant emotional problems associated with this illness.2 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) represent first-line psychopharmacologic treatment for GAD, followed by a switch to a different SSRI/SNRI, mirtazapine, buspirone, or benzodiazepines.9 However, with first-line treatment, remission is only achieved in one-third of patients, and 30% to 60% do not experience any response. In addition, these standard medications for GAD are associated with many significant side effects and risks. SSRIs, SNRIs, and buspirone can increase anxiety, agitation, gastrointestinal problems, sexual dysfunction, or fatigue. Benzodiazepines are associated with sedation, physical dependence, and rebound anxiety. In 1 study, less than half of anxious patients maintained remission after stopping benzodiazepine treatment.10 In those patients for whom first-line agents are no longer indicated, what is the evidence for next-step treatments for GAD? This article reviews psychopharmacologic alternatives to conventional treatment that relies heavily on newer antidepressants and benzodiazepines.
Articles reviewed were found by an OvidSP search using the MEDLINE database (1980 to week 4 of May 2010). The search was conducted using the keywords generalized anxiety disorder or GAD and therapeutics or treatment. Articles included adult patients with a GAD diagnosis that established chronicity of illness. These included a small number of studies that used Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III)11 criteria but added a chronicity of symptoms and included all studies that used DSM-III-R12 and DSM-IV-TR13 criteria.
Articles were excluded if they did not evaluate medication treatment for GAD or if they exclusively studied SSRIs, SNRIs, buspirone, mirtazapine, nefazodone, benzodiazepines, or herbal or investigational medications. Review articles were excluded as were non-English-language articles. The bibliographies of articles meeting criteria were reviewed to identify additional pertinent studies. Thirty-six articles were included. All of the references were then analyzed, and key portions were extracted.
Table 1 provides the medication, dosage, study design, measures, and findings for each of the 36 studies in this review.
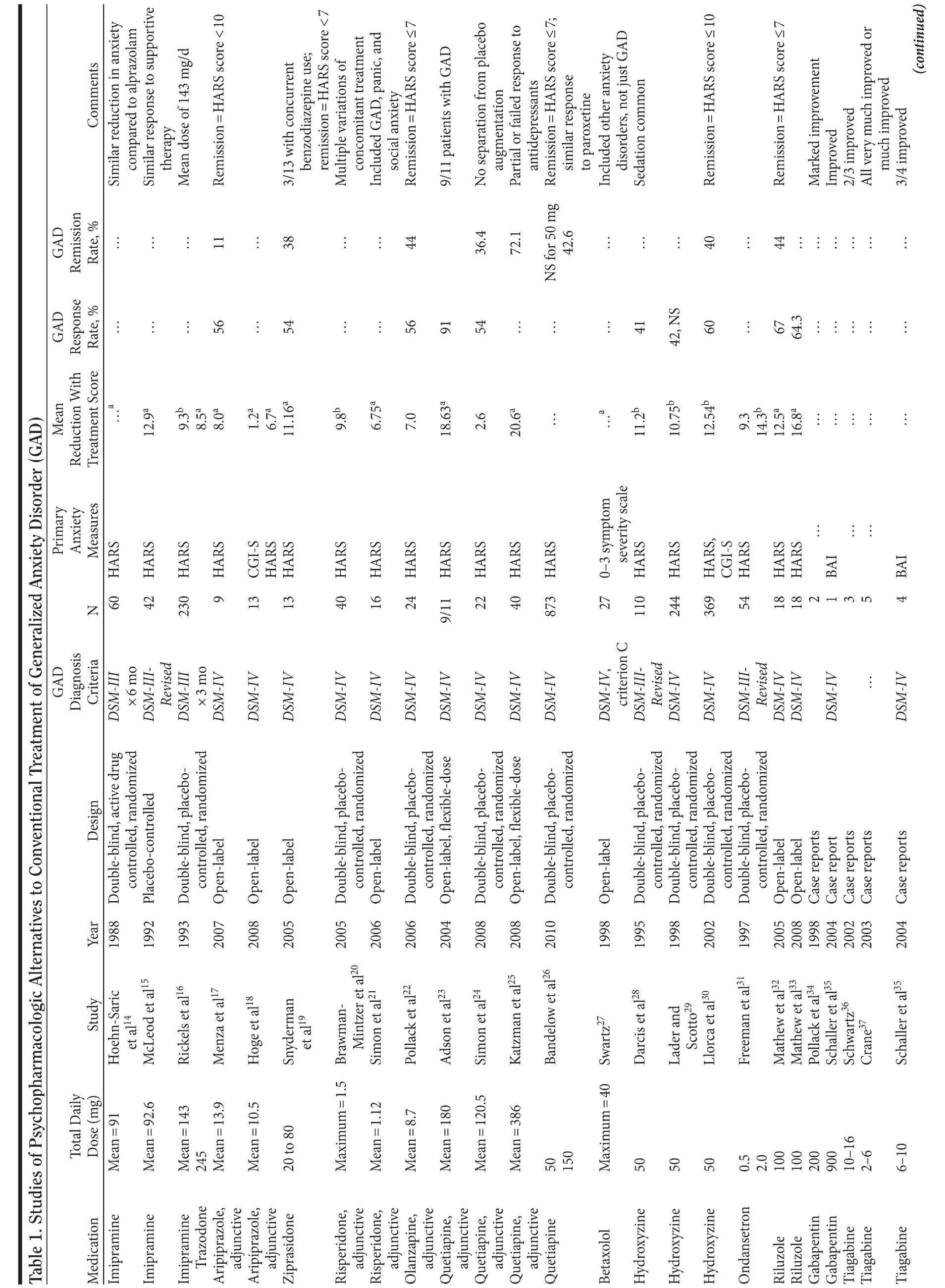
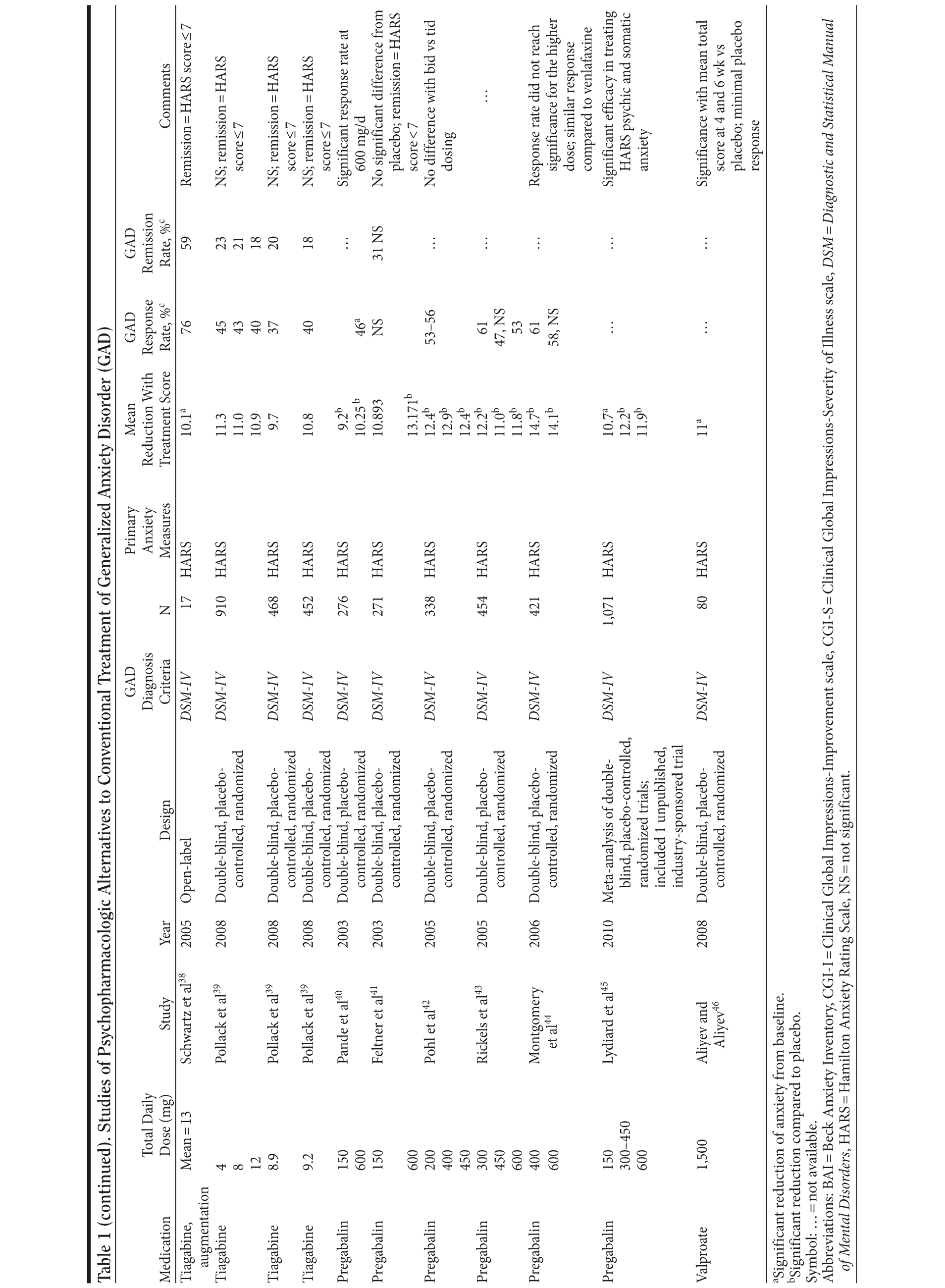
Tricyclic Antidepressants
Imipramine
Imipramine is the only tricyclic antidepressant (TCA) with substantial data and appears to be effective for reducing overall anxiety, especially the psychic symptoms (anxious mood, depression, fear, tension, insomnia, and difficulty concentrating). Hoehn-Saric et al14 in 1988 examined the effect of alprazolam and imipramine in GAD by enrolling 60 participants with DSM-III GAD for at least 6 months, without other major mental illness, in a prospective double-blind, randomized, flexible-dose study. While both medications showed a significant decline in the somatic (muscular or sensory disturbances; cardiovascular, respiratory, gastrointestinal, genitourinary, or autonomic symptoms) subscale and total Hamilton Anxiety Rating Scale (HARS) scores, imipramine was more effective than alprazolam in reducing anxiety symptoms as measured by the HARS at 2 weeks. However, this difference was not maintained at 6 weeks.14 In a small, double-blind, placebo-controlled study, McLeod and colleagues15 reported anxiety symptom reduction using the HARS with imipramine and alprazolam. However, no significant difference was found between the medications.
In 1993, Rickels et al16 evaluated imipramine, trazodone, and diazepam in GAD in a randomized, double-blind, placebo-controlled, flexible-dose, 8-week treatment study among 230 patients with a DSM-III diagnosis of GAD for at least 3 months and no concurrent depression or panic disorders. At week 3, all 3 active treatment modalities showed similar significant improvement over placebo. However, at weeks 4 and 8, only imipramine showed a sustained and significant improvement over placebo in reducing anxiety symptoms.16 These studies, using criteria that establish the chronic nature of GAD, indicate that imipramine continues to show evidence of its efficacy for GAD at a degree similar to or greater than benzodiazepines and should be considered a suitable option in the treatment of GAD at dosages of 90-135 mg. Frequent side effects such as sedation, anticholinergic effects, and cardiac conduction delay often limit the use of TCAs at therapeutic doses.
Antipsychotic Medication
Antipsychotic medications have been used to treat severe anxiety for many years, but their use has been associated with movement disorders such as tardive dyskinesia, akathisia, and extrapyramidal symptoms. While the risk for movement disorders is lower among newer antipsychotic medications, they are associated with metabolic side effects such as weight gain, diabetes, dyslipidemia, and fatigue. There have been many reports of second-generation antipsychotic medications reducing anxiety, but very few studies were specific to GAD. It has only been since February 2005 that studies specific to GAD using second-generation antipsychotic medication have been published. Many antipsychotic medication studies for GAD were primarily augmentation studies with the population skewed to treatment failures. Further, many of the studies were small, and the results are marginally significant.
Aripiprazole
Menza et al17 described a 6-week, open-label trial of aripiprazole augmentation for treatment-resistant GAD in 9 participants. All participants previously failed at least 1 antidepressant trial at a therapeutic dose. Aripiprazole was added to existing treatment. There was significant improvement in anxiety symptoms (HARS and Clinical Global Impressions [CGI]-Improvement scale). Five of 9 participants showed a ≥ 50% reduction in anxiety symptoms, with 1 participant going into remission.17 Hoge et al18 examined aripiprazole in an 8-week, open-label, prospective augmentation study that included GAD and/or panic disorder treatment failure participants. Thirteen participants had GAD or GAD with panic disorder. Flexible-dose aripiprazole was added to failed treatments, which were antidepressants and/or benzodiazepines. In both the GAD group and the panic disorder group, there was a significant reduction in anxiety symptoms and severity (HARS and CGI-Severity of Illness scale scores). These 2 studies demonstrate that aripiprazole has promise in augmentation at dosages starting at 10 mg daily.
Ziprasidone
One of the first second-generation antipsychotic medication studies evaluating effectiveness in the treatment of GAD was an open-label pilot study of ziprasidone for refractory GAD.19 Thirteen adult participants were enrolled in this 7-week, open-label study using ziprasidone. Thirty-eight percent of the participants reached remission (HARS score < 7), and 54% showed a favorable response with at least a 50% reduction in their anxiety symptoms as measured by the HARS. Three participants were concurrently treated with their usual benzodiazepine. The data suggest that ziprasidone at a daily dose range of 20 to 80 mg may be helpful for patients with GAD who did not have an adequate response to other medication treatment.19
Risperidone
Brawman-Mintzer et al20 evaluated risperidone augmentation for GAD in a randomized, double-blind, placebo-controlled prospective study with 40 participants who had a poor response to an anxiolytic or antidepressant, which was continued during the adjunctive 5-week risperidone study. Adjunctive risperidone was significantly more effective in reducing anxiety symptoms (HARS) from baseline to endpoint.20 Simon et al21 published an 8-week, open-label trial of risperidone augmentation for various anxiety disorders of which 16 of the 30 participants had a primary disorder of GAD. All participants had failed an adequate standard medication trial, and the addition of risperidone led to a significant reduction in anxiety symptoms (HARS).21 The effect, however, was small and below what is considered a clinical response (50% reduction in HARS score) or remission (HARS score < 7).21 Adjunctive risperidone could be tried in patients with poor response at titrated doses up to 3 mg daily.
Olanzapine
In 2006, Pollack and colleagues22 completed a randomized, double-blind, placebo-controlled study of olanzapine augmentation with 24 GAD patients who had failed fluoxetine treatment. The fluoxetine plus olanzapine group was significantly more likely to have a 50% reduction in HARS scores than those who took fluoxetine alone. A trend toward higher remission rate with olanzapine augmentation was also reported.22 Olanzapine augmentation at a mean dose of 8.7 mg daily may be helpful for patients who fail to respond to SSRIs alone.22
Quetiapine
Adjunctive quetiapine has shown mixed results. Adson et al23 examined the effect of quetiapine augmentation among 11 subjects with comorbid anxiety and depression who were refractory to antidepressant therapy. The most common anxiety disorder as defined by DSM-IV was GAD (9 participants), but the analyses did not separate the GAD group. By the second week, nearly all participants (91%) had reductions in anxiety and depressive symptoms (≥ 50% in scores on the HARS and the Hamilton Depression Rating Scale).23 In a randomized, placebo-controlled trial, Simon et al24 evaluated the effectiveness of quetiapine augmentation to paroxetine controlled release in 22 participants who failed to reach remission of GAD. At the end of 8 weeks, there was no significant difference between the quetiapine group and the placebo group, and this may be related to the relatively low dose of quetiapine (mean ± SD endpoint dose = 120.5 ± 100.5 mg/d) in this study. Katzman et al25 reported a 12-week, open-label, flexible-dose study of adjunctive quetiapine to antidepressant medication in 40 GAD participants who had not responded or only partially responded to treatment. Adjunctive quetiapine significantly reduced anxiety symptoms (HARS), and 72.1% of subjects attained remission at week 12 (HARS score < 10).25
In a large multicenter, double-blind, placebo-controlled study of 873 participants with GAD, Bandelow et al26 randomized participants into four 8-week monotherapy arms: quetiapine extended release (XR) 50 mg, quetiapine XR 150 mg, paroxetine 20 mg, and placebo daily. At 8 weeks, each active medication group produced significant reduction in symptoms (HARS) compared to placebo. Only the quetiapine XR 150-mg group showed a significant reduction in the HARS somatic subscale scores compared to placebo.26 Remission (HARS score ≤ 7) was significantly better for quetiapine XR 150 mg and paroxetine compared to placebo. Quetiapine XR 150-mg augmentation could be considered in patients not or partially responding to adequate dosages of SSRIs.26
Medication trials involving second-generation antipsychotic medications generally suffered from suboptimal study designs. Although most open-label trials showed improvement compared to baseline, the very few GAD studies with double-blind, placebo-controlled designs have had mixed success. However, these studies examined participants who had already failed 1 treatment and were more like to have difficulty responding to any treatment option. Given the metabolic risks of second-generation atypical antipsychotic medication, these studies do not support the widespread use of these medications for GAD, although with the large randomized trial, careful consideration may be given to quetiapine.26
Typical Neuroleptics
There were no studies examining the effectiveness of typical neuroleptics for the treatment of GAD.
β-Blockers
Betaxolol
An open-label study of betaxolol involved 31 patients, 27 with GAD as defined by criterion C of the DSM-IV (primary symptoms that define the disorder).27 Scored on a scale from 0-3, anxiety severity was reduced from moderately and severely ill to no more than marginally ill in 85% of the outpatients and in all of the inpatients within 2 days.27 Despite widespread use of propranolol for a variety of anxiety disorders, this study was the only one identified that examined the role of β-blockers in GAD treatment, and the results are promising to suggest the potential effectiveness of β-blockers as a class in the treatment of GAD.
Antihistamines
Hydroxyzine
Darcis et al for the French GP Study Group28 published a 4-week, randomized, double-blind, placebo-controlled study of 110 participants with GAD taking hydroxyzine 50 mg daily. There was a significant reduction in anxiety scores (HARS) compared to placebo. The number of responders (HARS score reduction ≥ 50%) was 41% for hydroxyzine and 18% for placebo.28 In a large, 4-week, double-blind, placebo-controlled, multicenter study of 244 participants with GAD, hydroxyzine 50 mg daily, buspirone 20 mg daily, and placebo were compared.29 The hydroxyzine group exhibited a significant reduction in HARS score but not in response rate (≥ 50% reduction in HARS score) compared to placebo.29
Llorca et al30 conducted a double-blind, placebo-controlled study comparing hydroxyzine 50 mg daily, bromazepam 6 mg daily, and placebo in 369 GAD outpatients for a 3-month period. Depression was excluded but other anxiety disorders were not. A significant decline in anxiety symptoms (HARS) was detected for hydroxyzine and bromazepam when compared to placebo. Response rate, defined as a 50% reduction in HARS score from baseline, differed significantly, approaching 60% for hydroxyzine and bromazepam and approximately 30% for placebo. The remission rates (HARS score ≤ 10) reached significance for the active medications over placebo, at 40% for hydroxyzine, 50% for bromazepam, and 30% for placebo.30 Overall, these 3 randomized, placebo-controlled, double-blind studies amounted to a total of 723 patients and consistently demonstrated the effect of hydroxyzine in reducing the anxiety symptoms associated with GAD. The primary deterrent to more widespread use appears to be only transient sleepiness. Existing evidence and its relative low cost would support use of hydroxyzine as an alternative to current standard options.
Serotonin Antagonists
Ondansetron
Ondansetron, a highly selective serotonin-3 (5-HT3) antagonist, was examined as a treatment alternative for anxiety and panic disorders. In a randomized, double-blind, placebo-controlled design, Freeman et al31 noted that ondansetron 1.0 mg bid showed a significant reduction in anxiety symptoms (mean HARS score) compared to placebo. Constipation was a common side effect. This multicenter study of 54 participants taking ondansetron shows promise.31 However, further inquiry is needed.
Glutamate-Modulating Agents
Riluzole
Riluzole is a presynaptic glutamate-releasing inhibitor used to treat amyotrophic lateral sclerosis. Mathew et al32 first reported on the efficacy of riluzole 100 mg daily in the treatment of 18 GAD patients in a small open-label, fixed-dose study over 8 weeks. Two-thirds of participants (67%) had a significant reduction in anxiety symptoms (HARS score decrease ≥ 50%), and 44% met criteria for remission (HARS score ≤ 7). Mathew et al33 replicated findings with 18 GAD patients, with 64.3% of participants showing a significant reduction in anxiety symptoms (HARS score decrease ≥ 50%). With 2 successful small open-label trials, riluzole 100 mg daily shows promise and may be an option for further investigation.
Anticonvulsants
Gabapentin
Pollack et al34 discussed 2 treatment-refractory cases of GAD that were successfully improved with gabapentin 100 mg tid. Benefit continued at 3-month follow-up.34 Schaller et al35 discuss a case series of 4 patients with DSM-IV GAD and treatment with tiagabine. One of the 4 patients had worsening of his anxiety with tiagabine and subsequently responded to an increase of gabapentin to 300 mg tid, serving as a single case study.35 These case studies preclude definitive conclusions and indicate a need for further research on gabapentin in the treatment of GAD.
Tiagabine
Tiagabine is a selective γ-aminobutyric acid (GABA) reuptake inhibitor with conflicting evidence of its effectiveness in treating GAD. Schwartz36 described a case series of 3 refractory GAD patients with tiagabine augmentation. One did not improve (10 mg daily), 1 showed marked improvement (8 mg bid), and the third showed moderate improvement (8 mg bid).36 Crane37 reported a case series of 5 patients with refractory GAD. Patients were very much improved or much improved after 4 weeks of tiagabine. Doses ranged from 2 mg daily to 6 mg daily. Schaller et al35 discussed a case series of 4 patients with DSM-IV GAD. Three of the 4 patients showed a sustained reduction in anxiety with 6-10 mg daily of tiagabine. One of the 4 patients had worsening of his anxiety.35 Schwartz et al38 followed up with 17 DSM-IV GAD patients in an 8-week, open-label trial of augmentation to SSRIs or benzodiazepines. The mean dose was 13 mg daily. By week 8, 76% responded with a ≥ 50% reduction in anxiety symptoms (HARS) and 59% achieved remission (HARS score ≤ 7).38
Pollack et al39 reported on 3 large 10-week, randomized, double-blind, placebo-controlled, parallel-group studies including 1 fixed-dose study and 2 flexible-dose studies. In the fixed-dose study, 910 patients received 4, 8, or 12 mg/d of tiagabine. No significant changes were detected compared to placebo in anxiety symptoms (HARS), disability (Sheehan Disability Scale), and anxiety/depression levels (Hospital Anxiety Depression Scale).39 In the 2 flexible-dose studies, a total of 920 participants were enrolled. The mean doses of tiagabine were 8.9 and 9.2 mg/d. Neither study found significant differences in anxiety symptoms when compared to placebo.39 Given the lack of improvement in anxiety symptoms in all of these larger experimental studies, tiagabine is not recommended for the treatment of GAD.
Pregabalin
There have been several industry-sponsored, multicenter, outpatient, prospective, randomized, double-blind, placebo-controlled studies. These studies defined response rate as a reduction in anxiety symptoms as measured by a HARS score ≥ 50%. Pande et al40 showed a significant improvement with pregabalin compared to placebo. However, no significant differences in response were observed when comparing pregabalin 50 mg tid to pregabalin 200 mg tid or lorazepam to pregabalin 200 mg tid. Both the pregabalin 200-mg tid and the lorazepam groups achieved early decline in anxiety symptoms by the first week.40 There were significantly more treatment responders with pregabalin 200 mg tid (46% response) and lorazepam 2 mg tid (61% response) when compared to placebo (27% response). The pregabalin 50-mg tid response rate did not separate from placebo. The most commonly associated adverse events with pregabalin were dizziness, somnolence, and headache.40
Feltner and colleagues41 compared pregabalin 50 mg tid, pregabalin 200 mg tid, lorazepam 2 mg tid, or placebo. They found a significant reduction in anxiety symptoms for pregabalin 200 mg tid versus placebo. However, pregabalin 50 mg tid did not show significant reduction in anxiety symptoms compared to placebo nor was pregabalin 200 mg tid significantly different from lorazepam. Only pregabalin 200 mg tid trended toward significance for remission at 31% (HARS score < 7).41 Pohl et al42 compared pregabalin 100 mg bid, 200 mg bid, and 150 mg tid to placebo. Anxiety symptoms (HARS scores) declined similarly in all 3 treatment arms, with 40% in each group showing a decline in anxiety symptoms in the first week and sustaining their response. This response represented a statistically significant decline when compared to placebo (53%-56% in treatment groups vs 34% in the placebo group).42
In a study of 454 participants with GAD, Rickels et al43 compared pregabalin 100 mg tid, pregabalin 150 mg tid, pregabalin 200 mg tid, alprazolam 0.5 mg tid, and placebo. All active treatment groups showed a significant reduction in anxiety symptoms compared to placebo. However, only pregabalin 100 mg tid (65%) and pregabalin 200 mg tid (53%) showed significant responses on the HARS compared to placebo (34%). Alprazolam (43%) and pregabalin 150-mg tid (47%) response trended toward significance.43
Montgomery et al44 compared pregabalin 200 mg bid, pregabalin 300 mg bid, venlafaxine 37.5 bid, and placebo and found a significant decline in anxiety symptoms. Response rate reached significance for pregabalin 200 mg bid (61%) and venlafaxine 37.5 bid (62%) compared to placebo (45%). Response rate for pregabalin 300 mg bid (58%) did not reach significance. All active treatment groups showed a significant decline in the psychic subscore compared to placebo, but only pregabalin 200 mg bid showed a significant reduction in somatic subscore compared to placebo.44
Pregabalin has had multiple large studies.45 A series of 5 randomized, placebo-controlled, double-blind studies of pregabalin with a total of 1,071 patients worldwide showed some reduction in HARS score compared to placebo in both psychiatric and somatic anxiety measures, although this was inconsistent at the low dose of 150 mg daily, and while the high dose of 600 mg daily is effective, the possibility of a therapeutic window is suggested. Pregabalin appeared to be fairly well tolerated, with dizziness, somnolence, and nausea side effects being the most common.45
In 2010, Lydiard et al45 combined data from 6 short-term, double-blind, placebo-controlled, fixed-dose trials of pregabalin for the treatment of GAD. Their review includes the 5 aforementioned studies as well as 1 additional study using data obtained directly from industry (N = 142). They concluded that pregabalin had significant efficacy in treating both HARS psychic and somatic anxiety measures. Furthermore, they indicated that a dose-response effect was evident for pregabalin that appeared to reach a plateau at a dose of 300 mg/d.45 Pregabalin appears to have some evidence to support its use as an alternative to current standards, but further inquiry is needed to clarify the optimum dosing strategy.
Valproate
Aliyev and Aliyev46 recently examined valproate in 80 male patients with GAD in a double-blind placebo-controlled design. At week 4, valproate separated from placebo by mean total HARS score, and at 6 weeks, the mean change in HARS score reached significance. Although a positive small response was seen, it may be exaggerated by a small placebo response.46 Further investigation is needed.
CONCLUSION
Generalized anxiety disorder is a severe and disabling illness. While there are no widely accepted treatment algorithms for GAD, initial treatment generally consists of an SSRI or SNRI, and second-tier options may consist of buspirone, mirtazapine, benzodiazepines, and/or a different SSRI or SNRI. This article reviewed published data for alternative psychopharmacology options for the treatment of GAD. Only some of the studies reviewed specifically addressed the question of refractory GAD; and all medications are considered off-label use of the medication. Many of the studies reported change in HARS scores. While response was consistently defined as a reduction in anxiety symptoms by 50% as measured by HARS score, remission was defined inconsistently, with HARS scores varying from less than 7 to 10. Although only a few evidence-based studies have been conducted for each of the alternative treatments for GAD, our review of the literature indicated that imipramine, hydroxyzine, and pregabalin provided the most consistent reduction in anxiety symptoms and the highest remission rates. Before referring a patient who has not responded to benzodiazepines or newer antidepressant medications to a psychiatrist, primary care providers may want to try pregabalin and hydroxyzine, given their relatively benign side effect profiles.
There are many potential options for the future treatment of GAD. If a patient fails the conventional GAD treatment, future directions may include reviewing the past treatments of anxiety symptoms with the patient and reevaluating the effectiveness of alternatives. To substantiate the effectiveness of the alternative medications reviewed, more clinical trials are needed to follow up the many open-label trials or small studies with large, double-blind, placebo-controlled studies. Interestingly, there are no studies with monoamine oxidase inhibitors and GAD. Other future directions may also include a variety of medications under investigation such as agomelatine, GABA-A-specific receptor modulators, benzodiazepine receptor agonists and partial agonists, buspirone-like partial 5-HT1A agonists, and antagonists for cholecystokinin B receptors of data.
Drug names: alprazolam (Niravam, Xanax, and others), aripiprazole (Abilify), betaxolol (Betoptic and others), bupropion (Aplenzin, Wellbutrin, and others), buspirone (BuSpar and others), diazepam (Valium and others), fluoxetine (Prozac and others), gabapentin (Neurontin and others), hydroxyzine (Vistaril and others), imipramine (Tofranil and others), lorazepam (Ativan and others), mirtazapine (Remeron and others), olanzapine (Zyprexa), ondansetron (Zofran, Zuplenz, and others), paroxetine (Paxil, Pexeva, and others), pregabalin (Lyrica), propranolol (Inderal, InnoPran, and others), quetiapine (Seroquel), riluzole (Rilutek and others), risperidone (Risperdal and others), tiagabine (Gabitril), trazodone (Oleptro and others), valproate (Depacon and others), ziprasidone (Geodon).
Author affiliation: Department of Psychiatry, University of Hawaii, Honolulu.
Potential conflicts of interest: Dr Huh has received grant/research support from Novartis. Dr Takeshita has served on the speakers or advisory boards of Forest. Drs Goebert, Lu, and Kang report no financial or other affiliations related to the subject of this article.
Funding/support: None reported.
REFERENCES
1. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed doi:10.1001/archpsyc.62.6.617
2. Wittchen HU, Kessler RC, Beesdo K, et al. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(suppl 8):24-34. PubMed
3. Yonkers KA, Dyck IR, Warshaw M, et al. Factors predicting the clinical course of generalised anxiety disorder. Br J Psychiatry. 2000;176(6):544-549. PubMed doi:10.1192/bjp.176.6.544
4. Mogotsi M, Kaminer D, Stein DJ. Quality of life in the anxiety disorders. Harv Rev Psychiatry. 2000;8(6):273-282. PubMed
5. DuPont RL, Rice DP, Miller LS, et al. Economic costs of anxiety disorders. Anxiety. 1996;2(4):167-172. PubMed doi:10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L
6. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60(7):427-435. PubMed doi:10.4088/JCP.v60n0702
7. Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety. 2002;16(4):162-171. PubMed doi:10.1002/da.10065
8. Yonkers KA, Warshaw MG, Massion AO, et al. Phenomenology and course of generalised anxiety disorder. Br J Psychiatry. 1996;168(3):308-313. PubMed doi:10.1192/bjp.168.3.308
9. Baldwin DS, Anderson IM, Nutt DJ, et al; British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567-596. PubMed doi:10.1177/0269881105059253
10. Rickels K, Case WG, Schweizer E, et al. Long-term benzodiazepine users 3 years after participation in a discontinuation program. Am J Psychiatry. 1991;148(6):757-761. PubMed
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition. Washington, DC: American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Washington, DC: American Psychiatric Association; 1987.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
14. Hoehn-Saric R, McLeod DR, Zimmerli WD. Differential effects of alprazolam and imipramine in generalized anxiety disorder: somatic versus psychic symptoms. J Clin Psychiatry. 1988;49(8):293-301. PubMed
15. McLeod DR, Hoehn-Saric R, Porges SW, et al. Effects of alprazolam and imipramine on parasympathetic cardiac control in patients with generalized anxiety disorder. Psychopharmacology (Berl). 1992;107(4):535-540. PubMed doi:10.1007/BF02245268
16. Rickels K, Downing R, Schweizer E, et al. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50(11):884-895. PubMed
17. Menza MA, Dobkin RD, Marin H. An open-label trial of aripiprazole augmentation for treatment-resistant generalized anxiety disorder. J Clin Psychopharmacol. 2007;27(2):207-210. PubMed doi:10.1097/01.jcp.0000248620.34541.bc
18. Hoge EA, Worthington JJ 3rd, Kaufman RE, et al. Aripiprazole as augmentation treatment of refractory generalized anxiety disorder and panic disorder. CNS Spectr. 2008;13(6):522-527. PubMed
19. Snyderman SH, Rynn MA, Rickels K. Open-label pilot study of ziprasidone for refractory generalized anxiety disorder. J Clin Psychopharmacol. 2005;25(5):497-499. PubMed doi:10.1097/01.jcp.0000177853.15910.de
20. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2005;66(10):1321-1325. PubMed doi:10.4088/JCP.v66n1016
21. Simon NM, Hoge EA, Fischmann D, et al. An open-label trial of risperidone augmentation for refractory anxiety disorders. J Clin Psychiatry. 2006;67(3):381-385. PubMed doi:10.4088/JCP.v67n0307
22. Pollack MH, Simon NM, Zalta AK, et al. Olanzapine augmentation of fluoxetine for refractory generalized anxiety disorder: a placebo controlled study. Biol Psychiatry. 2006;59(3):211-215. PubMed doi:10.1016/j.biopsych.2005.07.005
23. Adson DE, Kushner MG, Eiben KM, et al. Preliminary experience with adjunctive quetiapine in patients receiving selective serotonin reuptake inhibitors. Depress Anxiety. 2004;19(2):121-126. PubMed doi:10.1002/da.10137
24. Simon NM, Connor KM, LeBeau RT, et al. Quetiapine augmentation of paroxetine CR for the treatment of refractory generalized anxiety disorder: preliminary findings. Psychopharmacology (Berl). 2008;197(4):675-681. PubMed doi:10.1007/s00213-008-1087-x
25. Katzman MA, Vermani M, Jacobs L, et al. Quetiapine as an adjunctive pharmacotherapy for the treatment of non-remitting generalized anxiety disorder: a flexible-dose, open-label pilot trial. J Anxiety Disord. 2008;22(8):1480-1486. PubMed doi:10.1016/j.janxdis.2008.03.002
26. Bandelow B, Chouinard G, Bobes J, et al. Extended-release quetiapine fumarate (quetiapine XR): a once-daily monotherapy effective in generalized anxiety disorder: data from a randomized, double-blind, placebo- and active-controlled study. Int J Neuropsychopharmacol. 2010;13(3):305-320. PubMed doi:10.1017/S1461145709990423
27. Swartz CM. Betaxolol in anxiety disorders. Ann Clin Psychiatry. 1998;10(1):9-14. PubMed doi:10.3109/10401239809148813
28. Darcis T, Ferreri M, Natens J, et al; The French GP Study Group for Hydroxyzine. A multicentre double-blind placebo-controlled study investigating the anxiolytic efficacy of hydroxyzine in patients with generalized anxiety. Hum Psychopharmacol. 1995;10(3):181-187. doi:10.1002/hup.470100303
29. Lader M, Scotto JC. A multicentre double-blind comparison of hydroxyzine, buspirone and placebo in patients with generalized anxiety disorder. Psychopharmacology (Berl). 1998;139(4):402-406. PubMed doi:10.1007/s002130050731
30. Llorca PM, Spadone C, Sol O, et al. Efficacy and safety of hydroxyzine in the treatment of generalized anxiety disorder: a 3-month double-blind study. J Clin Psychiatry. 2002;63(11):1020-1027. PubMed
31. Freeman AM 3rd, Westphal JR, Norris GT, et al. Efficacy of ondansetron in the treatment of generalized anxiety disorder. Depress Anxiety. 1997;5(3):140-141. PubMed doi:10.1002/(SICI)1520-6394(1997)5:3<140::AID-DA7>3.0.CO;2-I
32. Mathew SJ, Amiel JM, Coplan JD, et al. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162(12):2379-2381. PubMed doi:10.1176/appi.ajp.162.12.2379
33. Mathew SJ, Price RB, Mao X, et al. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biol Psychiatry. 2008;63(9):891-898. PubMed doi:10.1016/j.biopsych.2007.09.012
34. Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155(7):992-993. PubMed
35. Schaller JL, Thomas J, Rawlings D. Low-dose tiagabine effectiveness in anxiety disorders. MedGenMed. 2004;6(3):8. PubMed
36. Schwartz TL. The use of tiagabine augmentation for treatment-resistant anxiety disorders: a case series. Psychopharmacol Bull. 2002;36(2):53-57. PubMed
37. Crane D. Tiagabine for the treatment of anxiety. Depress Anxiety. 2003;18(1):51-52. PubMed doi:10.1002/da.10104
38. Schwartz TL, Azhar N, Husain J, et al. An open-label study of tiagabine as augmentation therapy for anxiety. Ann Clin Psychiatry. 2005;17(3):167-172. PubMed doi:10.1080/10401230591002138
39. Pollack MH, Tiller J, Xie F, et al. Tiagabine in adult patients with generalized anxiety disorder: results from 3 randomized, double-blind, placebo-controlled, parallel-group studies. J Clin Psychopharmacol. 2008;28(3):308-316. PubMed doi:10.1097/JCP.0b013e318172b45f
40. Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160(3):533-540. PubMed doi:10.1176/appi.ajp.160.3.533
41. Feltner DE, Crockatt JG, Dubovsky SJ, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2003;23(3):240-249. PubMed doi:10.1097/00004714-200306000-00005
42. Pohl RB, Feltner DE, Fieve RR, et al. Efficacy of pregabalin in the treatment of generalized anxiety disorder: double-blind, placebo-controlled comparison of BID versus TID dosing. J Clin Psychopharmacol. 2005;25(2):151-158. PubMed doi:10.1097/01.jcp.0000155820.74832.b0
43. Rickels K, Pollack MH, Feltner DE, et al. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Arch Gen Psychiatry. 2005;62(9):1022-1030. PubMed doi:10.1001/archpsyc.62.9.1022
44. Montgomery SA, Tobias K, Zornberg GL, et al. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J Clin Psychiatry. 2006;67(5):771-782. PubMed doi:10.4088/JCP.v67n0511
45. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241. PubMed doi:10.1017/S1461145709990460
46. Aliyev NA, Aliyev ZN. Valproate (depakine-chrono) in the acute treatment of outpatients with generalized anxiety disorder without psychiatric comorbidity: randomized, double-blind placebo-controlled study. Eur Psychiatry. 2008;23(2):109-114. PubMed doi:10.1016/j.eurpsy.2007.08.001



