ABSTRACT
Objective: To build on the findings of our prior study of spontaneous posts made about brain zaps on a popular lay mental health website using questions specifically targeting brain zaps in a web-based questionnaire.
Methods: 3,141 responses were examined from an online questionnaire made available between June 2016 and February 2018. The questions probed the specifics of the medications taken, the temporal characteristics of medication taken, the symptoms associated with the brain zaps, the specifics of the “zap” experience itself, and their effect on quality of life. Special attention was paid to gathering data regarding the triggers of brain zaps, because in our previous study, eye movements triggering brain zaps emerged as an unexpected finding. As this was a convenience sample, qualitative analysis was primarily performed, except regarding the interaction between the half-life of antidepressants and the time to the onset of the first brain zaps, for which the numerical data appeared to be specific enough to allow such analyses.
Results: The data from the targeted questionnaire showed a pattern of responses that was very similar to that obtained from analysis of the spontaneous posts. These data include the types of medications taken, the length of time these medications were taken before the onset of the zaps, the length of the zaps, the feeling quality of the zaps, and the effect of gradual versus sudden discontinuation on their onset and presence. Lateral eye movement as a trigger emerged with even more clarity than in the previous study. The positive correlation between the time from onset of the brain zaps and the half-life of the drugs strongly suggests that brain zaps are indeed associated with antidepressant discontinuation.
Conclusions: Brain zaps remain a barely examined and poorly understood symptom of antidepressant discontinuation. Further studies are needed from both a prevention and treatment perspective. There is now an even stronger indication that brain zaps are typically triggered by lateral eye movements, which may open avenues for investigating this process.
Prim Care Companion CNS Disord 2022;24(1):21m02972
To cite: Papp A, Onton JA. Triggers and characteristics of brain zaps according to the findings of an internet questionnaire. Prim Care Companion CNS Disord. 2022;24(1):21m02972.
To share: https://doi.org/10.4088/PCC.21m02972
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of California, San Diego, La Jolla, California
bInstitute for Neural Computation, University of California, San Diego, La Jolla, California
*Corresponding author: Alexander Papp, MD, Department of Psychiatry, University of California, San Diego, UC San Diego, 9500 Gilman Dr, La Jolla, CA 92093 ([email protected]).
It was not always commonly accepted that sudden cessation of antidepressants (ADs), or even a slow taper, can cause significant adverse effects. Such reactions were not considered worthy of much clinical attention when they did occur. For instance, Haddad1 stated in 2001 that most symptoms were mild and only required reassurance, and even the most severe symptoms would disappear after 24 hours. However, things changed relatively quickly, and the opinion of the same author2 changed to “. . . in a minority of cases they can be severe, last several weeks and cause significant morbidity.”(p448)
Discontinuation symptoms are mostly neurologic symptoms. They can be of the classic kind (eg, vertigo) or unusual sensations that feel as though they are originating inside the brain or skull.
One of these unusual symptoms came to be called a brain zap. The term was coined by patients who posted on early internet forums and has now been accepted in the academic literature as well. Brain zaps are sensations described as brief electrical shocks or vibrations experienced as occurring inside the head. They can be accompanied by secondary sensations in the auditory modality and may involve a sense of momentary disconnection from alertness. They are typically reported by patients who stopped taking ADs or reduced their dose. They are inconsistently mentioned as one of the several discontinuation symptoms in the literature.
Although brain zaps are perhaps the most unique symptom of AD discontinuation, they are not included in the 43-item Discontinuation Emergent Signs and Symptoms Scale (DESS).3 When mentioned in the literature, they are not discussed or considered in any detail.
Davies and Read4 reported a meta-analysis of 24 publications published between 1996 and 2018 that provided usable data about the observed frequency, duration, and severity of discontinuation symptoms from ADs. The publications provided 1,540 data points. They found that more than half of the 1,540 AD users reported some withdrawal, and most of the users considered the symptoms (including brain zaps) “severe or moderately severe.” Of people who reported withdrawal effects, 40% experienced them for at least 6 weeks. None of the reviewed studies focused on brain zaps.
Our previous study5 (the MHD study) reported an analysis of unsolicited posts from a website (www.mentalhealthdaily.com) by persons who claim to suffer from brain zaps. Those who posted on that website were seeking or sharing information about brain zaps or simply expressing their frustration about their experience with brain zaps.
In our previous work,5 we referred to an online survey by Read et al6 that analyzed responses regarding adverse reactions. The questionnaire had 1,829 respondents and included questions about the subjective experiences, beliefs, and attitudes related to their ADs. Thirteen respondents (< 1%) reported “electric shock” experiences in this questionnaire, which did not distinguish between people taking or discontinuing ADs.6
Two new studies assessing online reports have been published since 2018. A study by Stockmann et al7 analyzed responses from a different lay website and focused on the differences in the pattern of responses between selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). They found a longer time to onset of withdrawal symptoms following SSRI discontinuation or reduction than for SNRIs, and withdrawal symptoms lasted significantly longer for those taking SSRIs than SNRIs. The authors7 suggest a protective effect regarding duration of symptoms from the norepinephrine reuptake inhibition, but it is also possible that the results were confounded given that SSRIs as a group (which includes fluoxetine and vortioxetine) tend to include ADs with longer half-lives than SNRIs, among which (des)venlafaxine is most frequently prescribed.
In 2020, Read8 reported the results of a study of 2,346 people who completed an online questionnaire investigating experiences with taking ADs. Close to 800 people reported negative discontinuation symptoms, 42.5% of whom reported having brain zaps. Less than 1% of the respondents reported that they received information from a professional about withdrawal effects.8
The objective of this study was to survey a population of respondents, similar to our MHD study,5 with an internet questionnaire designed to more precisely describe the brain zap phenomenon. The questions were designed to follow the themes from the narrative descriptions of symptoms and complaints in our previous study.5
METHODS
A questionnaire was designed to obtain answers that could be compared to the results of the MHD study and information from the literature. The questionnaire was open to responses between June 2016 and February 2018. Respondents voluntarily chose to answer questions once they found the link leading to the questionnaire. Demographic data were not collected. Due to the nature of the sample, our data allowed only for limited statistical analysis.
Sample
Following ethics approval from the University of California, San Diego, the anonymous questionnaire was placed online in Google Forms (the questionnaire is provided in Appendix 1). The questionnaire was advertised via a single post that was placed on the “brain zaps” discussion forum of the Mental Health Daily website announcing that the questionnaire was available and providing a link. Most of the respondents found the questionnaire by doing an internet search for brain zaps or via a link from Mental Health Daily.
The number of respondents who completed the survey was 3,141. The respondents had to state that they were aged ≥ 18 years, but no other demographic information was collected. Verbal responses made in languages other than English were translated using Google Translate. There were less than 10 such responses.
Instrument
The questionnaire had 49 questions presented in 5 sections: screening questions, general medication-related questions, description of brain zaps and their triggers, impact of the brain zaps on quality of life including the effect of attempts to ameliorate them, and a comments section. The screening questions were (1) aged ≥ 18 years, (2) have experienced “zap” or “shiver” type sensations, (3) the sensation is localized to the head or brain, and (4) the sensation is related to taking any of the listed medications (see Appendix 1 for the full list of AD medications). The questions were multiple choice or write-in. The write-in answers could be numerical or narrative.
The questions were designed to obtain answers that could be compared to both the results of the MHD study and data from the literature. We considered this comparison important due to the nature of our sample, which typically does not lend itself to statistical analysis. Because this descriptive study also does not, in most cases, lend itself to statistical analysis, corroboration in multiple areas if present would bolster the reliability of both studies. Thus, we hoped to replicate and further qualify our findings in the MHD study.
After the questionnaire closed, the data were downloaded into a spreadsheet and imported into a database application (FileMaker Pro) wherein all further processing (except the statistical analysis) took place.
Analysis
If a respondent named 2 different medications with 2 identifiable withdrawal events, the response generated 2 entries. Incorrect spellings of medications were normalized. All brand names were mapped to generics. Mistaken doses that were mappable to a more likely correct dose were corrected (eg, venlafaxine 0.75 mg to 75 mg). High but possible doses (eg, sertraline 400 mg) were accepted as valid entries.
For analysis purposes, we normalized length of time as days or months, depending on the question. Verbal qualifiers were converted to numbers by approximating the method described in the LSAT Terminology document9 (eg, couple = 2, few = 3, several and many = 5). Ranges given (eg, 4–5 days) were changed to the in-between value. Narrative responses were analyzed for content and standardized into keywords.
An entry was considered invalid if the medication reported was not from the provided list of ADs (except for bupropion, the medications on the list were serotonergic), if they also took medications that are known to have withdrawal effects, if they did not state the name of medication taken, if they reported 3 or more medications taken, if the medication’s name was unrecognizable, or if the answer was vague. In total, 214 responses were disqualified in this step.
An entry was also considered invalid if the brain zaps were reportedly not due to decreasing medication dose (eg, “when taking steady,” “I don’t know”). There were 660 such responses.
The remaining 2,267 responses were included in the analysis. Because the questionnaire allowed a single respondent to generate separate records for 2 different ADs, the total number of responses to some of the questions may be higher than 2,267. Conversely, because of missing or irrelevant responses, some individual questions have a lower number of answers than 2,267.
To test the hypothesis that zap onset latency differed based on medication half-life, the reported onset latencies were grouped for short half-life (paroxetine and [des]venlafaxine), long half-life (fluoxetine and vortioxetine), and all other ADs with in-between half-lives. Responses were normalized to average number of days and then submitted to a Kruskal-Wallis test for unequal variances to determine if the 3 groups came from the same or different distributions. A multiple comparisons test was used to determine which groups were significantly different from each other. The rest of the data were not amenable to statistical analysis and are simply summarized and presented to expose trends.
Some of the questions did not generate information that could be considered useful after the initial processing. The answers to those questions were not analyzed and are not included in this article. Only 114 of the valid respondents indicated that they filled out the questionnaire after consulting written records.
RESULTS
General Findings
The total number of respondents was 3,141, with a mean age of 38 years. All respondents were aged ≥ 18 and stated that they had experienced a zap or shiver sensation that seemed to localize to the head or brain. Furthermore, they stated their brain zaps were related to one of the AD medications in the list provided to them. Geographic information was not collected, but based on the brand names of medications mentioned, responses were received from Turkey, South Africa, and European countries. However, the majority of the responses appeared to come from the United States.
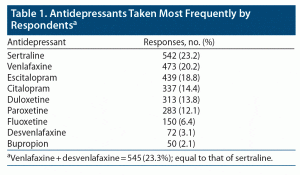
Table 1 shows the 9 most frequently mentioned ADs. Generic terms antidepressant or SSRI were mentioned 73 times. All other ADs were mentioned in low numbers (≤ 25). Some of the respondents were on more than 1 medication. The most common second ADs were bupropion and amitriptyline.
In most cases, respondents reported that the medications were taken for less than 2 years before the appearance of the brain zaps (255 [11.5%] < 60 days; 1,135 [51.3%] < 2 years). The remainder (818 respondents) reported longer than 2 years duration (487 [22%] 2–5 years; 215 [9.7%] 5–10 years; 116 [5.2%] ≥ 10 years).
Many respondents gave more than 1 answer to the question “What change in medication-taking led to the appearance of the zaps?” perhaps because many of them made separate attempts to stop the medication. Quick changes were more likely to result in discontinuation symptoms, but slow changes did not have a strong protective effect either. The number of cases occurring during a gradual weaning was 788, the number occurring during a rapid weaning was 528, the number occurring after sudden discontinuation was 909, and the number of respondents stating that they experienced zaps whenever they skipped a dose was 844.
Zap Onset Latency
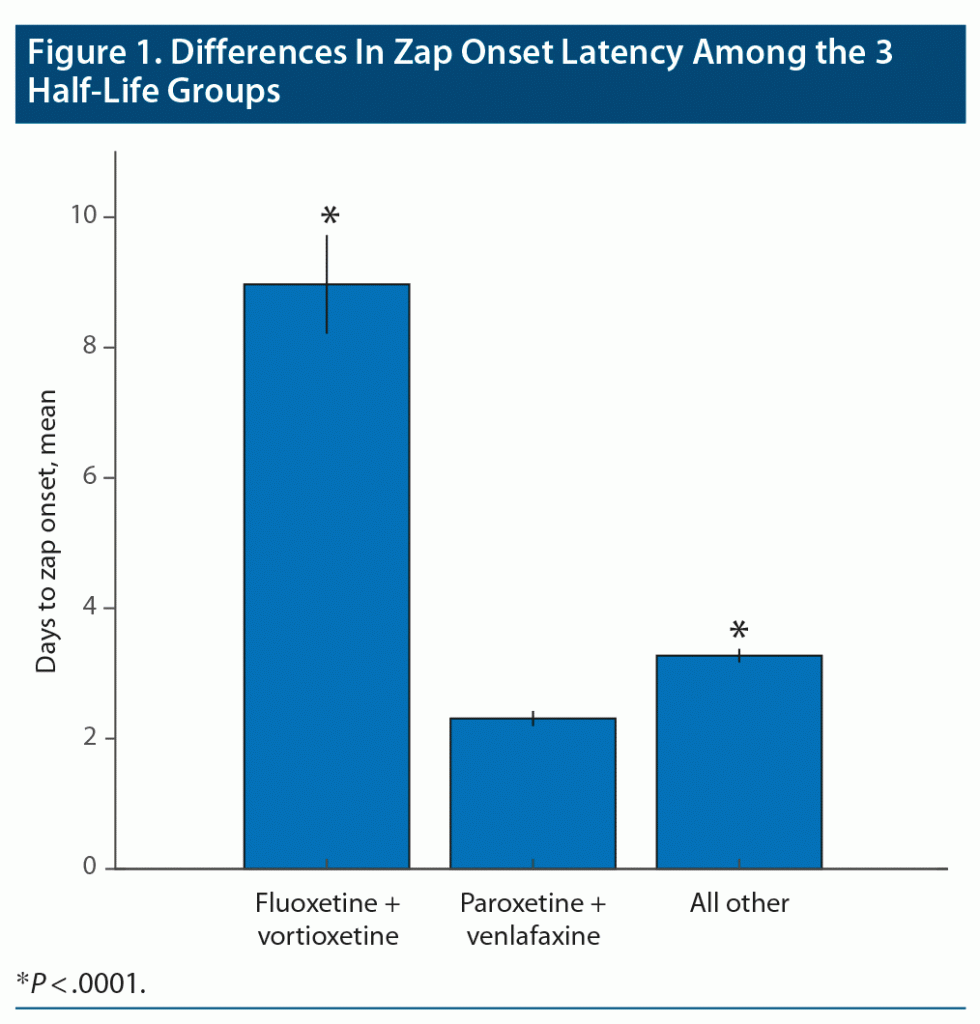
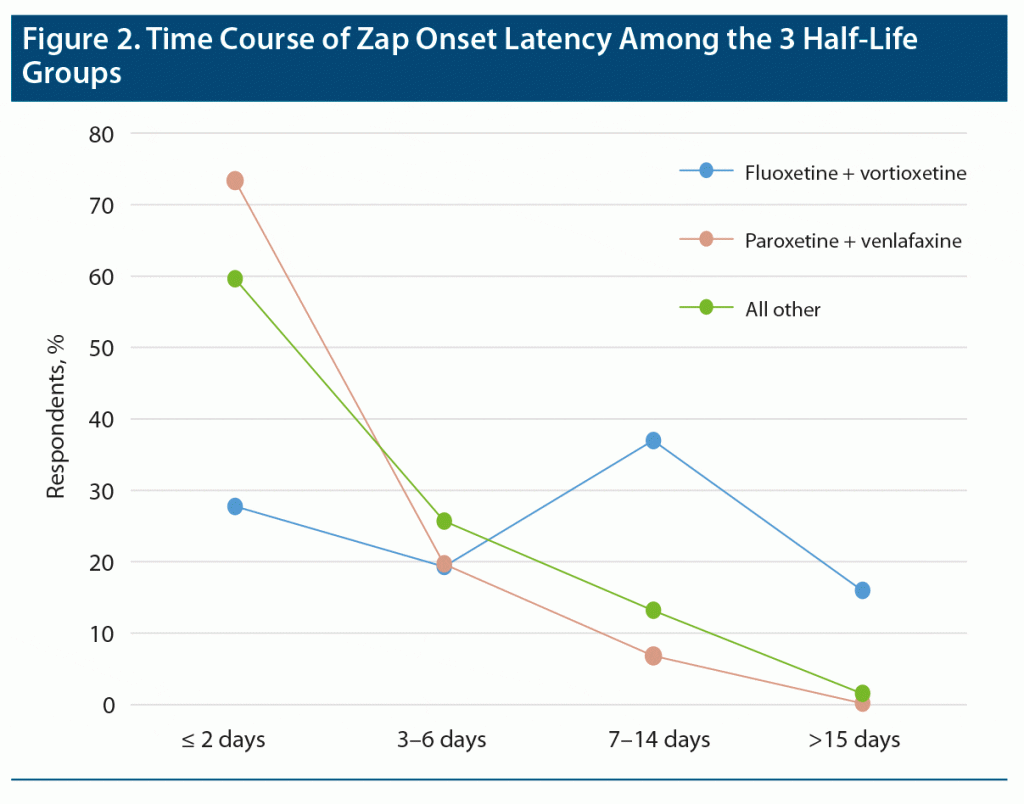
Zap onset latency (the length of time between the last dose of the medication and appearance of the first zap) provided one of the most robust results in our study. In this analysis, very short times (seconds, minutes) were ignored as mistaken responses. Moderately long times (few weeks) were accepted, but very long times (7 weeks and over) were ignored as very unlikely to be related to discontinuation. Zap onset latency for long, medium, and short half-life ADs were grouped separately and statistically compared by Kruskal-Wallis to reveal significant differences between the zap onset latency of the 3 half-life groups (Figure 1; P < .0001, χ2 = 144.6). Fluoxetine and vortioxetine, the long half-life medications, showed a dramatically longer latency to zap onset than the other 2 groups (P < .0001). The medium half-life drugs also showed significantly longer zap onset latency compared to short half-life medications (paroxetine and [des]venlafaxine) (P < .0001). Figure 2 shows the time courses of zap onset latencies of the 3 groups expressed in percent of each group. This figure highlights the prominent incidence of long-onset latencies for the long half-life medication group.
Methods Used to Reduce Brains Zaps and Their Efficacy
In an attempt to decrease brain zaps, about twice as many people reported that they restarted the medication than those who did not (1,468 vs 779). In most cases, restarting the medication helped (682 vs 86).
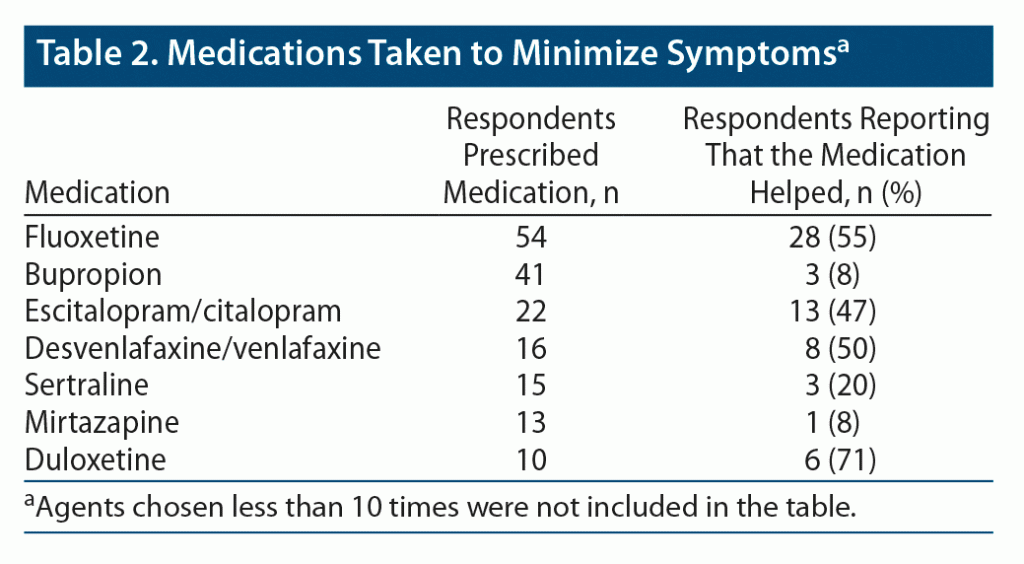
Of those starting a medication, 247 people were started on a different AD after the onset of the zaps. Quite often, this second agent was not an “official antidepressant,” but the answers were included in the analysis if the second agent was known to be an AD. Fluoxetine was the most frequent second serotonergic agent, chosen more than twice as often as (es)citalopram or (des)venlafaxine. All these medications were found to be effective in about 50% of the cases. It is notable that bupropion was chosen almost as frequently as fluoxetine, but it was found to be effective by only 8% of the respondents (Table 2).
Of the total 2,267 respondents, 2,103 (93%) reported that they were still having brain zaps at the time of completing the questionnaire. Approximately two-thirds of the respondents (876) stated that their condition was “as bad as ever” or “worse,” 436 said they had gotten a little bit better, 120 said they had gotten much better, 735 said they had gotten worse, and 173 gave no answer.
Characteristics of Brain Zaps
A main objective of the data collection was to obtain responses on the characteristics of the brain zaps. Regarding the length of a single brain zap, 1 second was the most frequently given answer, followed by a half second, 2 seconds, and 3 seconds. There were even higher durations given but in low numbers.
Responses asking about localization did not reveal clear tendencies. The highest number of responses was simply “head” or “brain” (599). Of the more specific localizations, there was a slight tendency to point to the “front” (the eyes themselves, the front of the head, or behind the eyes). A small number of responses were localized outside the head/brain, including arms/hands/fingers: 99, body: 74, throat/tongue/teeth/face/lips: 45, and legs: 35.
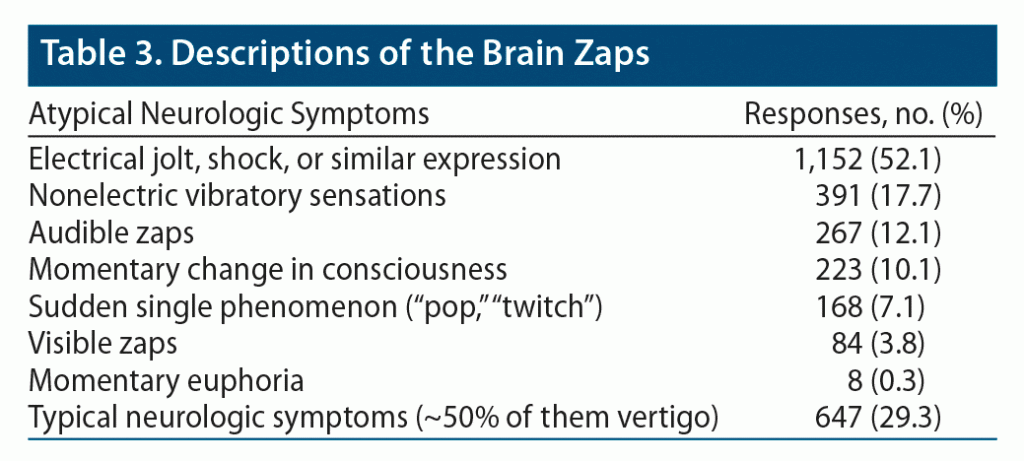
Respondents were asked to describe the brain zaps in an open-ended question, which required respondents to type their responses in the form of free text. Several clear groupings emerged (Table 3).
The vast majority described the sensation as an electrical jolt, shock, or shiver or used similar expressions (including zap). In a small subset of responses, the vibratory sensations took a nonelectric form, such as experiencing “strobe without light” or “card shuffle.” Some described hearing the zaps, sometimes sounding like a smooth “swoosh” or “whoosh” sound and other times like a sharp “crackle” or “rattling box full of tic tacs.” About 10% of respondents described a momentary change in consciousness, like confusion or disorientation occurring for a split second, but about half of them described what we termed a skip-restart-blink experience, using wording such as “brain skipping a beat,” “mind/brain blinking,” and “like when video is buffering for a moment.” Fewer responses still described a sudden single phenomenon (“pop,” “twitch”) and a relatively low number of responses described perceiving light briefly (as a “flash of light,” “strobe light”).
There were 647 descriptions of neurologic signs, almost half of them being vertigo. On the curious side, there were 8 reports of momentary euphoria, 2 of them with orgasm-like sensation.
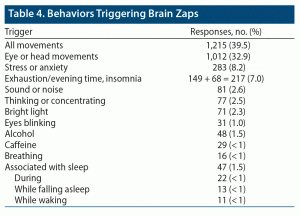
Behaviors Triggering Brain Zaps
Another open-ended question asked about behaviors triggering brain zaps. For 1,669 respondents, a specific trigger was associated with zap onset. By far, the greatest number of reports was for eye or head movements (1,012). Head movements were included with eye movements because they tend to trigger the vestibulo-ocular reflex, which moves the eyes. There were 203 unspecified “movement” responses, making a total of 1,215 responses that specifically, or possibly, refer to the movement of eyes as a trigger. Other triggers are shown in Table 4.
Effects of Brain Zaps on Quality of Life
The last section of the questionnaire regarded the effect of brain zaps on quality of life. Most of the 2,267 respondents reported a negative effect. Of these, 17% were “overwhelming,” causing very significant interference with functioning, and about 40% each were “significant or “some” negative effects, respectively. Only 54 respondents reported no noticeable effect, and 11 reported that the brain zaps were enjoyable.
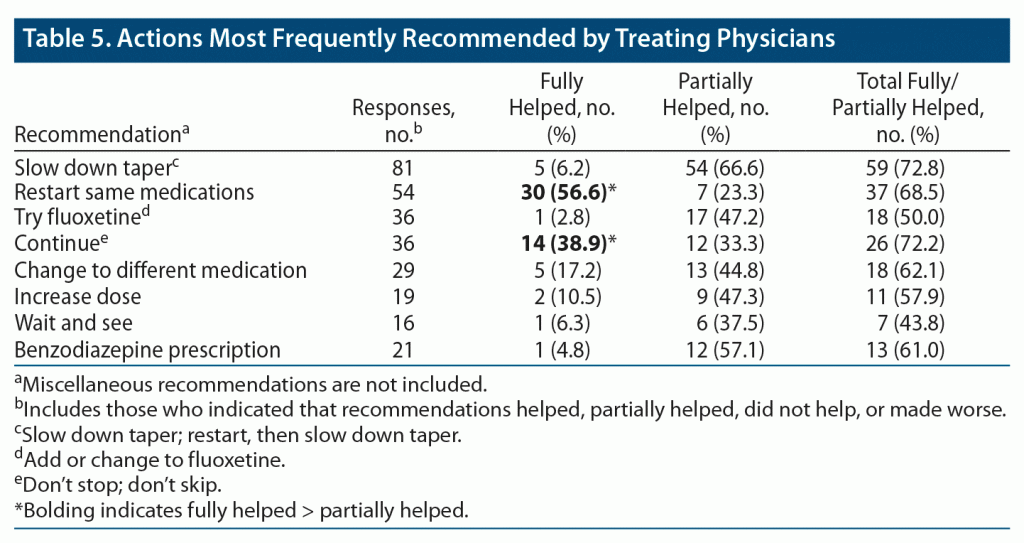
Of all the respondents, 1,313 stated that they did not report the symptoms to a professional; 897 said that they did, of whom 615 told the prescriber. The total number of times something was recommended by the prescriber was 413. The breakdown of the most frequent recommendations is provided in Table 5.
As for other suggestions, “see neurologist” was recommended 11 times. Relatedly, meclizine was the most frequently prescribed non–AD medication, which was noted to be of very little help. There were 86 miscellaneous recommendations, including tests such as hearing test, brain imaging, echocardiogram, and electrocardiogram, as well as a large variety of psychotropics and vitamins. These highly heterogenous recommendations had only one thing in common: they were barely or not at all helpful.
DISCUSSION
In the past, authors such as Christmas10 expressed concern that brain zap is a subjective lay term popularized on the internet that people with vaguely similar experiences simply adopted, hence the appearance of a regularly occurring phenomenon. However, in this article, we present compelling evidence that brain zaps are real phenomena and related to serotonergic mechanisms, based on the correlation between zap onset latency and medication half-life.
Our statistical analysis revealed that long half-life medications resulted in generally longer zap onset latencies. This finding is remarkable because even if patients knew about the half-life of the medication they used, it is doubtful that they would apply that knowledge to their memory of when the zaps first occurred after discontinuation of the medication. This finding represents the first time that zap onset latency has been statistically linked to medication half-life, thus further legitimizing the idea that the brain zap phenomenon is linked to AD discontinuation. Even the shortest half-life medications showed significantly shorter zap onset latencies compared to all other medium half-life medications, even though their trajectories over time were very similar. This finding shows that even moderate differences in half-life can affect the zap onset latency, further implicating this class of medications in the brain zap discontinuation symptom.
Many of our findings in the MHD study were corroborated in our current study. In both studies, respondents described zaps as electrical sensations, “brain rebooting/blinking,” and vibratory sensations or reported “hearing” the zaps. Vertigo was also the most frequently described neurologic symptom, and there were a few descriptions of euphoria or orgasm-like experiences. Also corroborated were the medications most likely to generate brain zaps, how long the medication was taken before the appearance of the zaps, what change in medication taking led to their appearance, the typical length of a zap, and the effect of brain zaps on quality of life.
One of the more striking findings in both studies was the number of reports referring to eye movements. In most cases, the eye movements were further specified as the side-to-side kind. We would like to emphasize that people reported this trigger in both of our studies in open-ended responses, with no prompting. We are unaware of this feature of brain zaps being reported in any previous publication.
Both in the MHD study and in this study, people reported “hearing their eyes move.” At this point we cannot provide an explanation for this phenomenon. Superior canal dehiscence syndrome11 is a very rare condition wherein people hear their eyes move, but that is only 1 of many other neurologic symptoms of a condition that has a well-known pathology. It is unlikely that it would be relevant to the consequences of discontinuing ADs.
The responses in this study indicate that restarting the same AD was helpful in most cases when it was attempted. Switching to a different antidepressant was reported to be more effective when the second AD was an SSRI versus another class (eg, bupropion or mirtazapine). These strategies appear to be the most effective in clinical practice to alleviate the AD withdrawal symptoms, which resonates with the general findings of the existing literature summarized in our MHD article. In addition, based on our findings, we suggest asking patients whether lateral eye movements trigger the complaint they are describing. A positive answer would likely identify true brain zaps, avoiding inefficient treatments, unnecessary referrals, or dismissive comments.
From a clinical standpoint, it is important to realize that while these complaints will diminish and disappear after a few months for most patients, for a minority it becomes a years-long, perhaps decades-long, ordeal, with currently little to no help provided from the medical profession.
Limitations
Our study analyzed responses obtained from an online survey. Such surveys utilize a self-selected convenience sample, which, while having the capacity to provide qualitative data, are generally not suitable for quantitative analysis. By the nature of the sampling method itself, there is a high risk of selection bias.12 Only a small number of our respondents indicated that they consulted written records to answer questions, which makes the reliability of individual answers impossible to gauge. For this reason, with one exception, we were able to make judgments only on the general similarities in responses obtained and their corroboration of the findings from the MHD study.
CONCLUSION
We report the analysis of data obtained from a questionnaire that was available on the internet between 2016 and 2018. The goal was to follow up and further specify a pattern of findings derived from analyzing spontaneous posts about brain zaps on an internet forum. The results obtained from the questionnaire confirm the findings derived from our earlier study.
We were specifically able to confirm that lateral eye movement is reported as a trigger in a majority of the cases. It is even more remarkable that those responses were given without the questionnaire offering any hint as to what responses were expected. It seems reasonable to conjecture that lateral eye movement relates to something significant in the generation of a brain zap and understanding its biological mechanism would be an important step in treating people who suffer from this consequence of AD discontinuation.
For the future, we recommend the following 3 actions: (1) revise the DESS so it includes brain zaps, because we believe that it is a strongly associated symptom and clearly the most unique. (2) On the basis of our findings, create and validate a short, simple questionnaire that could help clinicians to identify brain zaps (a concept of such a questionnaire is provided in Appendix 2). (3) Devise studies exploiting the apparent strong association between lateral eye movements and brain zaps, with the goal of elucidating its pathomechanism and providing the opportunity to create effective treatments.
Submitted: March 18, 2021; accepted September 2, 2021.
Published online: February 10, 2022.
Potential conflicts of interest: None.
Funding/support: None.
Supplementary material: See accompanying pages.
Clinical Points
- Brain zaps are real clinical phenomena with typical symptomatic characteristics, which can aid the clinician in their identification.
- Restarting the offending antidepressant or switching to a serotonergic antidepressant with a longer half-life may be effective strategies for brain zap symptoms; very slow tapering seems to have some advantage over a fast one.
- Brain zaps are tolerable and tend to improve with time for most patients; however, for some they can be disabling and last many years.
References (12)

- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183–197. PubMed CrossRef
- Haddad PM, Anderson IM. Recognizing and managing antidepressant discontinuation symptoms. Adv Psychiatr Treat. 2018;13(6):447–457. CrossRef
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87. PubMed CrossRef
- Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–121. PubMed CrossRef
- Papp A, Onton JA. Brain zaps: an underappreciated symptom of antidepressant discontinuation. Prim Care Companion CNS Disord. 2018;20(6):18m02311. PubMed CrossRef
- Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67–73. PubMed CrossRef
- Stockmann T, Odegbaro D, Timimi S, et al. SSRI and SNRI withdrawal symptoms reported on an internet forum. Int J Risk Saf Med. 2018;29(3-4):175–180. PubMed CrossRef
- Read J. How common and severe are six withdrawal effects from, and addiction to, antidepressants? The experiences of a large international sample of patients. Addict Behav. 2020;102:106157–3. PubMed CrossRef
- LSAT Quantity Terminology: some, few, several, many. PowerScore website. Accessed October 2019. https://www.powerscore.com/lsat/help/lsat-quantity-terminology.cfm
- Christmas DMB. “Brain shivers”: from chat room to clinic. Psychiatr Bull. 2005;29(6):219–221. CrossRef
- Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. 2017;8:177. PubMed CrossRef
- Jauhar S, Hayes J. The war on antidepressants: what we can, and can’t conclude, from the systematic review of antidepressant withdrawal effects by Davies and Read. Addict Behav. 2019;97:122–125. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite