Disulfiram is 1 of 3 drugs approved by the US Food and Drug Administration for the treatment of alcohol dependence. It is efficient for the supervised treatment of alcohol dependence and is recommended for those who are motivated to discontinue alcohol use. Disulfiram irreversibly inhibits aldehyde dehydrogenase, which is an enzyme of the major oxidative pathway of alcohol metabolism that converts acetaldehyde to acetate. This increase in serum acetaldehyde during the first 12 hours of administration results in diaphoresis, palpitations, facial flushing, nausea, vertigo, hypotension, and tachycardia, which is known as disulfiram ethanol reaction.1
Insistently, patients are advised to abstain from any alcoholic beverages and the use of any alcohol-containing products during treatment with disulfiram.2 Recently, disulfiram ethanol reactions with alcohol-based hand sanitizers have been reported.3
In this report, we discuss the case of a patient on disulfiram treatment who developed erythematous plaques and papules after consumption of noodles containing vinegar.
Case Report
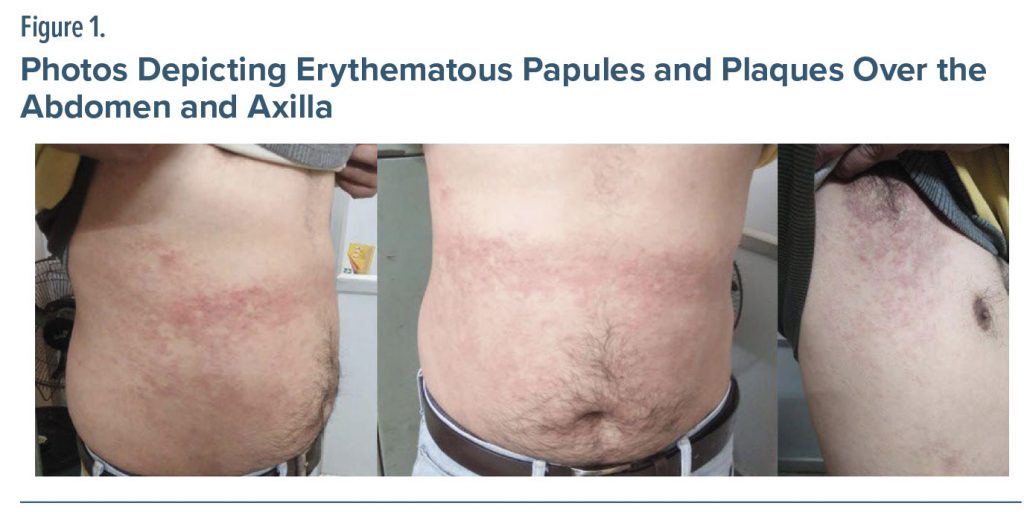
A 42-year-old married man known to have alcohol dependence syndrome was admitted to the hospital for detoxification for 10 days. Subsequently, after the detoxification phase he was started on the antiaversive agent disulfiram 250 mg once/day after providing written consent. The evening after discharge, he consumed noodles. The next morning, he noticed multiple rashes over his abdomen and axilla (Figure 1). He immediately went to the hospital, and on examination, several erythematous papules and plaques were observed all over the abdomen and axilla. He was kept in the hospital due to a suspected diagnosis of drug-related intertriginous and flexural exanthema in relation to disulfiram. The tablet disulfiram was stopped, and he was managed conservatively. Consequently, a complete resolution of the plaques and papules was seen after 7 days with slight hyperpigmentation. His Naranjo score was 6.4
Discussion
Unlike other anticraving drugs, disulfiram does not modulate neurobiological mechanisms of addiction but rather acts by producing an aversive reaction when combined with alcohol.2 In addition to its interaction with alcohol, disulfiram also has its own side effects but with an overall relatively good safety profile. However, several studies2 report various dermatologic, neurologic, psychiatric, and cardiac events.
Except for papular acne, other disulfiram-induced dermatologic side effects have been rarely reported. In our case, the patient developed intertriginous and flexural erythematous papules and plaques overnight after intake of noodles containing vinegar. He had taken only 2 doses of disulfiram with a cumulative dose of 500 mg before the development of skin rash. Skin eruptions resolved within a span of 7 days after discontinuation of disulfiram, albeit with some residual hyperpigmentation. Radaschin and colleagues5 reported a similar dermatologic reaction, a type IV hypersensitivity reaction, after taking disulfiram 500 mg daily for 2 weeks. Severe skin reactions like Stevens-Johnson syndrome and toxic epidermal necrolysis have also been described in a similar context.6
Vinegar is produced by 2 biotechnical processes: alcoholic fermentation and acetous fermentation. Vinegar contains varied concentrations of acetic acid and alcohol regulated by the respective food safety and standard authorities.7 Although the alcohol content in the vinegar in our case was minute, it still can be implicated in the patient’s skin reaction.
Conclusion
In conclusion, we present a curious case of disulfiram-induced short-lasting nonmalignant skin reaction with a single exposure to vinegar. To our knowledge, this is an isolated report linking vinegar consumption and disulfiram. Clinicians should be aware of this potential association, which can be included in psychoeducation modules.
Article Information
Published Online: November 30, 2023. https://doi.org/10.4088/PCC.23cr03537
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(6):23cr03537
Submitted: April 2, 2023; accepted July 18, 2023.
To Cite: Pandey E, Garg S. Vinegar-induced disulfiram reaction. Prim Care Companion CNS Disord. 2023;25(6):23cr03537.
Author Affiliations: Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Uttarakhand, India (both authors).
Corresponding Author: Shobit Garg, MD, DPM, Shri Guru Ram Rai Institute of Medical and Health Sciences, 248001, Uttarakhand, India 8958534261 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Informed verbal consent was received from the patient for publication of this case report and photos. Information has been de-identified to protect anonymity.
ORCID: Eshani Pandey: https://orcid.org/0009-0003-9881-9056; Shobit Garg: https://orcid.org/0000-0001-5913-9021
References (7)

- Stokes M, Abdijadid S. Disulfiram. In: StatPearls. Treasure Island, FL: StatPearls Publishing; October 24, 2022.
- Lanz J, Biniaz-Harris N, Kuvaldina M, et al. Disulfiram: mechanisms, applications, and challenges. Antibiotics (Basel). 2023;12(3):524. PubMed CrossRef
- Ghosh A, Mahintamani T, Balhara YPS, et al. Disulfiram ethanol reaction with alcohol-based hand sanitizer: an exploratory study. Alcohol Alcohol. 2021;56(1):42–46. PubMed CrossRef
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Radaschin DS, Bujoreanu FC, Tatu AL. Disulfiram-induced baboon syndrome. Am J Ther. 2020;29(2):e272–e273. PubMed CrossRef
- Mutschler J, Grosshans M, Soyka M, et al. Current findings and mechanisms of action of disulfiram in the treatment of alcohol dependence. Pharmacopsychiatry. 2016;49(4):137–141. PubMed CrossRef
- Ho CW, Lazim AM, Fazry S, et al. Varieties, production, composition and health benefits of vinegars: a review. Food Chem. 2017;221:1621–1630. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite