Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Clozapine is an important tool in the treatment of refractory patients, but clinicians need to be sophisticated in managing its many possible adverse drug reactions. Clozapine can cause falls due to orthostatic hypotension or sedation, particularly in geriatric patients. A less-recognized cause of falls in clozapine patients is myoclonus, which manifests as knee buckling or leg folding.
Unexpected Falls During Clozapine Treatment Explained by Myoclonus
To the Editor: Clozapine is an important tool in the treatment of refractory patients, but clinicians need to be sophisticated in managing its many possible adverse drug reactions.1 Clozapine can cause falls due to orthostatic hypotension or sedation, particularly in geriatric patients.2 A less-recognized cause of falls in clozapine patients is myoclonus, which manifests as knee buckling or leg folding.3-5 Although there are no high-quality studies, clozapine-induced myoclonus can (1) be dose related6 or, more precisely, serum concentration related; (2) be the first sign of clozapine intoxication; and (3) evolve into generalized tonic-clonic seizures7 if the dose (or serum concentration) is not reduced.
This case report reminds clinicians using clozapine that myoclonus can be a cause of unexpected falls and supports the concept that myoclonus is a serum concentration-dependent adverse drug reaction that can be safely managed with therapeutic drug monitoring (TDM).
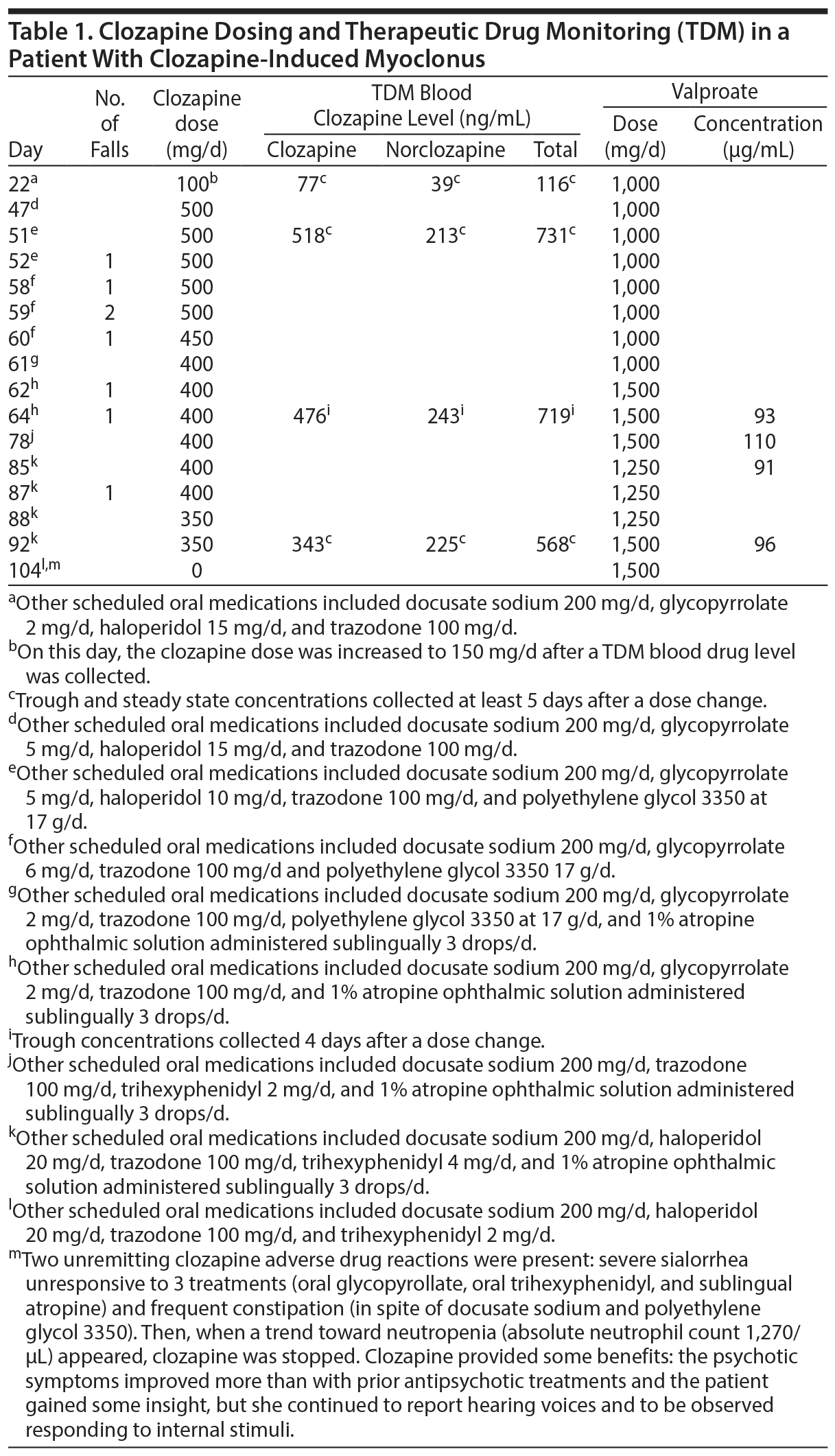
Case report. A 22-year-old white female nonsmoker with a history of treatment-resistant schizophrenia and substance abuse was referred for her fifth admission to a psychiatric hospital. After a brief stay in jail due to public intoxication, she required treatment for auditory hallucinations and thought blocking. Because she had previously failed multiple antipsychotic trials (including single and polytherapy) with oral and long-acting injectable formulations, a clozapine trial was started. Beginning on day 1 of the trial, clozapine was slowly titrated up as olanzapine and haloperidol were discontinued on day 6 and day 56, respectively. Despite no history of prior falls, 4 falls occurred between days 58 and 60 (Table 1). The patient refused vital sign monitoring for 3 days, thus orthostasis was initially considered the cause of the falls. However, when she complied with monitoring, her systolic and diastolic blood pressure were found to be within normal limits (110/92 mm Hg). After the senior author (J.d.L.) described myoclonus as another cause of falls, the second author (J.B.) diagnosed the patient with myoclonus after witnessing a fall with knee buckling (the legs appeared to lose strength, the knees bent, and the patient fell to the floor). Two days later, the patient experienced jerking arm movements, causing her to drop items.
A neighboring university hospital completed most of the laboratory tests, but clozapine TDM using liquid chromatography/mass spectroscopy/mass spectroscopy was performed at a contracted commercial laboratory. Early morning (before medication intake) collections are used as trough clozapine TDM measures in our hospital. We aspire to have at least 5 half-lives with no dose changes to reach drug steady state. Assuming that clozapine’s half-life may be up to 24 hours,6 5 days may be required to reach steady state, which was reached on days 22, 51, and 92. The TDM on day 64 occurred only 4 days after the last dose change.
Table 1 shows that the patient was taking 1,000 mg/day of valproate, but no valproate concentrations on that dose were measured. On day 62, the valproate dose was increased to 1,500 mg/day, which provided definitive therapeutic concentrations of valproate (ranging from 91-110 μg/mL), but the patient had 2 myoclonic jerks with these therapeutic valproate concentrations with a clozapine dose of 400 mg/day. Conversely, the myoclonic jerks disappeared when the clozapine dose was decreased to 350 mg/day. Therefore, myoclonus occurred despite the patient’s use of valproate, with clozapine doses ranging from 500-400 mg/d, while it remitted when the clozapine dose was decreased to 350 mg/day. We followed a guideline8 that recommends a therapeutic clozapine range of 350-600 ng/mL, thus the clozapine TDM associated with myoclonus in this patient did not appear high, as the clozapine concentration was approximately 500 ng/mL, and the total concentration was around 700 ng/mL. The only available clozapine TDM on a 350-mg/day dose that did not cause myoclonus provided clozapine and total concentrations of 343 and 568 ng/mL, respectively. On day 104, although myoclonus was no longer present, clozapine was finally discontinued after its risks were considered greater than its benefits.
Wong and Delva9 recommended lowering the clozapine dosage when myoclonus is present and described valproate as the most common antiepileptic drug used for clozapine-induced seizure. This case and 2 prior cases4,10 suggest that (1) valproate treatment may not protect from clozapine-induced myoclonus and (2) clozapine-induced myoclonus may disappear after reducing clozapine dosage/concentrations. On the other hand, myoclonus occurred without abnormally high serum clozapine concentrations only in this case.
References
1. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. PubMed CrossRef
2. Pitner JK, Mintzer JE, Pennypacker LC, et al. Efficacy and adverse effects of clozapine in four elderly psychotic patients. J Clin Psychiatry. 1995;56(5):180-185. PubMed
3. Antelo RE, Stanilla JK, Martin-Llonch N. Myoclonic seizures and "leg folding" phenomena with clozapine therapy: report of two cases. Biol Psychiatry. 1994;36(11):759-762. PubMed CrossRef
4. de Leon J, Diaz FJ. Serious respiratory infections can increase clozapine levels and contribute to side effects: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1059-1063. PubMed CrossRef
5. Liang CS, Hsieh TH. Myoclonus as an indicator of infection in patients
with schizophrenia treated with clozapine. J Psychiatry Neurosci. 2011;36(1):E1-E2. PubMed CrossRef
6. Sabaawi M, Singh NN, de Leon J. Guidelines for the use of clozapine in individuals with developmental disabilities. Res Dev Disabil. 2006;27(3):309-336. PubMed CrossRef
7. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128. PubMed CrossRef
8. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235. CrossRef
9. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463. PubMed CrossRef
10. Cooke C, de Leon J. Adding other antipsychotics to clozapine. J Clin Psychiatry. 1999;60(10):710. PubMed CrossRef
aEastern State Hospital, Lexington, Kentucky
bUK Mental Health Research Center, Eastern State Hospital, Lexington, Kentucky
cPsychiatry and Neurosciences Research Group, Institute of Neurosciences, University of Granada, Granada, Spain
dBiomedical Research Centre in Mental Health Net (CIBERSAM), Santiago Apóstol Hospital, University of the Basque Country, Vitoria, Spain
Author contributions: Dr McCollum wrote the first draft, and Dr de Leon modified it to fit the journal’s style. All of the authors contributed to the interpretation of the case and approved the final manuscript.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Lorraine Maw, MA, at the UK Mental Health Research Center, Lexington, Kentucky, for editorial assistance. Ms Maw reports no conflicts of interest related to the subject of this report.
Patient consent: The patient provided oral permission for publication of this case. The patient’s state guardian (the patient had been declared incompetent by the court) signed a consent form to allow publication.
Published online: January 18, 2018.
Prim Care Companion CNS Disord 2018;20(1):17l02151
To cite: McCollum B, Barclay J, de Leon J. An unexpected fall during clozapine treatment explained by myoclonus. Prim Care Companion CNS Disord. 2018;20(1):17l02151.
To share: https://doi.org/10.4088/PCC.17l02151
© Copyright 2018 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite