Prim Care Companion CNS Disord 2021;23(6):21cr03059
To cite: Gómez L, Vidal B, Cabrera Y, et al. Successful treatment of post-COVID symptoms with transcranial direct current stimulation. Prim Care Companion CNS Disord. 2021;23(6):21cr03059.
To share: https://doi.org/10.4088/PCC.21cr03059
© Copyright 2021 Physicians Postgraduate Press, Inc.
aClinical Neurophysiology Department and Non-Invasive Brain Stimulation Unit, International Center for Neurological Restoration, Havana, Cuba
bMental Health Service, Borrás-Marfán Hospital, Havana, Cuba
*Corresponding author: Lázaro Gómez, MD, Clinical Neurophysiology Department and Non-Invasive Brain Stimulation Unit, International Center for Neurological Restoration, 15805, 25 Ave, Playa, Havana 11300, Cuba ([email protected]).
Anxiety, phobic episodes, fatigue, cognitive difficulties, and sleep disturbances are common features among patients after the acute phase of coronavirus disease 2019 (COVID-19).1 Anxiety and depression are probably the most common psychiatric diagnoses after COVID-19 (12.8% and 9.9%, respectively).2 It has been suggested that noninvasive brain stimulation could be a valuable tool for the management of the postacute phase of patients with COVID-19, ameliorating musculoskeletal pain and releasing the mental distress triggered by the surrounding psychosocial stressors related to COVID-19.3,4 The use of transcranial direct current stimulation (tDCS) is becoming a common practice among professionals. Although tDCS has been approved only for research purposes, many trials have demonstrated that repeated sessions of tDCS improved mood and anxiety in some patients.5–7 We chose to use tDCS as an adjuvant therapy in a patient with post-COVID symptoms.
Case Report
The patient was a 58-year-old man with history of arterial hypertension and premorbid anxiety signs, who was diagnosed as PCR (polymerase chain reaction) positive for severe acute respiratory coronavirus 2. He was admitted to the hospital and maintained in an isolation ward due to mild COVID-19 symptoms. A few days after diagnosis, he reported tiredness and dry cough and received appropriate supportive interventions during the hospitalization. After 14 days, he was completely asymptomatic, his PCR test was negative, and he was discharged. A couple of weeks later, he developed severe anxiety symptomatology and fatigue, with phobic episodes when going out for short walks, which developed into agoraphobia as he refused to leave his home even for short periods of time. He also described loss of memory and motivation to return to normal activities, feeling depressive with a decrease in his cognitive performance, and severe insomnia. When evaluated by the clinical staff, the patient was diagnosed with moderate intensity post-COVID syndrome (per the World Health Organization),8 with high levels of anxiety. All the test results were within normal limits by that time, including chest x-ray, electrocardiogram, brain computed tomography, and blood tests. He was recommended to start psychotherapy and medication (sertraline and clonazepam) to control his anxiety disorder. He partially refused the medication and accepted to take clonazepam 1 mg each evening to aid sleeping.
Alternatively, he accepted and gave his written informed consent to be considered for tDCS therapy as a nonpharmacologic alternative to treat anxiety and depressive symptoms. He received 1 daily session of tDCS (2 mA, 20 minutes) over 20 days. A montage of anode F3 and cathode F4 was selected (NeuroConn tDCS device, Berlin, Germany), as previous research has described it as a moderate effective montage for control of anxiety and depressive symptoms.9,10
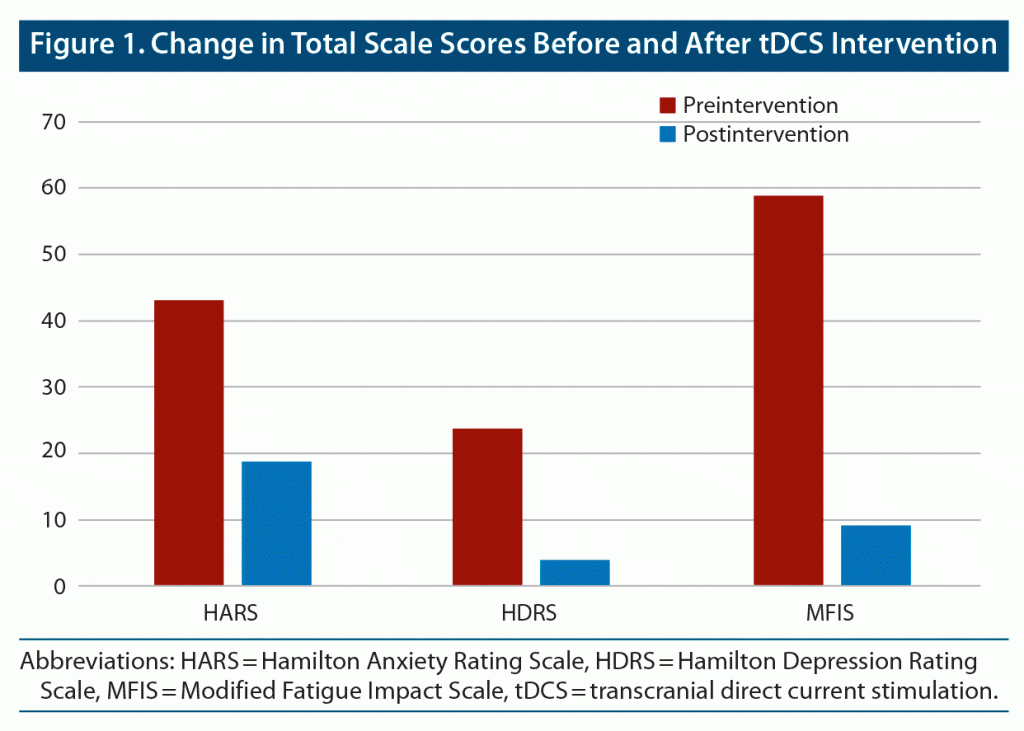
The Hamilton Anxiety Rating Scale (HARS),11 Hamilton Depression Rating Scale (HDRS),12 and Modified Fatigue Impact Scale (MFIS)13 were administered before and after the patient received the last tDCS session. A progressive and sustained tendency to improvement in anxiety, mood, and social relationships was observed during the intervention. After the last tDCS session, a clinically significative decrease in scores of the HARS, HDRS, and MFIS was observed. The patient’s anxiety score decreased 24 points according to the HARS, while his HDRS score decreased 20 points and MFIS decreased 50 points (Figure 1 and Table 1). In parallel, the patient was feeling very well, with less anxiety and better physical and cognitive performance. No other phobic episodes were described during or after the intervention.
Conclusion
The present case documents, to our knowledge, the first utilization of tDCS as an add-on approach in the management of anxiety and depressive symptoms in a patient with post-COVID symptoms. Our findings support the development of broader research in a large group of patients with post-COVID symptoms.
Received: June 21, 2021.
Published online: December 2, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Jim Clifford, LCPC, and Tlalli Mota, BS (both of Kaiser Permanente, San Diego, California) for proofreading the report. They report no conflicts of interest related to the subject of this case report.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (13)

- Ismael F, Bizario JCS, Battagin T, et al. Post-infection depressive, anxiety and post-traumatic stress symptoms: A prospective cohort study in patients with mild COVID-19. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110341. PubMed CrossRef
- Taquet M, Luciano S, Geddes JR, et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. PubMed CrossRef
- Pilloni G, Bikson M, Badran BW, et al. Update on the use of transcranial electrical brain stimulation to manage acute and chronic COVID-19 symptoms. Front Hum Neurosci. 2020;14:595567. PubMed CrossRef
- Baptista AF, Baltar A, Okano AH, et al. Applications of non-invasive neuromodulation for the management of disorders related to COVID-19. Front Neurol. 2020;11:573718. PubMed CrossRef
- Loo CK, Alonzo A, Martin D, et al. Transcranial direct current stimulation for depression: 3-week, randomized, sham-controlled trial. Br J Psychiatry. 2012;200(1):52–59. PubMed CrossRef
- Sampaio-Junior B, Tortella G, Borrione L, et al. Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Bipolar Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(2):158–166. PubMed CrossRef
- Pavlova EL, Menshikova AA, Semenov RV, et al. Transcranial direct current stimulation of 20- and 30-minutes combined with sertraline for the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:31–38. PubMed CrossRef
- World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. Accessed November 4, 2021. https://apps.who.int/iris/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf
- Zhou Q, Yu C, Yu H, et al. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020;70:17–26. PubMed CrossRef
- Sharafi E, Taghva A, Arbabi M, et al. Transcranial direct current stimulation for treatment-resistant major depression: a double-blind randomized sham-controlled trial. Clin EEG Neurosci. 2019;50(6):375–382. PubMed CrossRef
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. PubMed CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
Please sign in or purchase this PDF for $40.
Save
Cite