ABSTRACT
Objective: Over-the-counter (OTC) and prescription sleep medications are frequently used as treatments for chronic insomnia, despite risks and limited long-term efficacy. Investigating mechanisms underlying this predilection for pharmacotherapy may uncover strategies to decrease reliance on sleep aids. The objective of this study was to determine how time monitoring behavior (TMB; clock-watching) and associated frustration may interact with insomnia symptoms to drive the use of sleep aids.
Methods: Patients (N = 4,886) presenting for care at a community-based, private sleep medical center between May 2003 and October 2013 completed the Insomnia Severity Index (ISI) and Time Monitoring Behavior-10 (TMB-10) and reported their frequency of sleep medication use (OTC and prescription, separately). Mediation analyses examined how clock-watching and related frustration could be associated with insomnia symptoms and medication use.
Results: The relationship between TMB and sleep medication use was significantly explained by ISI (P < .05), in that TMB (especially related frustration) appears to aggravate insomnia, which in turn leads to sleep aid use. Similarly, but to a lesser extent, the relationship between ISI and sleep medication use was explained by TMB, in that ISI may lead to increased TMB, which may in turn lead to sleep aid use.
Conclusions: TMB and the associated frustration it engenders may perpetuate a negative cycle of insomnia and sleep aid use. Future longitudinal and interventional research is necessary to examine the developmental course of these clinical symptoms and behaviors and to test whether decreasing frustration by limiting TMB reduces the proclivity for pharmacotherapy.
Prim Care Companion CNS Disord 2023;25(3):22m03344
To cite: Dawson SC, Krakow B, Haynes PL, et al. Use of sleep aids in insomnia: the role of time monitoring behavior. Prim Care Companion CNS Disord. 2023;25(3):22m03344.
To share: https://doi.org/10.4088/PCC.22m03344
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychological and Brain Sciences, Indiana University, Bloomington, Indiana
bSleep and Human Health Institute, Savannah, Georgia
cSleep Arts & Sciences, Savannah, Georgia
dDepartment of Psychiatry and Behavioral Health, Mercer University School of Medicine, Savannah, Georgia
eHealth Promotion Sciences, University of Arizona, Tucson, Arizona
fThe Initiative on Stress, Trauma, and Resilience (STAR), Department of Psychiatry and Human Behavior, Center for Behavioral and Preventive Medicine, The Miriam Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island
gDepartment of Orthopaedics and Rehabilitation, The University of New Mexico, Albuquerque, New Mexico
*Corresponding author: Barry Krakow, MD, 211 Early St, Savannah, GA 31405 ([email protected]).
Insomnia is a common disorder affecting 3.9%–22.1% of adults.1 Beyond nighttime symptoms and daytime impairment, insomnia is associated with long-term health problems, including cardiovascular disease,2,3 diabetes,2 depression,4,5 acute myocardial infarction,6 and increased mortality risks.7 Treatment guidelines recommend cognitive-behavioral therapy for insomnia (CBT-I) for adult chronic insomnia.8,9 However, barriers persist in accessing CBT-I,10,11 especially compared to sleep aids.
Recent evidence demonstrates serious risks with the use of prescription and over-the-counter (OTC) sleep aids.12–19 OTC antihistamine risks include cognitive decline12 and possibly dementia.13 Further concerns associated with prescription sleep aids include cognitive impairment,20 dementia,14 Alzheimer’s disease,15 cancer,16 psychiatric disorders,17 and mortality.18,21 Despite these troubling observations, use of sleep aids remains high.19
While tolerance to sedative effects of diphenhydramine (most common OTC sleep aid) emerges after 3 days,22 prescription sleep aids have demonstrated efficacy in reducing nighttime insomnia symptoms for 12 months or longer.23 Thus, it is no surprise that prescribing rates of benzodiazepines for insomnia remained stable from 2003 to 2015, with 1 in 4 medical visits for insomnia resulting in a prescription.19 Notwithstanding, approximately half of patients with insomnia taking prescription sleep aids do not attain remission.24 Accordingly, many patients using prescription or OTC medications may be eager and willing to discontinue these drugs, and so research examining modifiable behavioral risk factors to reduce sleep aid use may prove useful. Time monitoring behavior (TMB) is one such factor.
TMB, clock-watching during periods of sleeplessness, is common among individuals with insomnia.25–27 TMB appears as an effort to predict or control sleep duration, arising from the unpredictability and fear of sleep loss. Clock-watching may offer a temporary sense of control through mental calculations about lost sleep or sleep still to be achieved. Though no prospective research exists on the developmental origins of TMB, both theory and clinical experience suggest ways in which clock-watching may arise during insomnia episodes. For example, the 3P (predisposing, precipitating, perpetuating) model of insomnia posits specific responses to sleep loss perpetuate chronic insomnia,28 while the attention-intention-effort model suggests attempts to control sleep cause or exacerbate insomnia.29
TMB is probably an adaptive short-term response, allowing one to compensate for sleep loss by hoping for more sleep through planning, usually to extend time in bed, based on knowing the time of night. This can be a precise analysis, for example, “If I can fall back asleep within 15 minutes, reset my alarm by 30 minutes, I can still gain 60 more minutes of sleep, but if . . .” and so on.26 This clock-watching and mental arithmetic directly aggravate sleeplessness by spending time engaged in stressful waking activities instead of sleeping.
The behavior results in additional negative consequences—reinforcing the belief that self-initiation of sleep is impossible and promoting psychophysiologic conditioning30 by creating frustration about sleep loss. Frustration may then aggravate sleeplessness,31 perpetuating more TMB and frustration, a cycle known as “losing sleep over losing sleep.”26 This emotional response may explain more about how TMB adversely influences insomnia and use of sleep aids than the actual act of clock-watching or arithmetic calculations in bed.
As frustration fuels greater sleep loss, sleep aids may be used to gain control over sleep and break this cycle. Taking a hypnotic allows a patient to relax and thereby promote sleep32,33; however, continued TMB and efforts to force sleep could counteract hypnotic effects or natural sleepiness.29 Worse, despite the variable range of benefits that sleeping pills provide, discontinuing hypnotics can result in rebound insomnia.34 While this exacerbation of insomnia may last just 1 or 2 nights,35 it may aggravate TMB and the cycle of losing sleep over losing sleep. With no alternative means to initiate or return to sleep, resumption of sleep medications may be immediate.
Vochem et al36 examined a sample of treatment-seeking insomnia patients compared to a cohort of good sleepers and demonstrated a significant correlation between greater scores on the Time Monitoring Behavior-10 (TMB-10) scale and worse insomnia severity, without examining sleep aid use. Another work evaluated TMB in a large group of patients with mixed sleep disorders presenting to a sleep medical center.25 Among those reporting clinically meaningful posttraumatic stress symptoms, more severe TMB, associated frustration, and insomnia were noted compared to those with minimal or no posttraumatic stress symptoms,25 a salient finding as sleep disturbance is a core feature of PTSD37 and is highly prevalent across psychiatric diagnoses.38
Sleep disturbance in psychiatric disorders requires special attention, as (1) insomnia is a very common residual symptom following pharmacologic and psychological treatments for major depressive disorder,39 (2) insomnia is a risk factor for relapse in alcohol use disorder,40 and (3) nocturnal wakefulness is associated with risk of suicide.41 Further underscoring the importance of TMB to insomnia comorbid with psychiatric disorders, such patients receive more prescriptions for benzodiazepines,19 and their insomnia is less likely to remit with medications compared to patients without psychiatric comorbidities.24
In the current study, we used a large sample of patients with mixed sleep disorders to examine the relationship between TMB, insomnia symptoms, and use of sleep aids. In addition to examining whether TMB was associated with sleep aid use, we investigated how TMB and insomnia symptoms may together influence use. We hypothesized TMB would aggravate insomnia severity, leading to increased use of sleep aids.
METHODS
Participants
This retrospective chart review was conducted for patients presenting at Maimonides Sleep Arts & Sciences, Ltd (MSAS), a community-based, private sleep medical center in Albuquerque, New Mexico, between May 2003 and October 2013. The University of Arizona Institutional Review Board determined this research exempt from further human subject review. As per standard MSAS protocol, all patients provided written consent for their medical information to be used anonymously for research purposes. All patients completed online questionnaires regarding sleep and medical history. The sample comprised 4,886 patients at least 18 years of age who completed the questionnaires.
Measurements
All data were extracted from questionnaires. Prevalence of an insomnia complaint was based on a single question asking whether patients reported this sleep problem and for how long. Patients were also asked whether they had psychiatric disorders. Medication use frequency was reported on a scale from 0 (rarely/never) to 5 (nightly) in response to “How often do you use prescription medication to help you sleep?” The same scale assessed frequency of non-prescription or natural remedies to help with sleep. To compare frequency, weekly or greater use of sleep medication was classified as regular use. For the mediation analyses, the full scale was used as a continuous variable.
The Insomnia Severity Index (ISI), a validated measure of insomnia symptoms,42 quantified insomnia symptom severity. The ISI is a 7-item scale with higher scores indicating greater symptom severity (score range, 0–28).
The TMB-1025 is a 10-item measure of TMB and related frustrations. The 10 items evaluate both TMB engaged in before sleep onset (sleep onset insomnia) and during nocturnal awakenings (sleep maintenance insomnia). For each period, (sleep initiation and sleep maintenance), 2 questions assess behavior, and 3 questions assess associated frustration. The TMB-10 has a range of 0–30, with higher scores indicating greater TMB and associated frustration. Prior research43 found that the TMB-10 can be split into 3 factors: behavior (TMB-BX: actual clock-watching and mental calculations), sleep onset frustration (TMB-SOF), and sleep maintenance frustration (TMB-SMF).
For additional analysis, chronic insomnia disorder was defined by insomnia symptoms of at least moderate severity (ISI > 14), with insomnia-related daytime impairment (ISI item 5 ≥ 1) and a minimum of 3 months duration, which is consistent with current nosologies.44,45
Data Analysis
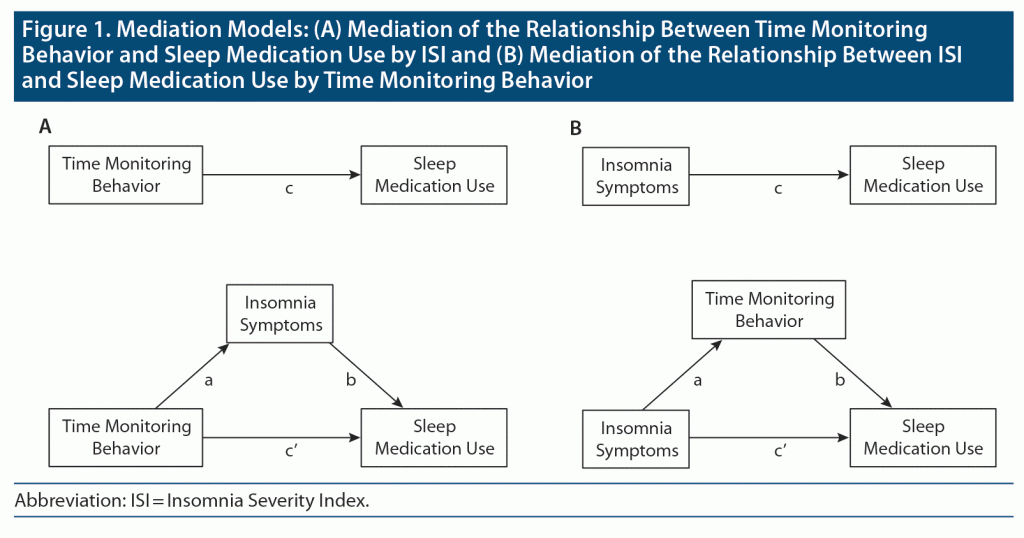
To characterize how TMB (and aspects thereof) and insomnia symptom severity may influence one another with regard to medication use, a series of mediation analyses were conducted, depicted graphically in Figure 1. Each analysis compared a direct path between independent and dependent variables (IV and DV, respectively) to an indirect path that included a mediator. The indirect path was tested for statistical significance using bootstrapping.46 Full mediation occurred if the direct path was not statistically significant after controlling for the indirect path; otherwise, partial mediation was judged to have occurred.47 Full mediation suggests that the mediator may be able to fully explain the relationship between IV and DV. Partial mediation suggests that the relationship between IV and DV cannot be fully explained by the mediator; a direct relationship remains between the IV and DV. The proportion of mediation (PM) is an estimate of the percent of the total path that could be accounted for by the indirect path; it may exceed 1.0 depending on the relationship between the direct path between IV and DV and the total relationship between IV and DV.48 Accordingly, greater values of PM may be interpreted as indications of a more robust mediation and a weaker direct relationship between the IV and DV.
Mediation models differed in 3 aspects: (1) whether prescription or OTC medication use was being tested as the sleep medication use outcome, (2) the directionality of the mediation model (ie, insomnia mediating the relationship between TMB and medication use versus TMB mediating the relationship between insomnia symptoms and medication use), and (3) whether the full TMB-10 scale or one of its 3 subscales was being examined. Follow-up analyses were conducted in 3 separate groupings: (1) the full sample, (2) those patients reporting psychiatric diagnosis, and (3) those patients who met study criteria for chronic insomnia disorder.
RESULTS
Sample Descriptive Statistics
The sample was 45.5% female, with an average age of 50.1 (SD = 13.8) years. Insomnia complaint was reported by 2,086 participants, and 1,558 met study criteria for chronic insomnia disorder. Psychiatric disorders were reported by 2,295 participants. For the entire sample, the mean ISI score was 14.77 (SD = 6.22), and the mean score on the TMB-10 was 11.73 (SD = 8.76). Also, 37.8% of the sample used any sleeping medications at least weekly, and 26.2% used prescription sleep medication at least weekly.
Consistent with the hypothesis that TMB would be related to medication use, scores on the TMB-10 were higher among patients reporting regular use of any sleep medication (mean = 13.91, SD = 8.93) than those who did not (mean = 10.41, SD = 8.39, t3702.7 = 13.57, P < .0001, Δ=0.42). Similarly, those patients who reported regular use of prescription sleep medication had higher scores on the TMB-10 (mean = 14.07, SD = 9.10) than those who did not (mean = 10.90, SD = 8.49, t2118.5 = 10.88, P < .0001, Δ=0.37).
TMB and Medication Use in Patients With Reported Insomnia or Psychiatric Diagnoses
Regular use of sleep medication was more common in patients reporting insomnia than not (58.10% vs 22.61%, χ21 = 640.82, P < .0001). Among those reporting insomnia, regular use of sleep medication was more common in those reporting psychiatric diagnosis than not (67.01% vs 44.48%, χ21 = 103.95, P < .0001). Similarly, regular use of prescription sleep medication was more common in patients who reported insomnia complaints than not (44.15% vs 12.82%, χ21 = 606.89, P < .0001). And again, among those with insomnia, regular use of prescription sleep medication was more common in those who had comorbid psychiatric diagnoses than not (53.45% vs 29.94%, χ21 = 111.79, P < .0001).
In a 2 x 2 analysis of variance, both insomnia complaint and psychiatric diagnoses were associated with higher scores on the TMB-10 (F3, 4882 = 144.79, P < .0001, η2 = 0.08). There were statistically significant main effects for both insomnia (mean = 14.44, SD = 8.98 vs mean = 9.71, SD = 8.03, F1, 4882 = 295.64, P < .0001, η2partial = 0.0571) and psychiatric diagnosis (mean = 13.23, SD = 9.00 vs mean = 10.40, SD = 8.33, F1, 4882 = 52.34, P < .0001, η2partial = 0.0106), but the interaction between the 2 variables was not significant (F1, 4882 = 0.79, P = .3742, η2partial = 0.0002).
Mediation Analysis
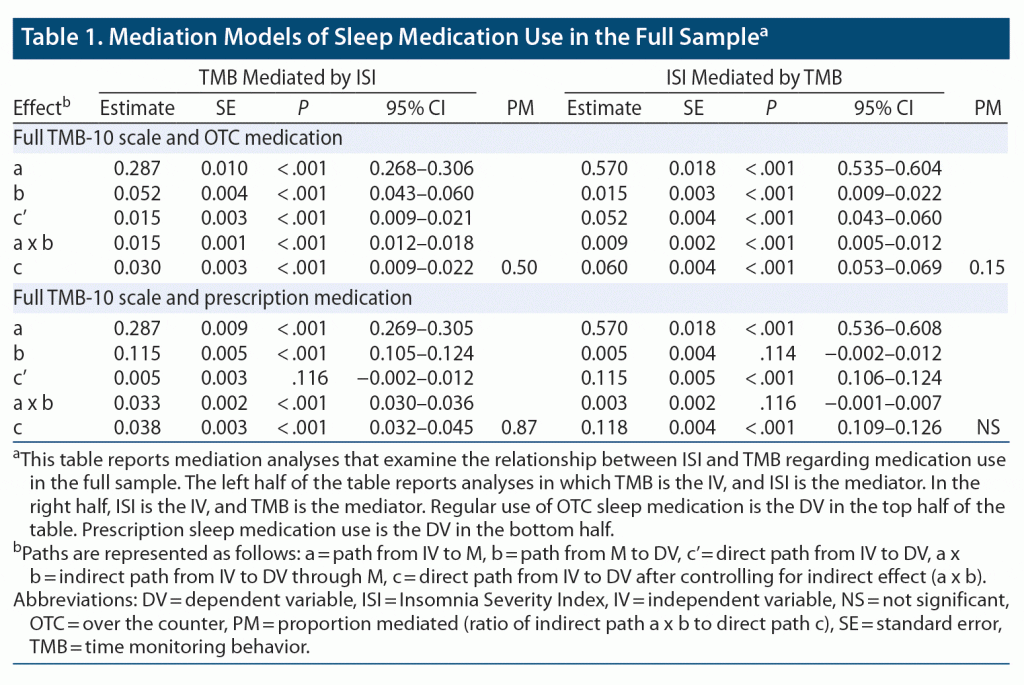
The mediation models for the TMB-10, the ISI, and both OTC sleep and prescription sleep medications are shown in Table 1. Taken together, the models show greater effect sizes for a TMB to ISI to medication use path than ISI to TMB to medication use, with the greatest effect size demonstrated for prescription medication.
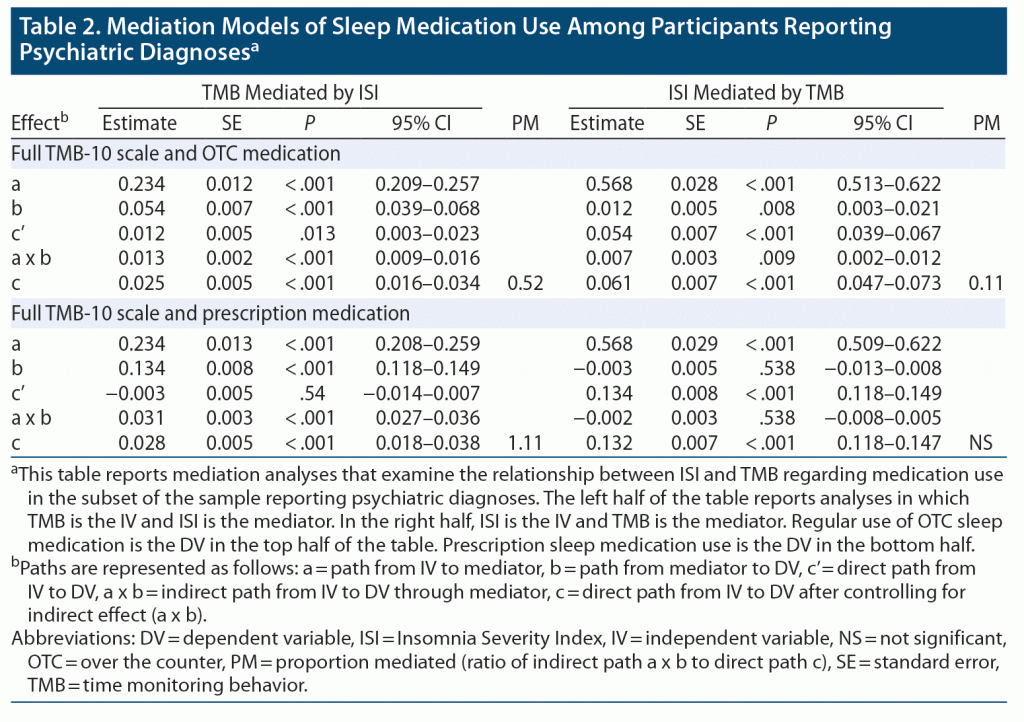
The mediation models for the TMB-10, the ISI, and both OTC sleep and prescription sleep medications among patients with self-reported psychiatric diagnoses are shown in Table 2. The effect sizes followed the same pattern as for the whole sample.
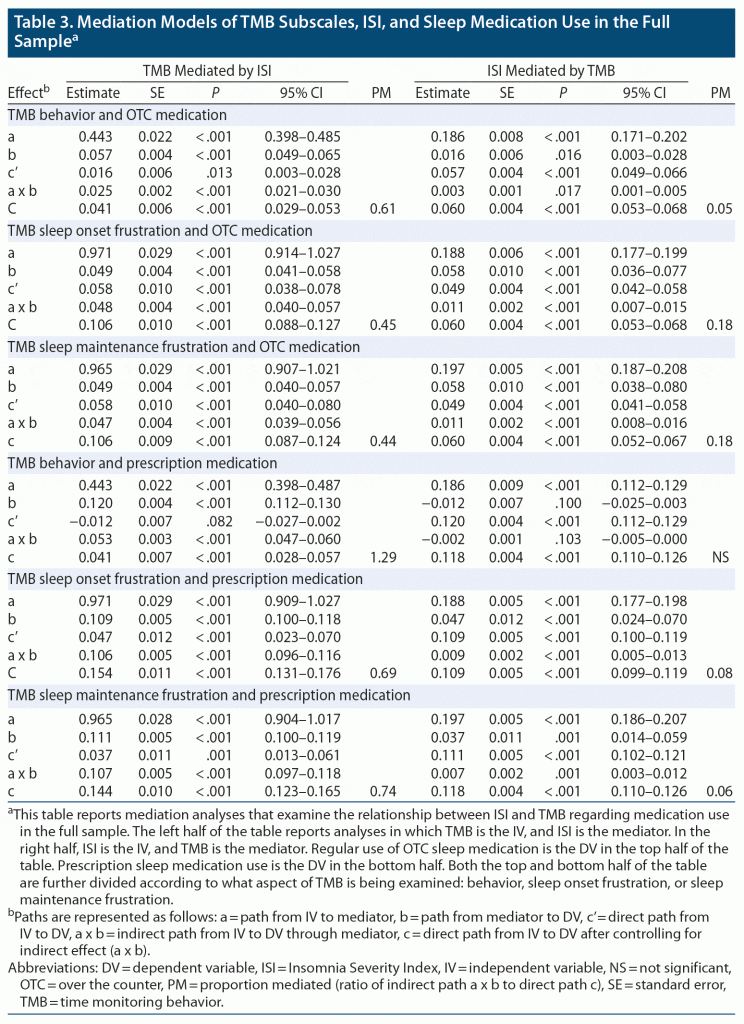
The mediation models for the TMB subscales, the ISI, and both OTC sleep and prescription sleep medications are shown in Table 3. A similar pattern was shown in that mediation effect sizes were greater for a TMB to ISI to medication use path and for prescription medication use. In addition, greater mediation was found for TMB-BX than for the frustration-related factors: TMB-SOF and TMB-SMF.
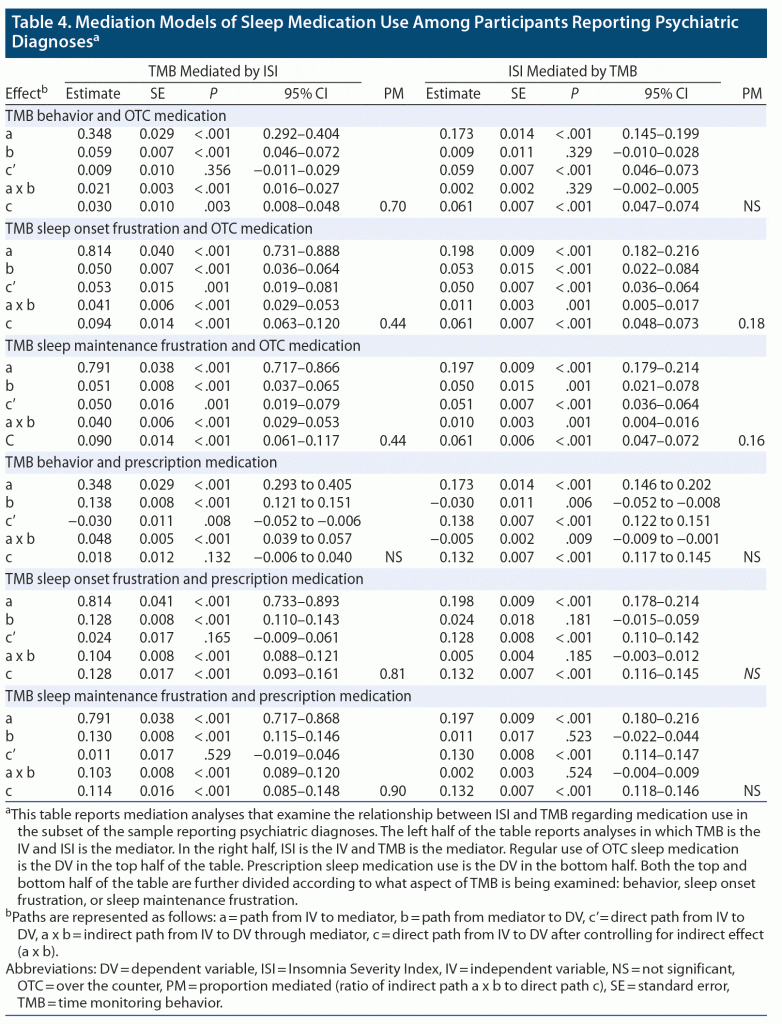
The mediation models for the TMB subscales, the ISI, and both OTC sleep and prescription sleep medications among patients with self-reported psychiatric diagnoses are shown in Table 4. Once again, mediation effect sizes were greater for TMB to ISI to medication use path and for prescription medication use. Also, greater mediation was again found for TMB-BX than for the frustration-related factors: TMB-SOF and TMB-SMF.
See the Supplementary Material for more detailed description of the above mediation models and for results of mediation analyses focused on patients who met study criteria for chronic insomnia disorder. In brief, the majority of analyses focused on chronic insomnia disorder did not show statistically significant mediation.
DISCUSSION
The current study demonstrated TMB is of particular importance to patients with insomnia who use sleep medications. First, sleep medication was more common among patients reporting both insomnia and psychiatric conditions. Second, TMB was higher in those same patients. Finally, our findings suggest a bidirectional relationship between TMB and insomnia symptom severity in the context of sleep medication use. Primarily, TMB appears to be related to sleep medication use due to its relationship with insomnia symptom severity. That is, TMB may provoke worsening of insomnia, which leads to greater use of sleep aids. To a lesser extent, as insomnia symptoms worsen, so too may TMB, potentially resulting in greater sleep aid use. In sum, while insomnia symptoms and TMB may feed off one another, TMB may be upstream of insomnia with regard to association with sleep medication use.
Whereas many factors influence patients’ decisions to use sedating drugs, frustration due to TMB seems noteworthy. Evidence pointing to this emotional response was consistently observed across all mediation models for sleep aid use when using TMB frustration instead of TMB alone. Furthermore, the relationship between frustration and sleep medication use was more likely to be partially, rather than fully, mediated by insomnia symptom severity. This finding suggests TMB frustration retains a significant relationship with sleep medication use, outside of its relationship with insomnia symptom severity. Overall, the observed relationships tended to be stronger and more consistent when examining the OTC use, rather than prescription sleep medications, as well as among the entire sample, rather than only those with psychiatric diagnoses or chronic insomnia. These differences are likely due to greater availability of OTC medications, greater complexity of psychiatric patients, and reduced range of ISI scores among chronic insomnia patients, as a cutoff of ISI > 14 was used to define this group.
The importance of sleep-related frustration aligns with research where clock-watching by people with insomnia increased both worry and subjective sleep latency,31 either of which may exacerbate TMB, creating a vicious cycle of clock-watching and sleep loss. These findings are relevant to clinicians treating insomnia and those providing CBT-I, because long-term CBT-I benefits are optimized after sleep medication discontinuance,49 albeit CBT-I can be effectively combined with medication tapering.50
A standard recommendation is to turn around the clock or remove it from the bedroom to reduce TMB. Notwithstanding, the present results suggest the behavior itself is not the sole driver of medication use; rather, frustration is at least as important, if not more so. Clinically, 2 potential approaches could effectively address frustration. Cognitive restructuring may alleviate frustration related to probability overestimation or catastrophizing,51 or an emotion-focused approach may be helpful to address maladaptive emotional processing.26 For patients with psychiatric disorders, for whom CBT-I has been found effective,52,53 reducing frustration may prove particularly helpful.
In the past decade, numerous studies and review articles raised questions about the efficacy of specific sleep aids and adverse effects associated with OTC and prescription sleep medications.12–18,54,55 Regardless of agreement or disagreement with these critiques, the vexing nature of insomnia clearly leads a substantial proportion of patients or medical professionals to seek or to prescribe sleep medications.56,57 Given the potential role of TMB-related frustration in the exacerbation of insomnia, we speculate interventions to diminish TMB may be helpful for patients who wish to improve insomnia symptoms and discontinue use of sleep aids. If the frustration arising from TMB contributes to the patient’s insomnia and drive to take a sleeping pill, then defusing this frustration may improve insomnia and reduce sedative use.
Frustration associated with TMB is likely an important area of research and clinical attention for insomnia patients. It remains a question whether reducing TMB-induced frustration would decrease insomnia symptoms or sleep aid use; nonetheless, the current findings suggest this hypothesis should be tested. Such research must involve longitudinal studies of TMB, insomnia symptoms, and medication use as well as the effects of different therapeutic pathways. Since previous experimental research demonstrated deleterious effects of TMB on sleep in just one night,31 benefits of reducing TMB may emerge in a similar time frame. Moreover, randomized controlled trials of TMB interventions could test whether such treatments reduce insomnia and facilitate tapering off sleep aids.
Limitations
Measures were limited to self-report and did not include other constructs likely to influence medication use, such as beliefs about sleep, attitudes toward medication use in general, availability of medications, which medication(s) patients used, and dosage instructions, frequency, and duration of use for prescription medications. In addition, psychiatric diagnoses were self-reported rather than based on symptom scales or structured interviews. As a cross-sectional study, our findings are correlational and cannot address causality.
CONCLUSIONS
The US public health burden of insomnia and associated sleep aid use is substantial and appears to be worsening. The identification of specific, modifiable behaviors that confer risk for insomnia has potential to reduce this burden. Results from this study demonstrated TMB may be such a factor, because TMB and insomnia symptom severity were reciprocally associated with sleep medication use. Mediation models provided best support for a pathway by which TMB and the associated frustration may contribute to insomnia symptom severity. Finally, data from this large-scale sleep clinic sample provide solid proof-of-concept for future longitudinal studies to assess the role of TMB in the prevention or treatment of insomnia as well as its possible impact on decreasing sleep medication use.
Submitted: June 22, 2022; accepted November 7, 2022.
Published online: May 16, 2023.
Relevant financial relationships: Dr Krakow owns and operates several websites that market books and videos for the treatment of sleep disorders, including insomnia. Dr Rojo-Wissar was supported by the National Institute of Mental Health’s Psychiatric Epidemiology Training Program (5T32MH014592-39) and subsequently by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Training Program in Childhood Stress, Trauma, and Resilience (T32HD101392). Drs Dawson, Haynes, and McIver and Mr Ulibarri report no relevant financial relationships.
Funding/support: None.
Previous presentation: An earlier version of this work was presented in at the Association of Processional Sleep Societies meeting; June 4, 2014; Minneapolis, Minnesota.
Supplementary material: See accompanying pages.
Clinical Points
- Time monitoring behavior (TMB) is linked to insomnia severity.
- Because of its influence on insomnia severity, TMB, and especially the frustration it causes, may amplify demand for sleeping pills.
- TMB may negatively influence efforts to discontinue sleeping pills.
- Simple behavioral steps (remove bedroom timepieces) or targeted psychotherapy (address frustration response) may potentially alleviate adverse TMB effects on insomnia symptoms and on sedative/hypnotic use.
References (57)

- Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. PubMed CrossRef
- Grandner MA, Jackson NJ, Pak VM, et al. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21(4):427–433. PubMed CrossRef
- Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–935. PubMed CrossRef
- Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. PubMed CrossRef
- Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep (Basel). 2014;37(8):1295–1304. PubMed CrossRef
- Laugsand LE, Vatten LJ, Platou C, et al. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. PubMed CrossRef
- Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–75.e2. PubMed CrossRef
- Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. PubMed CrossRef
- Buysse DJ, Rush AJ, Reynolds CF 3rd. Clinical management of insomnia disorder. JAMA. 2017;318(20):1973–1974. PubMed CrossRef
- Kathol RG, Arnedt JT. Cognitive behavioral therapy for chronic insomnia: confronting the challenges to implementation. Ann Intern Med. 2016;165(2):149–150. PubMed CrossRef
- Thomas A, Grandner M, Nowakowski S, et al. Where are the behavioral sleep medicine providers and where are they needed? a geographic assessment. Behav Sleep Med. 2016;14(6):687–698. PubMed CrossRef
- Basu R, Dodge H, Stoehr GP, et al. Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry. 2003;11(2):205–213. PubMed CrossRef
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergic medications and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. PubMed CrossRef
- Wu C-S, Wang S-C, Chang I-S, et al. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry. 2009;17(7):614–620. PubMed CrossRef
- Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349(sep09 2):g5205. PubMed CrossRef
- Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. PubMed CrossRef
- Chung K-H, Li C-Y, Kuo S-Y, et al. Risk of psychiatric disorders in patients with chronic insomnia and sedative-hypnotic prescription: a nationwide population-based follow-up study. J Clin Sleep Med. 2015;11(5):543–551. PubMed CrossRef
- Kripke DF. Chronic hypnotic use: deadly risks, doubtful benefit: review article. Sleep Med Rev. 2000;4(1):5–20. PubMed CrossRef
- Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. PubMed CrossRef
- Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(suppl 2):9–13. PubMed
- Kripke DF, Klauber MR, Wingard DL, et al. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43(9):687–693. PubMed CrossRef
- Richardson GS, Roehrs TA, Rosenthal L, et al. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22(5):511–515. PubMed CrossRef
- Roth T, Walsh JK, Krystal A, et al. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6(6):487–495. PubMed CrossRef
- Pillai V, Roth T, Roehrs T, et al. Effectiveness of benzodiazepine receptor agonists in the treatment of insomnia: an examination of response and remission rates. Sleep (Basel). 2017;40(2):zsw044. PubMed CrossRef
- Krakow B, Krakow J, Ulibarri VA, et al. Nocturnal time monitoring behavior (“clock-watching”) in patients presenting to a sleep medical center with insomnia and posttraumatic stress symptoms. J Nerv Ment Dis. 2012;200(9):821–825. PubMed CrossRef
- Krakow B. Sound Sleep, Sound Mind: 7 Keys to Sleeping through the Night. 1st ed. Wiley; 2007.
- Woods H, Marchetti LM, Biello SM, et al. The clock as a focus of selective attention in those with primary insomnia: an experimental study using a modified Posner paradigm. Behav Res Ther. 2009;47(3):231–236. PubMed CrossRef
- Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–553. PubMed CrossRef
- Espie CA, Broomfield NM, MacMahon KMA, et al. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10(4):215–245. PubMed CrossRef
- Perlis ML, Giles DE, Mendelson WB, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–188. PubMed CrossRef
- Tang NKY, Anne Schmidt D, Harvey AG. Sleeping with the enemy: clock monitoring in the maintenance of insomnia. J Behav Ther Exp Psychiatry. 2007;38(1):40–55. PubMed CrossRef
- Winkler A, Rief W. Effect of placebo conditions on polysomnographic parameters in primary insomnia: a meta-analysis. Sleep (Basel). 2015;38(6):925–931. PubMed CrossRef
- Khader W, Culnan E, Morales K, et al. A meta-analysis of placebo effects across hypnotic RCTs: A first pass analysis. Sleep (Basel). 2017;40(suppl 1):A127. CrossRef
- Hajak G, Clarenbach P, Fischer W, et al. Rebound insomnia after hypnotic withdrawal in insomniac outpatients. Eur Arch Psychiatry Clin Neurosci. 1998;248(3):148–156. PubMed CrossRef
- Roehrs T, Vogel G, Roth T. Rebound insomnia: its determinants and significance. Am J Med. 1990;88(suppl 1):39S–42S. PubMed CrossRef
- Vochem J, Strobel C, Maier L, et al. Pre-Sleep Arousal Scale (PSAS) and the Time Monitoring Behavior-10 scale (TMB-10) in good sleepers and patients with insomnia. Sleep Med. 2019;56:98–103. PubMed CrossRef
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. PubMed CrossRef
- Roth T, Jaeger S, Jin R, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60(12):1364–1371. PubMed CrossRef
- Carney CE, Segal ZV, Edinger JD, et al. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68(2):254–260. PubMed CrossRef
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7(6):523–539. PubMed CrossRef
- Perlis ML, Grandner MA, Brown GK, et al. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):e726–e733. PubMed CrossRef
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. PubMed CrossRef
- Dawson SC, Krakow B, McIver ND, et al. Time monitoring behavior: factor analysis and relationship to sleep medication use. Sleep (Basel). 2014;37(S1):A178.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
- American Psychiatric Association. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association; 2013.
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. PubMed CrossRef
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58(1):593–614. PubMed CrossRef
- Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. PubMed CrossRef
- Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. PubMed CrossRef
- Morin CM, Bastien C, Guay B, et al. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332–342. PubMed CrossRef
- Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Therapist Guide. 2nd ed. Oxford University Press; 2006.
- Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. PubMed CrossRef
- Kaplan KA, Harvey AG. Behavioral treatment of insomnia in bipolar disorder. Am J Psychiatry. 2013;170(7):716–720. PubMed CrossRef
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225–233. PubMed
- Chen P-L, Lee W-J, Sun W-Z, et al. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLoS One. 2012;7(11):e49113. PubMed CrossRef
- Roehrs T, Roth T. ‘Hypnotic’ prescription patterns in a large managed-care population. Sleep Med. 2004;5(5):463–466. PubMed CrossRef
- Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep (Basel). 2014;37(2):343–349. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite