Prim Care Companion CNS Disord 2021;23(6):20cr02897
To cite: Lhamu T, Sadh K, Dahale AB, et al. Valproate-induced pancytopenia and phenytoin toxicity in a young adult with intellectual disability and mesial temporal lobe sclerosis. Prim Care Companion CNS Disord. 2021;23(6):20cr02897.
To share: https://doi.org/10.4088/PCC.20cr02897
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India
*Corresponding author: Kamaldeep Sadh, MD, Department of Psychiatry, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, 560029, India ([email protected]).
The prevalence of epilepsy in patients with intellectual developmental disorder is higher than in the general population and is known to increase with the severity.1–3 Evaluation and management of such patients pose multiple challenges. One of the major challenges is to monitor the side effect profile of the antiepileptic drugs and prevent toxicity. There are few case reports from India reporting toxicities of antiepileptic drugs, especially valproate or phenytoin.4–6 Here, we present a case of a young adult with intellectual disability and seizures, who developed toxicity to both these antiepileptic drugs prescribed for seizure control.
Case Report
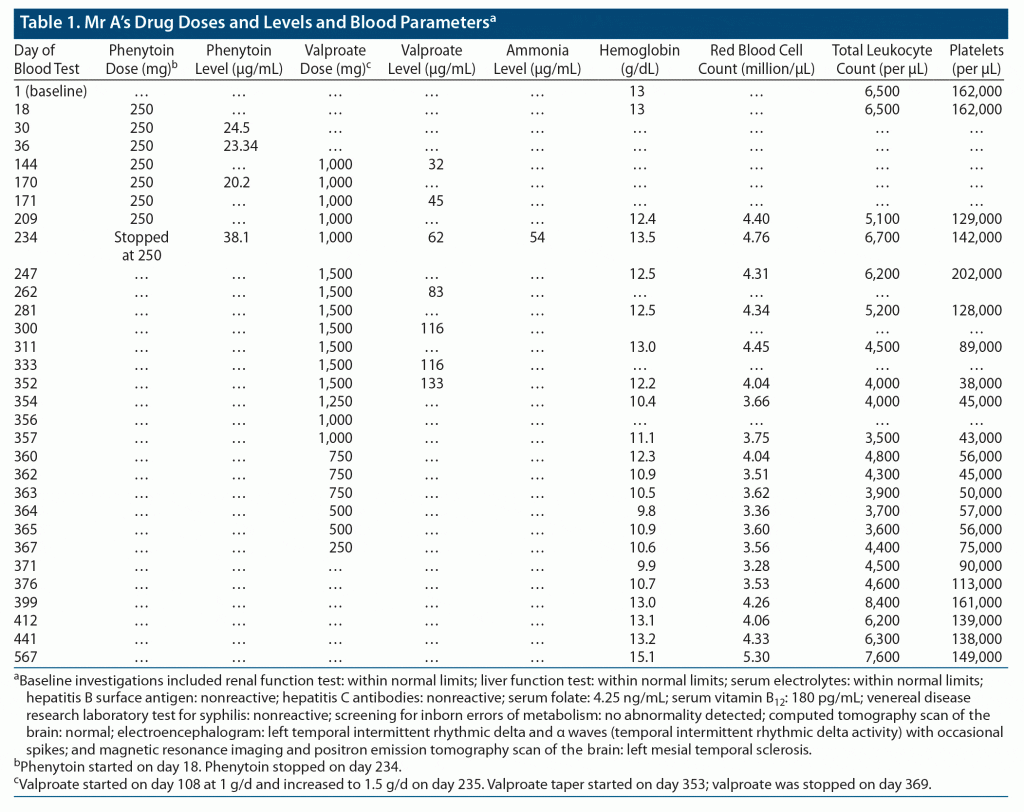
Mr A, a 20-year-old male patient, was admitted to the long-term care ward in our department after having been found abandoned on the roadside with unusual behavior. On examination, he was oriented and had minor physical anomalies (facial dysmorphism, low-set ears, and high-arched palate). He could speak a few words of his native language and obey simple commands. The systemic examination was normal. The mental status examination revealed no features of psychosis or mood disorder. Mr A was able to carry out only basic activities of daily living. After an IQ assessment, he was diagnosed with severe intellectual developmental disorder. Soon after admission, the patient had an episode of focal seizure. Neuroimaging was suggestive of left mesial temporal sclerosis (results of his computed tomography, electroencephalography, magnetic resonance imaging, and positron emission tomography scans are provided in Table 1). He was started on phenytoin up to 250 mg after a neurology consultation. Antipsychotics were given on an as-needed basis for agitation. Vitamin B12 and folate supplements were also added. Due to inadequate control of seizures, valproate 1 g/d was added. Mr A had no further seizure episodes. After 3 months of taking these antiepileptic drugs, he suddenly developed ataxia and multiple falls and was diagnosed with phenytoin toxicity (serum phenytoin levels = 38.1 mg/mL at 250 mg/d). A neurology consultation was conducted, and phenytoin was stopped. Valproate was increased to 1.5 g/d, and clobazam 15 mg/d was added (Table 1).
Complete blood counts at 6, 10, and 15 weeks after increasing valproate showed decreasing platelet counts from 1.28 L/mL to 38,000/mL along with a white blood cell count of 4,000/mL and hemoglobin of 12.2 g/dL. Mr A had bilateral fine tremors but no clinical signs of valproate toxicity or bleeding manifestations (serum valproate of 133 mg/mL, ammonia of 52 mg/mL). There was no history of fever, joint pain, rashes, or gastrointestinal symptoms. Tests for chikungunya IgM and dengue IgM were negative. After detection of reduction in cell counts, an immediate neurology referral was made, and valproate was planned to be tapered and stopped (Table 1).
Mr A was thereafter maintained on levetiracetam 1,000 mg/d and clobazam 20 mg/d and subsequently did not have seizures. Blood investigations were monitored regularly (Table 1). From the 11th day after stopping valproate, his platelet count started to noticeably increase. His platelet count, hemoglobin, and total white blood cell counts stably normalized from the 28th day, 47th day, and 15th day onward, respectively. At 3 months, a full investigation panel including complete blood count; renal, liver, and thyroid function tests; serum electrolytes; fasting glucose level; and lipid profile was noted to be within normal limits.
Discussion
The thrombocytopenia induced by valproate has been reported; however, other hematologic toxicities like macrocytosis, neutropenia, and pure red cell aplasia have rarely been reported.4–7 Although the prevalence of thrombocytopenia ranged from 12% to 18% in some studies,3,6–10 there is currently a lack of research regarding its clinical implications. The thrombocytopenia induced by valproate is assumed to be a dose-dependent side effect; however, the exact mechanism of this adverse effect is unclear as is its timeline of appearance. Adjustment of dosage has been shown to partially reverse the thrombocytopenia.8 Shifting to other antiepileptics like phenytoin helps when cost is a major limiting factor for prescribing the newer antiepileptic drugs. Phenytoin can rarely cause hematologic side effects.9
Notably, our patient developed neurotoxicity on phenytoin 250 mg after 2 months of initiation of treatment and later developed hematologic toxicity on valproate. Hematologic parameters started worsening a few weeks after increasing the dose of valproate and started improving within 11 days of stopping the drug.
Conclusion
There is a need for vigilant and regular monitoring for toxicity of antiepileptic drugs like valproate and phenytoin, especially in individuals with intellectual developmental disorder. Close monitoring is required even when the patient is stable. Monitoring is needed more frequently in the initial year, probably monthly.10 Exploring newer antiepileptic drugs for efficacy, affordability, and a safer side effect profile would be beneficial.
Published online: December 23, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: As the patient was diagnosed to have intellectual disability and the family members could not be traced despite multiple efforts, consent was obtained from 2 independent psychiatrists not related to the treating team. All information has been de-identified to protect anonymity.
References (9)

- Bowley C, Kerr M. Epilepsy and intellectual disability. J Intellect Disabil Res. 2000;44(Pt 5):529–543. PubMed CrossRef
- Robertson J, Hatton C, Emerson E, et al. Prevalence of epilepsy among people with intellectual disabilities: a systematic review. Seizure. 2015;29:46–62. PubMed CrossRef
- Yoshimura Y, Hara K, Akaza M, et al. Effects of antiepileptic monotherapy on hematological and biochemical parameters. Epilepsy & Seizure. 2019;11(1):1–13. CrossRef
- Goyal SK, Badyal DK. Significant thrombocytopenia with sodium valproate in an adult patient with alcohol dependence. Indian J Psychiatry. 2018;60(2):252–253. PubMed CrossRef
- Mittal K, Mehta P, Aggarwal HK, et al. Valproate induced thrombocytopenia and renal dysfunction: case report. Eur J Biomed Pharm Sci. 2017;4(1):449–450.
- Abdulla MC. Multiple adverse events of valproate in a patient with bipolar affective disorder. Ann Indian Psychiatry. 2020;4(1):102–103.
- Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62–65. PubMed CrossRef
- Buoli M, Serati M, Botturi A, et al. The risk of thrombocytopenia during valproic acid therapy: a critical summary of available clinical data. Drugs R D. 2018;18(1):1–5. PubMed CrossRef
- Verrotti A, Scaparrotta A, Grosso S, et al. Anticonvulsant drugs and hematological disease. Neurol Sci. 2014;35(7):983–993. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite