Objective: To collate and analyze randomized controlled trials (RCTs) that evaluated pharmacologic interventions to reduce weight gain in patients with severe mental illness (SMI).
Data Sources: Searches were conducted in PubMed, Web of Science, and PsycINFO databases from inception through May 9, 2019, using the terms ("severe mental disease" OR "severe mental illness" OR "severe mental disorder" OR schizophre* OR bipolar OR antipsychotic*) AND (weight) AND (pharmacologic* OR treatment). There was no language restriction, and the electronic search was complemented by a manual search for additional articles in reference lists and previous reviews.
Study Selection: Fifty-two studies investigating different pharmacologic weight loss interventions in SMI were retrieved. Only RCTs assessing pharmacologic interventions to manage weight gain in adult subjects with SMI and reporting change in body weight as a primary outcome were included.
Data Extraction: Two reviewers independently extracted data about the name and dose of the pharmacologic agent used to manage weight gain, trial duration, agent used for index disease, psychiatric diagnostics, and the mean change in body weight over the course of the trial. A meta-analysis was performed using a random effects model to pool mean body weight change over the course of the trial.
Results: The most-studied agent was metformin (14 studies), followed by topiramate (6 studies), nizatidine (4 studies), and sibutramine (3 studies). Other agents were investigated in 1 or 2 isolated studies. A meta-analytical procedure showed a significant pooled mean difference of −3.27 kg (95% CI, −4.49 to −2.06) for metformin compared with placebo and −5.33 kg (95% CI, −7.20 to −3.46) favoring topiramate.
Conclusions: Metformin and topiramate were the most-studied agents for weight control in SMI and were considered efficacious and safe in promoting weight reduction compared to placebo in this population. More studies are required with larger sample sizes and in line with the recommendations from research from the obesity and metabolic field to better define guidelines for use of pharmacologic interventions to reduce weight gain in patients with SMI.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Effectiveness of Pharmacologic Interventions in the Management of Weight Gain in Patients With Severe Mental Illness:
A Systematic Review and Meta-Analysis
ABSTRACT
Objective: To collate and analyze randomized controlled trials (RCTs) that evaluated pharmacologic interventions to reduce weight gain in patients with severe mental illness (SMI).
Data Sources: Searches were conducted in PubMed, Web of Science, and PsycINFO databases from inception through May 9, 2019, using the terms ("severe mental disease" OR "severe mental illness" OR "severe mental disorder" OR schizophre* OR bipolar OR antipsychotic*) AND (weight) AND (pharmacologic* OR treatment). There was no language restriction, and the electronic search was complemented by a manual search for additional articles in reference lists and previous reviews.
Study Selection: Fifty-two studies investigating different pharmacologic weight loss interventions in SMI were retrieved. Only RCTs assessing pharmacologic interventions to manage weight gain in adult subjects with SMI and reporting change in body weight as a primary outcome were included.
Data Extraction: Two reviewers independently extracted data about the name and dose of the pharmacologic agent used to manage weight gain, trial duration, agent used for index disease, psychiatric diagnostics, and the mean change in body weight over the course of the trial. A meta-analysis was performed using a random effects model to pool mean body weight change over the course of the trial.
Results: The most-studied agent was metformin (14 studies), followed by topiramate (6 studies), nizatidine (4 studies), and sibutramine (3 studies). Other agents were investigated in 1 or 2 isolated studies. A meta-analytical procedure showed a significant pooled mean difference of −3.27 kg (95% CI, −4.49 to −2.06) for metformin compared with placebo and −5.33 kg (95% CI, −7.20 to −3.46) favoring topiramate.
Conclusions: Metformin and topiramate were the most-studied agents for weight control in SMI and were considered efficacious and safe in promoting weight reduction compared to placebo in this population. More studies are required with larger sample sizes and in line with the recommendations from research from the obesity and metabolic field to better define guidelines for use of pharmacologic interventions to reduce weight gain in patients with SMI.
Prim Care Companion CNS Disord 2019;21(6):19r02483
To cite: Hiluy JC, Nazar BP, Gonçalves WS, et al. Effectiveness of pharmacologic interventions in the management of weight gain in patients with severe mental illness: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2019;21(6):19r02483.
To share: https://doi.org/10.4088/PCC.19r02483
© Copyright 2019 Physicians Postgraduate Press, Inc.
aObesity and Eating Disorders Group—Institute of Psychiatry, Federal University of Rio de Janeiro and State Institute of Diabetes and Endocrinology of Rio de Janeiro, Rio de Janeiro, Brazil
*Corresponding author: Joao C. Hiluy, MD, Instituto de Psiquiatria da UFRJ, GOTA—Grupo de Obesidade e Transtornos Alimentares, Av Venceslau Bras 71, Fundos, Botafogo, Rio de Janeiro, Brazil 22290-160 ([email protected]).
Research demonstrates that the prevalence of overweight and obesity is increasing rapidly worldwide.1 Patients with severe mental illness (SMI), defined as a group of serious mental conditions characterized by length of duration and the disability produced (in this study, we used this term to describe a subgroup of patients with schizophrenia, schizoaffective disorder, and bipolar disorder), tend to present a higher prevalence of metabolic disorders such as overweight, obesity, and metabolic syndrome compared to the general population.2-4 Evidence shows that ethnic differences may play an important role in this weight gain.5 In a systematic review, Janssen et al6 calculated the mean pooled prevalence of overweight and obesity in SMI populations to be 40.4% for obesity, whereas the estimates for clinical measurement in the US general population is 35.7%.7 Further, it is important to highlight that cardiovascular diseases represent the leading cause of death in patients with SMI,8,9 and obesity and overweight are important risk factors for this outcome.
Weight gain effects of pharmacologic agents used to treat SMI have been documented,4 mainly among atypical antipsychotics, mood stabilizers, and antidepressants. However, even after controlling for medication effects, a complex interplay between obesity and SMI emerges when considering factors such as lifestyle (social isolation, improper diet, sedentary lifestyle) and neurobiological mediators, which are involved in both mental disorders and food intake.10-12
Although weight gain is a frequent issue for patients with SMI, it is usually neglected in their treatment plan.13 Several nonpharmacologic interventions have demonstrated positive results in patients with SMI based on diet/nutritional counseling, exercise, cognitive-behavioral therapy, and psychoeducation.14 Pharmacologic agents for the treatment of weight gain in SMI have also been studied, as patients with SMI may have difficulty implementing nonpharmacologic interventions and combining both may potentially offer additive benefits to the weight loss treatment.15
A series of systematic reviews16-18 previously summarized information regarding the use of pharmacologic agents to counteract weight disturbances observed in patients with SMI. Mizuno and colleagues18 conducted the last systematic review (in 2014) of pharmacologic agents for the management of weight gain in patients with schizophrenia. This meta-analysis18 included 40 trials with 19 different interventions. The results showed that metformin was the most extensively studied drug regarding body weight, with a mean difference of −3.17 kg (95% CI, −4.44 to −1.90 kg) compared to placebo. Additional analysis demonstrated that topiramate, sibutramine, aripiprazole, and reboxetine were also different compared to placebo. In addition, metformin and rosiglitazone improved insulin resistance, and metformin and sibutramine decreased blood lipid levels. Since the publication of this review,18 several randomized controlled trials (RCTs) on this topic have been published. To update the current evidence, we conducted a systematic review and meta-analysis on the effectiveness of add-on medications to treat weight gain in the broad spectrum of patients with SMI, focusing on the clinical relevance of these findings.
METHODS
Literature Search
We adhered to the PRISMA reporting guidelines19 and conducted a systematic literature search in PubMed, Web of Science, and PsycINFO from database inception through May 9, 2019. Two authors (J.C.H. and W.S.G.) independently used the following search terms: ("severe mental disease" OR "severe mental illness" OR "severe mental disorder" OR schizophre* OR bipolar OR antipsychotic*) AND (weight) AND (pharmacologic* OR treatment). A search limit was set for "clinical trials," and there was no language restriction. The electronic search was complemented by a manual search for additional articles in reference lists and previous reviews.
Inclusion and Exclusion Criteria

- Weight gain in patients with severe mental illness is an important and frequent clinical condition that is usually neglected during treatment.
- Pharmacologic interventions for weight gain in patients with severe mental illness are an important part of their overall treatment plan.
- Metformin currently has a robust body of evidence showing effectiveness in the management of weight gain in severe mental illness, followed by topiramate.
The criteria for inclusion of studies in this systematic review were RCTs assessing pharmacologic interventions to manage weight gain in patients with SMI (schizophrenia, bipolar I disorder, schizoaffective or schizophreniform disorder) and reporting change in body weight as a primary outcome measure. We excluded (1) noncontrolled studies, (2) studies with children, and (3) reviews (although they were used as references) and trials in which body weight change was not a primary endpoint.
Data Collection and Extraction
Two reviewers (J.C.H. and W.S.G.) independently screened and selected the studies on the basis of title and abstract. After consensus on the primary selection, both authors independently reviewed the full text of the selected studies to determine suitability for inclusion based on established selection criteria. Disagreements between the 2 reviewers were resolved by discussion with each other and the senior author (J.C.A.) until consensus was reached.
One researcher (J.C.H.) extracted data from each study using an extracting data form designed for the purpose of this review. Extracted data included sociodemographic and clinical characteristics of trial participants; pharmacologic agents used for treatment of the index disease, as well as name and dose of the agent used to manage weight gain; trial duration; and the mean change in body weight over the course of the trial.
Quality Assessment
Methodological quality of the included studies was assessed by using a 10-item list based on the Delphi List For Quality Assessment of Randomized Clinical Trials (Delphi List).20 The Delphi List was developed as a standardized list to assess the quality of RCTs. From the initial set of 9 items, we added an extra question to check if the authors performed a sample size calculation. Thus, our assessment ranged from 0 (minimum) to 10 (maximum). Two reviewers (J.C.H. and J.C.A.) independently rated the methodological quality of the included studies. Discrepancies between raters were discussed until a consensus was reached.
Statistical Analysis
All analyses were performed using Review Manager Software (Revman), version 5.3 from the Cochrane Collaboration Group (http://ims.cochrane.org/revman). The primary outcome measure was defined as the mean change difference in body weight over the course of the trial. A meta-analysis was conducted with each particular agent if it was tested in at least 3 different placebo-controlled RCTs. When a specific study did not provide a standard deviation (SD) for primary outcome, it was excluded and sequentially included in a new analysis in which we imputed the missing SD using the Revman calculator.
Heterogeneity was assessed using I2 statistics, and publication bias was analyzed with a visual inspection of funnel plots. To control for study heterogeneity, a random effects meta-analysis was chosen, using inverse variance and reporting 95% confidence intervals (95% CIs) to express the mean differences across active drug and placebo groups between baseline and postintervention body weight. To compare between subgroup analyses, we used the 95% CIs and analyzed if they contained the null value. The primary outcome was further investigated in a subgroup analysis exploring the following: (1) long-term versus short-term duration trials and (2) high-quality versus low-quality trials based on the quality assessment. Finally, we analyzed if the inclusion of studies with imputed SD changed outcomes.
RESULTS
Search Results
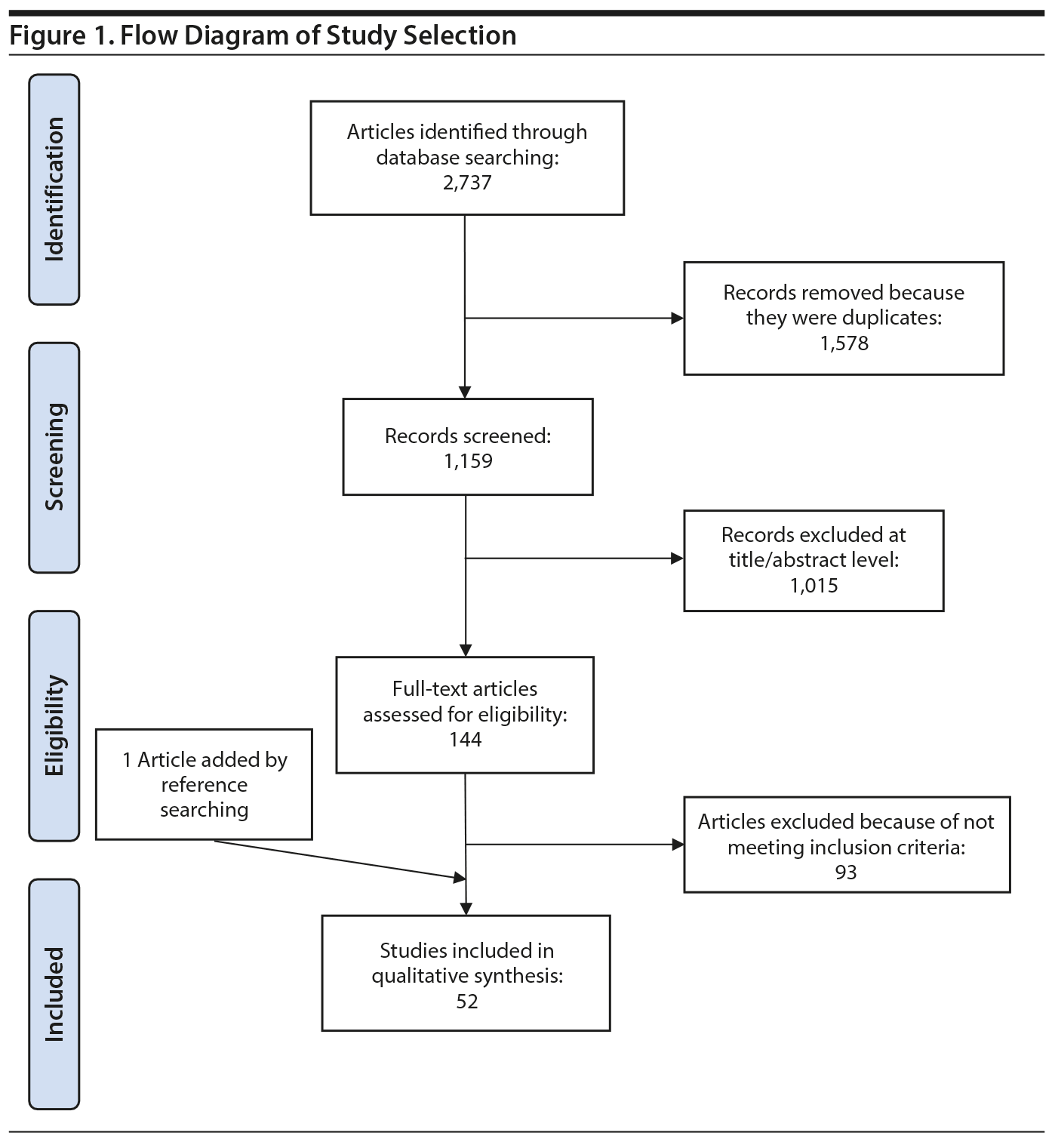
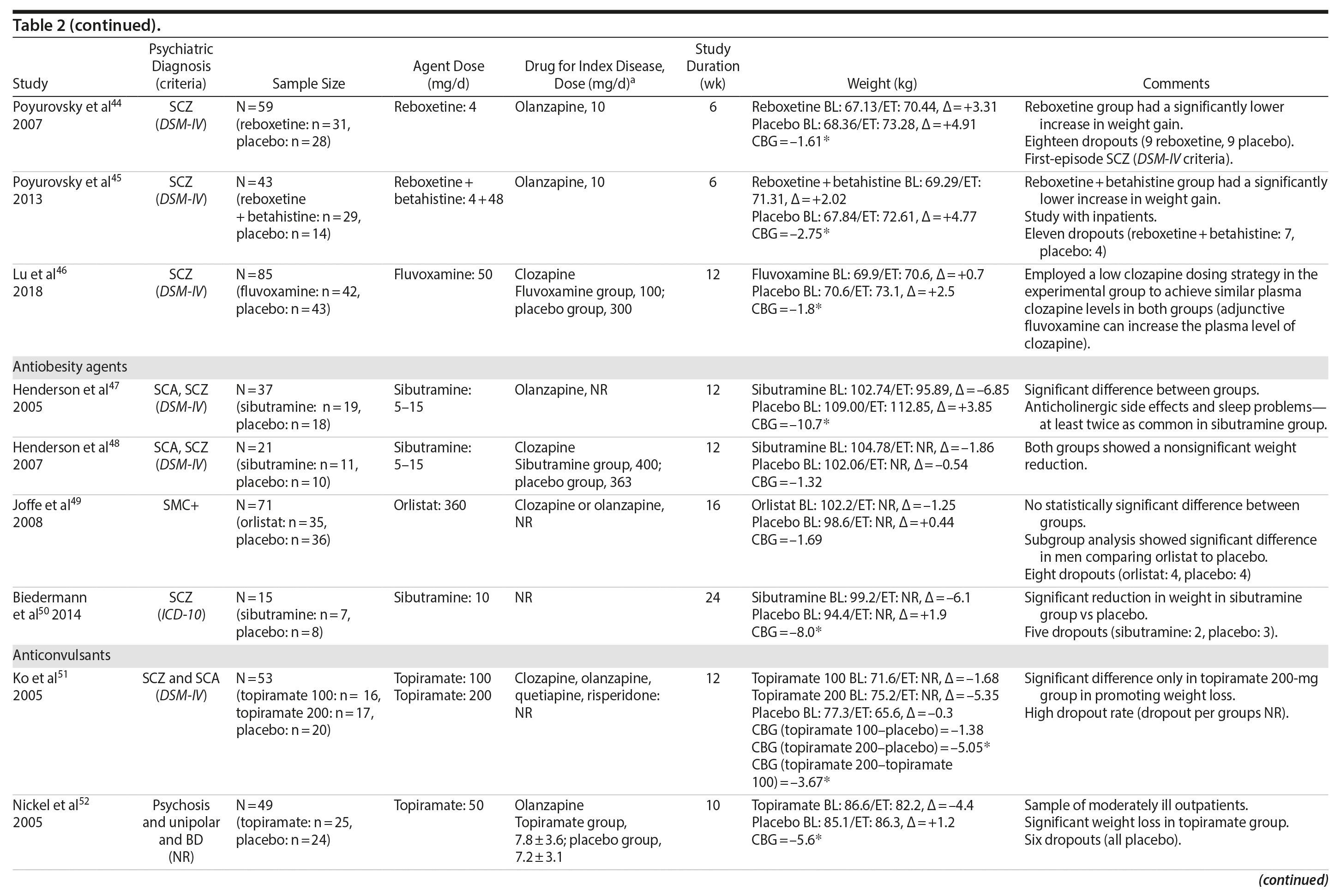
The literature search identified 2,737 references. After extracting the duplicates (1,578 references), 1,015 articles were excluded based on title and abstract. Of the 144 eligible articles, 93 were excluded for several reasons, and 1 study was added after reference searching. Thus, 52 studies15,21-70 (Figure 1) were eligible for the final qualitative synthesis with the following pharmacologic agents: amantadine, aripiprazole, atomoxetine, betahistine, exenatide, famotidine, fluoxetine, fluvoxamine, melatonin, metformin, naltrexone, nizatidine, orlistat, ramelteon, ranitidine, reboxetine, rosiglitazone, sibutramine, topiramate, and zonisamide. In these studies, the primary outcome was the mean difference of weight loss over the course of the study. Additional secondary variables were also assessed in these studies (ie, body mass index [BMI], fasting glucose, blood pressure, lipid profile). We describe the results according to pharmacologic classes of agents.
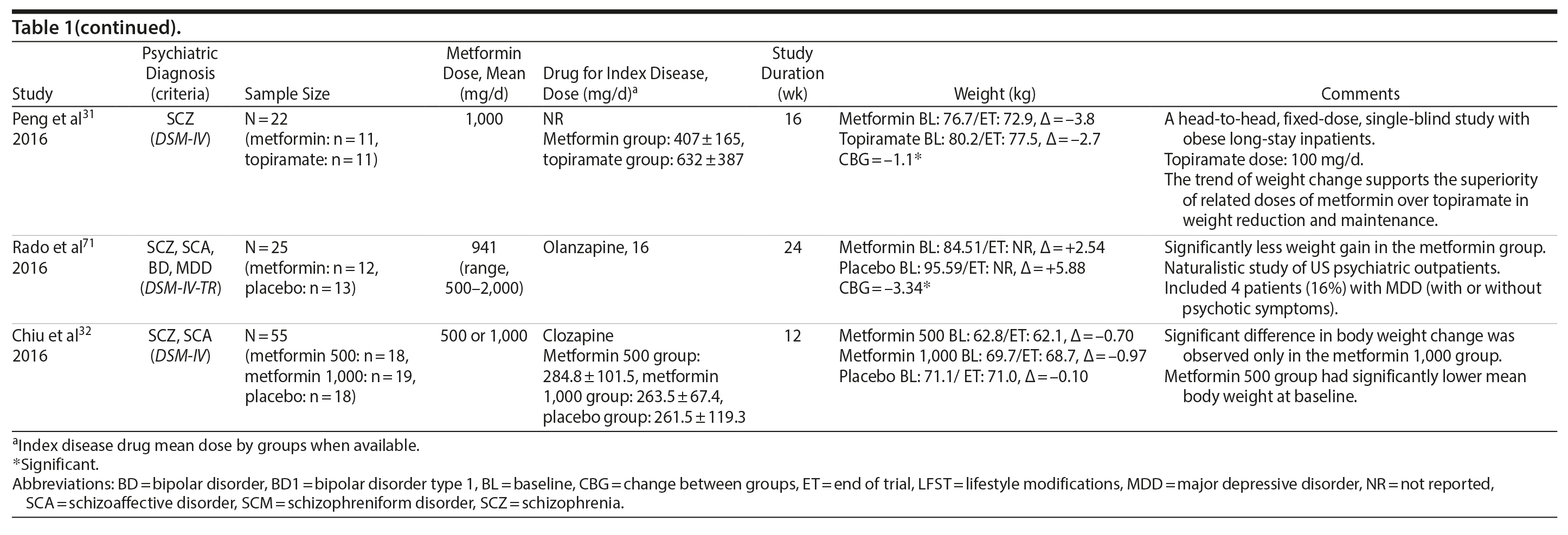
Metformin
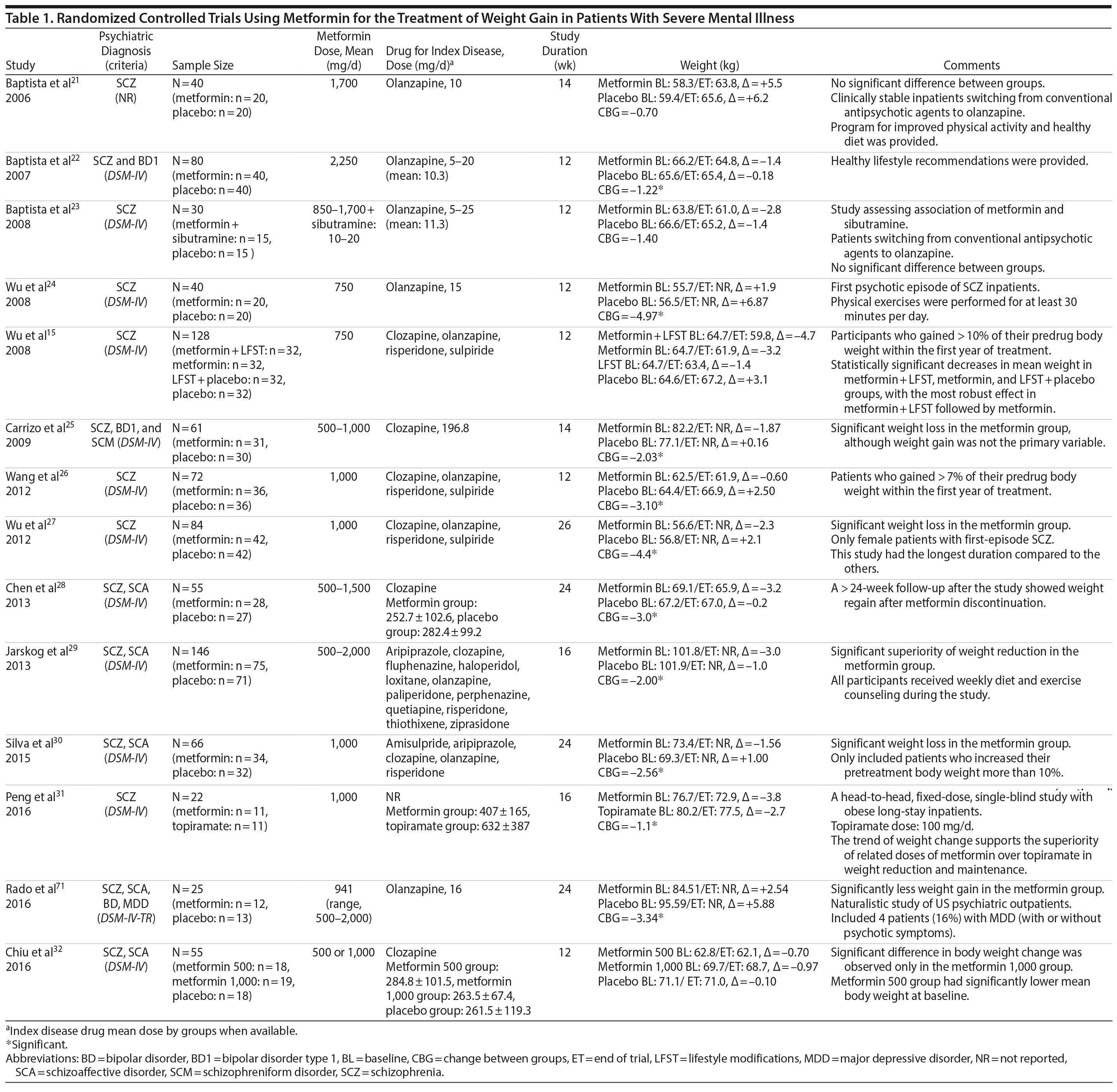
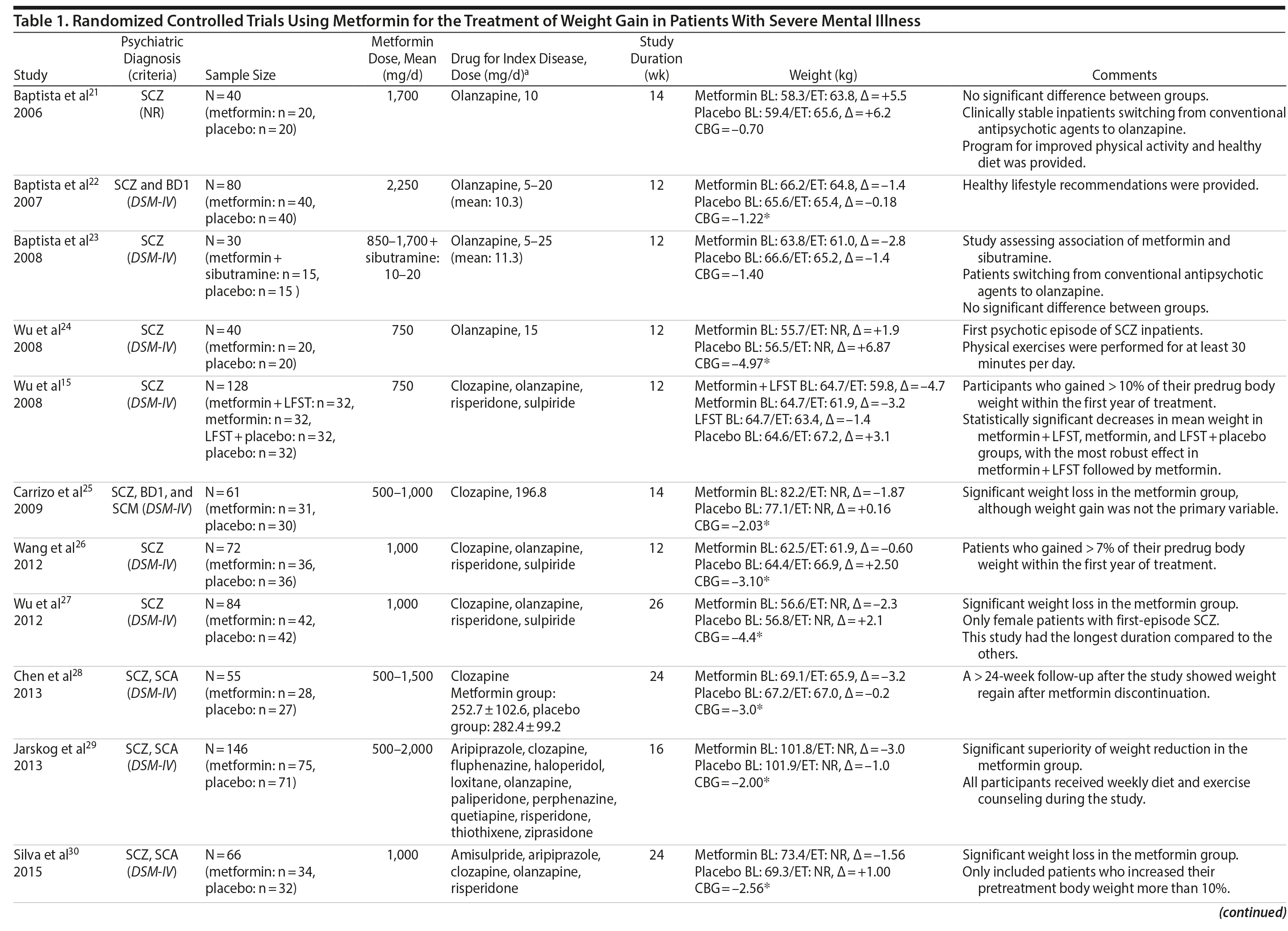
Metformin was the most-studied agent in the treatment of weight gain in patients with SMI and was tested in 14 RCTs15,21-32,71 (Table 1). Metformin is a biguanide commonly used as a first-line treatment for type 2 diabetes. It decreases hepatic glucose output and increases insulin-mediated glucose utilization in peripheral tissues. The rationale for using metformin to treat weight gain in patients with SMI is supported by evidence that metformin can reduce body weight in patients with type 2 diabetes and in obese individuals without diabetes.72,73 Metformin also does not seem to increase leptin, as seen in olanzapine-treated patients,74 and in a recent study it was found that metformin may alter the composition of intestinal microbiota.75 These findings show that other uncertain mechanisms may contribute to metformin’s effect on reducing body weight. In addition, since metformin lacks central nervous system action, the risk of mental illness worsening is potentially minimized.
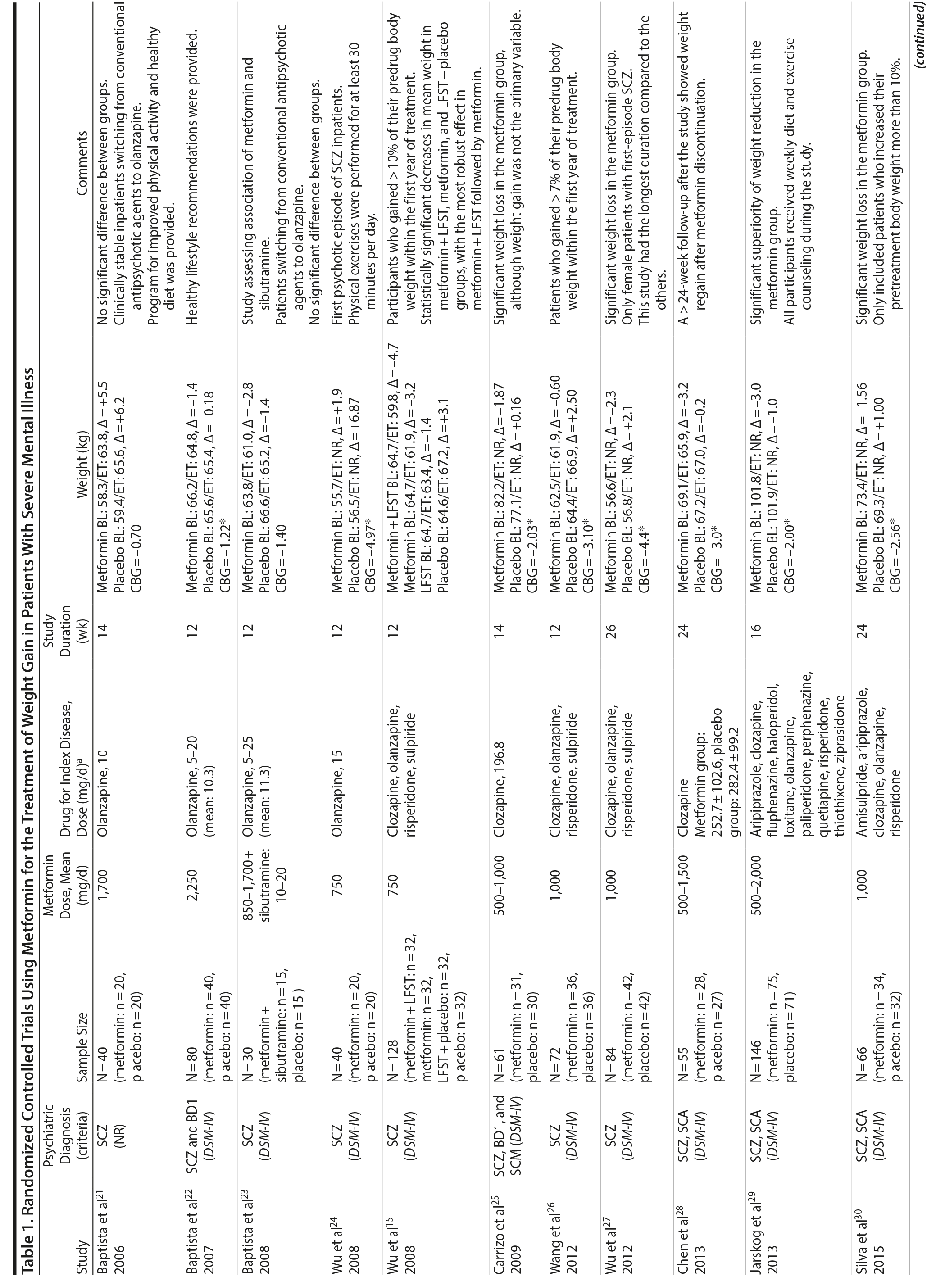
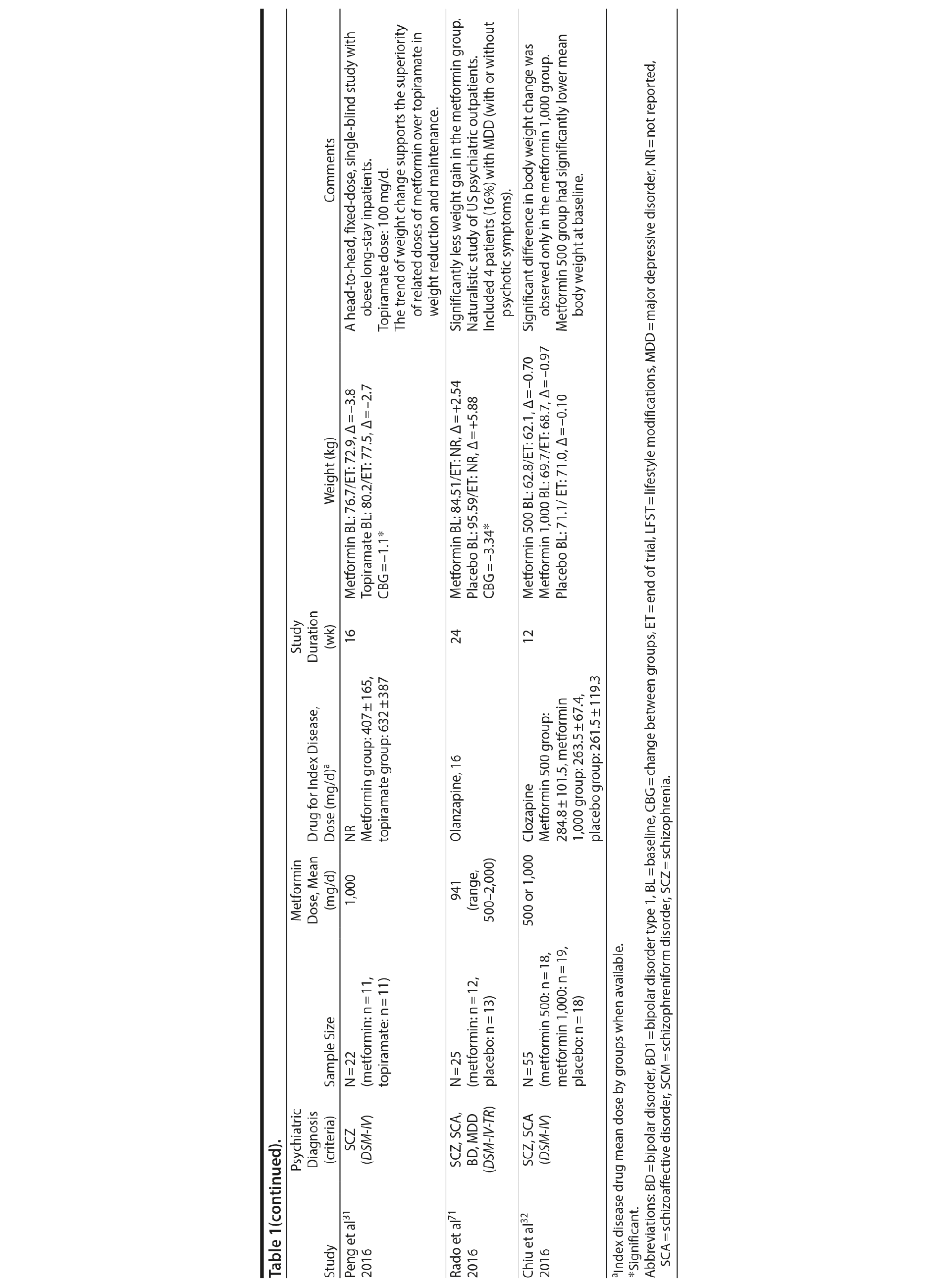
A growing body of evidence supports the effectiveness of metformin in the treatment of weight disorders in SMI. After first conducting a negative study using metformin to manage weight gain in schizophrenia, Baptista et al21 performed another trial 1 year later in a larger sample22 and found a significant weight loss induced by metformin versus placebo. Overall, from the 14 metformin studies15,21-32,71 included in this review, 12 showed significant weight loss compared to placebo15,22,24-32,71 (Table 1).
Metformin studies in SMI included a total of 904 patients (497 exposed to metformin and 407 to placebo). Thirteen RCTs15,21-30,32,71 were placebo controlled, and 1 RCT31used topiramate as an active comparator. In terms of duration, metformin studies were predominantly short-terms trials, ranging from 12 to 14 weeks. However, it is important to highlight that metformin was the only pharmacologic agent tested for weight management of SMI with 4 longer-duration trials (24 or 26 weeks).27,28,30,71 Metformin dose ranged from 500 mg to 2,250 mg daily.
Twelve trials evaluated metformin in monotherapy,21,22,24-32,71 whereas 2 studies assessed the combination of this agent with sibutramine23 and lifestyle intervention.15 Baptista et al23 compared the effectiveness of metformin plus sibutramine versus metformin plus placebo in weight management of SMI. They found that although both groups achieved significant weight reduction, there was no significant difference between the treatment arms. In contrast, Wu et al24 compared metformin plus lifestyle intervention to placebo plus lifestyle intervention and to placebo alone and reported significantly greater weight loss with the combination of metformin plus lifestyle intervention in patients with schizophrenia. Of note, this was the only trial24 that compared pharmacologic and nonpharmacologic interventions among the studies included in this meta-analysis.
The 4 metformin trials27,28,30,71 with longer duration (24-26 weeks) helped to fill the gap with regard to the action of pharmacologic agents for weight control in SMI. In general, these were small sample size studies employing median doses of metformin in monotherapy (approximately 1,000 mg/d). Overall, they demonstrated a consistent and extended weight reduction over a period of nearly 6 months. Of note, the only study28 to include a follow-up of 24 weeks after study drug discontinuation reported significant weight regain after stopping the medication.
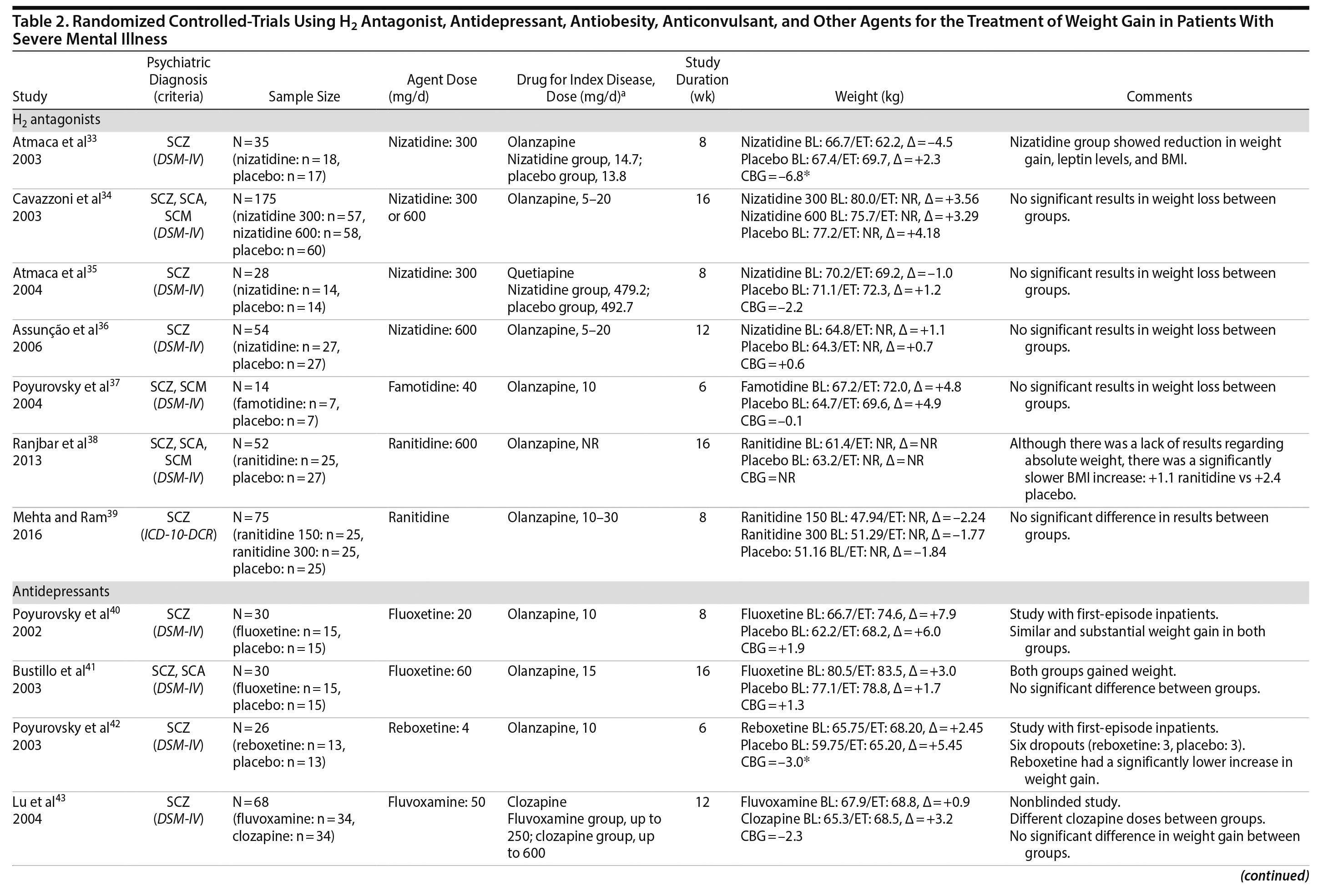
H2 Antagonists
Histamine-2 (H2) antagonists are a class of compounds that reduce or inhibit the secretion of gastric acid by binding competitively to histamine H2 receptors.34 The H2 receptor also appears to play a role in the regulation of feeding behaviors. Rodent studies suggest that the effects of H2 antagonism on weight loss may be mediated by increases in cholecystokinin,76 which implicates a gastric-central nervous system feedback circuit in explaining the putative weight-reducing effects of H2.39 Cimetidine, an H2 antagonist, has been reported to reduce weight in overweight healthy subjects as well as in overweight patients with type 2 diabetes mellitus.77 Using this theoretical background, it has been hypothesized that weight gain in patients with SMI might be treated with H2 antagonists.
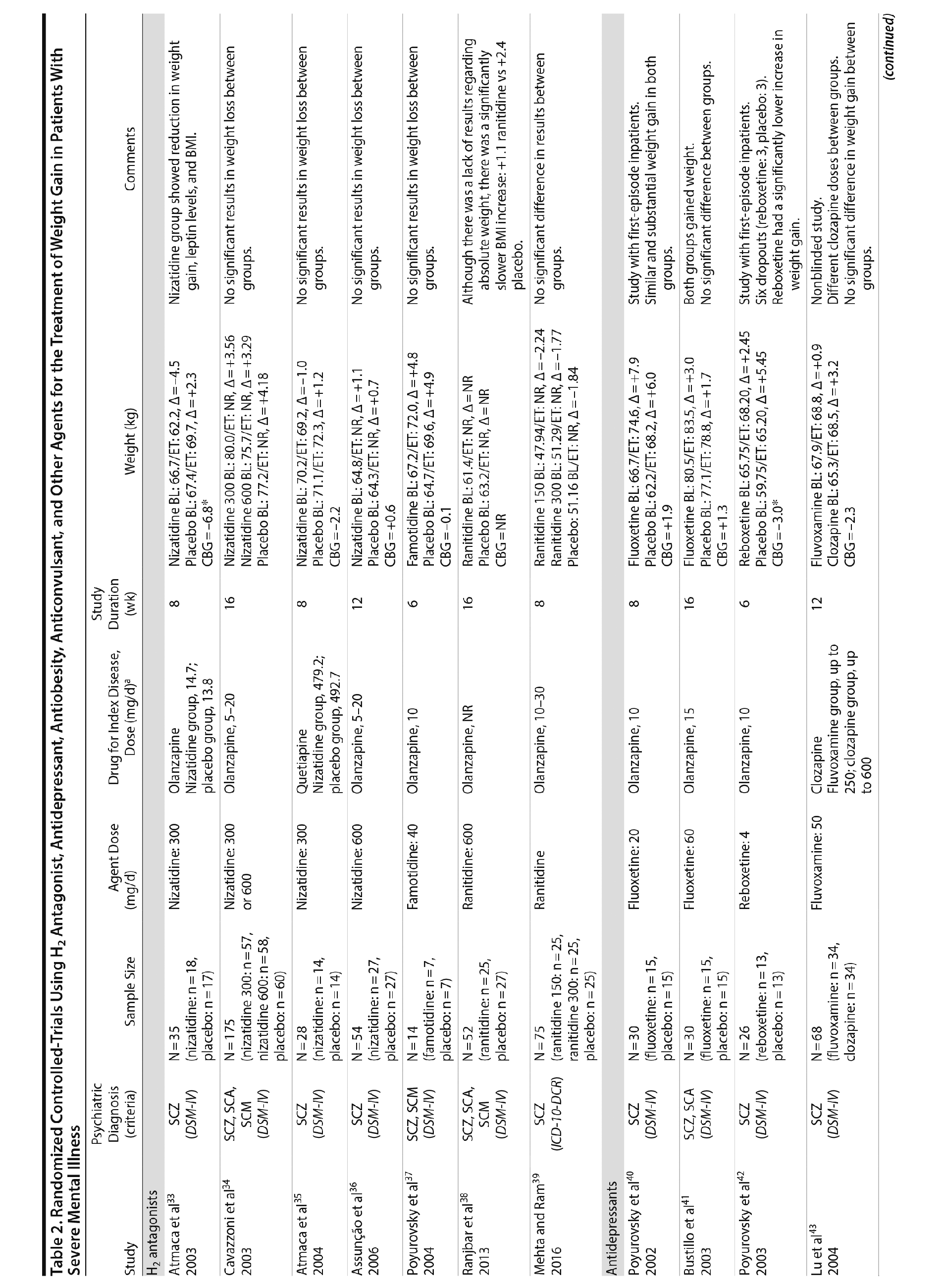
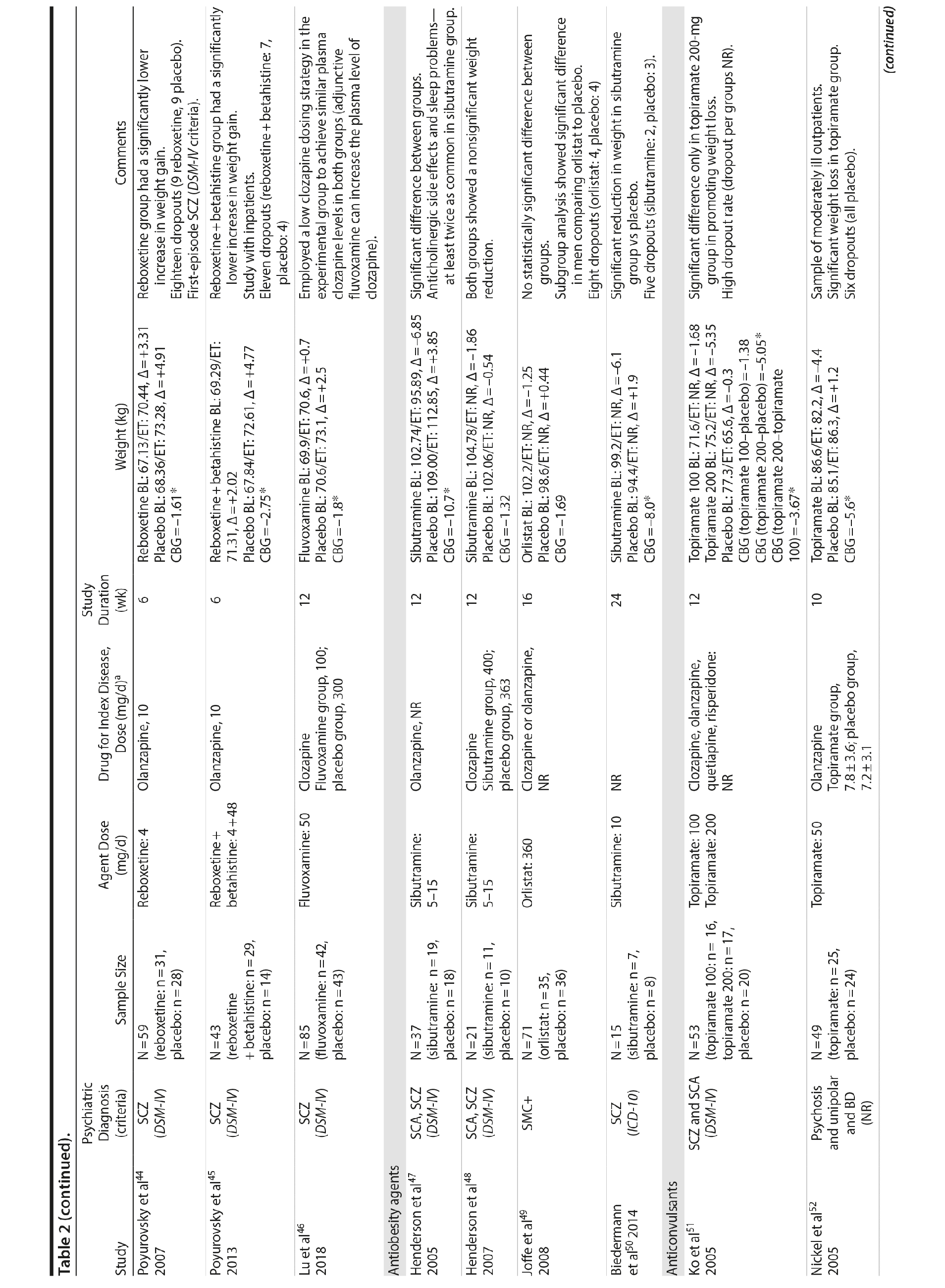
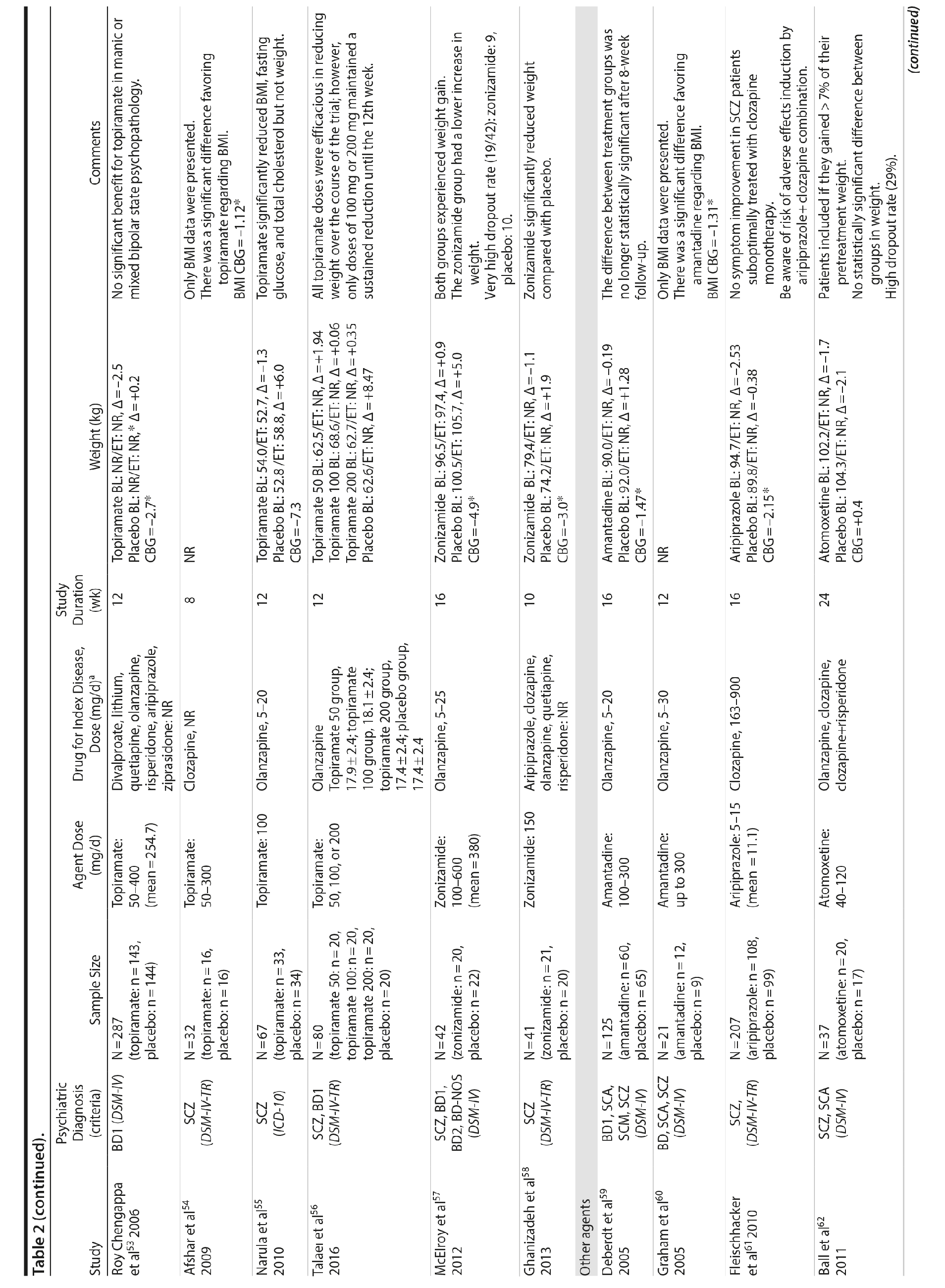
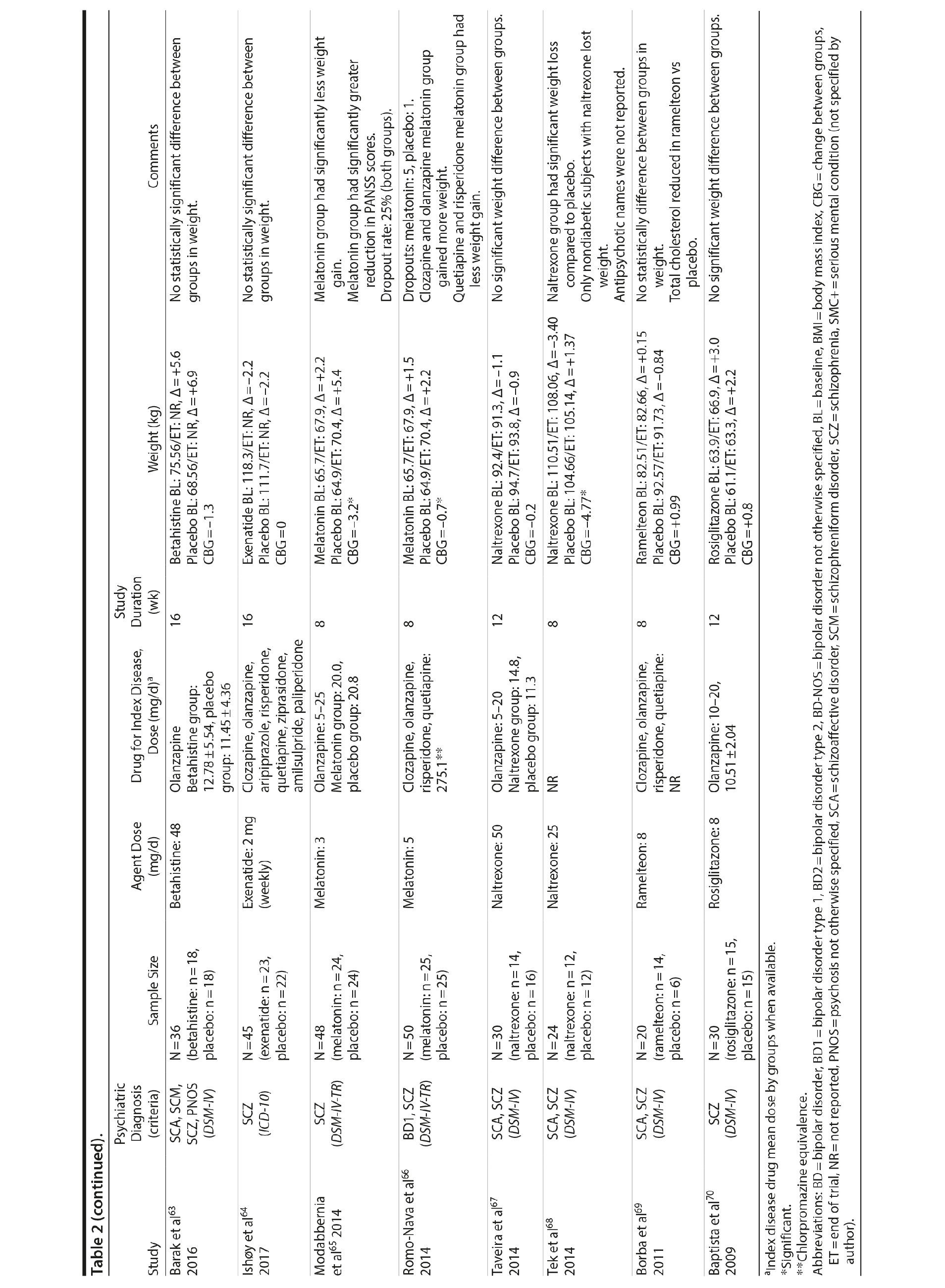
Seven studies33-39 tested H2 antagonists in the treatment of weight gain in patients with SMI. Overall, these were short-term trials ranging in duration from 6 to 16 weeks. Three agents were investigated in this therapeutic class. Nizatidine was the most-studied compound with 4 RCTs,33-36 ranitidine was evaluated in 2 studies,38,39 and famotidine was assessed in 1 study.37 However, the promising initial positive results of nizatidine33 in reducing weight in a group of patients with schizophrenia were not confirmed by subsequent RCTs with this agent and others from the same class34-36 (Table 2).
Antidepressants
Two classes of antidepressants were studied in the management of weight disorders in SMI: selective serotonin reuptake inhibitors (SSRIs) and selective-norepinephrine reuptake inhibitors (SNRIs). It is well established that drugs that enhance serotoninergic activity may act on food intake and food selection.78 One possible mechanism for the effect of SSRIs on weight is that by increasing synaptic levels of serotonin, these agents indirectly activate some 5-HT receptors (5-HT2C/5-HT1B) involved in appetite regulation, resulting in short-term weight loss.79 Other mechanisms have been proposed to explain the anorexic effects of SSRIs, such as mediation of neuropeptide Y80 or corticotrophin-releasing factor.81 In the case of SNRIs, there is some evidence suggesting a potential weight loss role of reboxetine in a bipolar patient82 and in a group of obese individuals with binge-eating disorder.83
Fluoxetine, fluvoxamine, and reboxetine were evaluated in 2 studies each.40,41,43-46 While both studies with fluoxetine40,41 and 1 with fluvoxamine43 were negative, both studies performed with reboxetine42,44 and 1 with fluvoxamine46 showed significantly less weight gain in the active drug group compared to placebo. However, these medications failed to promote weight reduction in these trials. An additional study45 was developed adding betahistine to reboxetine, an antivertigo agent, but it failed to show any advantage of this association (Table 2).
Antiobesity Agents
Two antiobesity agents, sibutramine and orlistat, were evaluated in the treatment of weight gain in patients with SMI.23,47-50 Sibutramine is a tertiary amine originally developed as a potential antidepressant84 that was withdrawn from the market, except from Brazil, because of cardiovascular safety concerns. Orlistat is a lipase inhibitor and a potent inhibitor of pancreatic and gastric lipases, acting locally in the gut lumen with minimal absorption, approved for weight loss. Orlistat inhibits dietary triglyceride hydrolysis by 30%, decreasing fat absorption proportionally.85,86
Once both drugs showed positive results in reducing weight in nonpsychiatric patients, 5 studies evaluated these agents in patients with SMI.23,47-50 In 3 studies47,48,50 the use of sibutramine was associated with a positive weight loss; however, this difference was significant in only 2 of those studies.47,50 It is important to mention that another study23 failed to demonstrate any advantage of combining sibutramine with metformin for weight management in a group of patients with schizophrenia. The only study49 that evaluated orlistat in the treatment of weight gain in SMI showed no significant difference between this agent and placebo (Table 2).
Anticonvulsants
The rationale for using anticonvulsants in the treatment of patients with SMI is that some agents of this class have weight-loss properties. Topiramate and zonisamide are anticonvulsants that demonstrate such effects. Although the exact mechanism of action of topiramate on weight is unknown, negative modulation of glutamate AMPA/kainate receptors and inhibition of carbonic anhydrase have been proposed as possible mechanisms involved in weight reduction and reduced appetite in patients taking this drug.78 It has also been suggested that topiramate reduces fat deposition by reducing food intake or stimulating energy expenditure.87 Zonisamide is an antiepileptic agent with serotoninergic and dopaminergic properties that also blocks sodium and calcium channels and inhibits carbonic anhydrase activity.88 The effects on brain serotonin and dopamine systems may explain, at least in part, its weight loss properties.89 Furthermore, zonisamide has shown efficacy in promoting weight loss in clinical trials involving patients with seizure disorders88 and in obese individuals without comorbid neuropsychiatric conditions.90
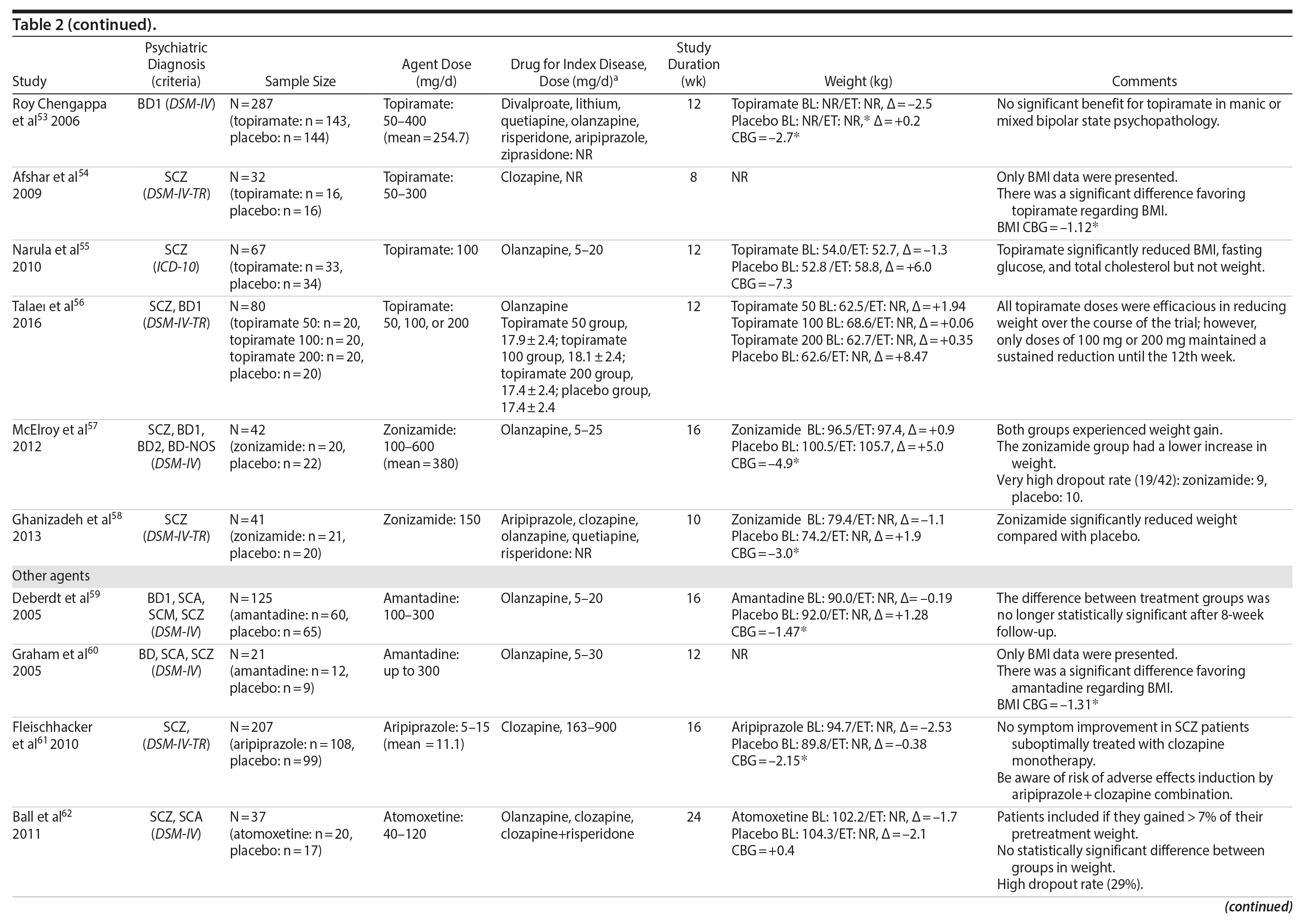
Topiramate was evaluated in 6 studies.51-56 In a short-term RCT evaluating SMI patients with weight problems, Ko et al51 tested topiramate in 2 different doses (100 and 200 mg/d) versus placebo and showed that only the 200-mg topiramate group achieved a significant weight loss compared to placebo. Following this report, Talaei et al56 also evaluated different doses of topiramate and found that although this agent was effective in the 3 doses tested, the 100- and 200-mg daily doses were superior to the 50-mg daily doses on weight parameters. Two additional RCTs52,55 demonstrated the weight loss properties of fixed doses (50 and 100 mg/day) of topiramate in patients with SMI.
Two RCTs57,58 evaluated the effects of zonisamide on weight in patients with SMI. In the first study,57 although both groups experienced weight gain, the authors reported that patients treated with zonisamide gained less weight than those taking placebo. A major concern in this study was the high dropout rate in both groups. In the second study,58 a significant mean weight difference of -3.0 kg favoring the zonisamide group was found. In contrast to the previous study,57 treatment with zonisamide was well tolerated, and a very low attrition rate was reported (Table 2).58
Other Agents
Several other agents (amantadine,59,60 aripiprazole,61 atomoxetine,62 betahistine,63 exenatide,64 melatonin,65,66 naltrexone,67,68 ramelteon,69 and rosiglitazone70) were also investigated for their potential role in weight management in SMI patients. Unlike the classes presented previously, these agents were investigated in only 1 or 2 RCTs and are discussed together (Table 2).
Rosiglitazone is another antidiabetic agent studied in the management of weight disorders in a patient with SMI.70 In contrast to studies with metformin,15,22,24-32,71 the only study70 using rosiglitazone 8 mg/d compared this compound with placebo in a 12-week trial and found that body weight significantly increased in both groups (with no significant differences between the groups). Exenatide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), was evaluated in 1 trial.64 The GLP-1RAs are registered for treatment of both obesity and type 2 diabetes. Ish׸y and colleagues64 performed a clinical trial with exenatide 2 mg weekly in patients with schizophrenia. The exenatide group presented a similar weight gain compared to the placebo group after 3 months.64 The authors64 concluded that exenatide did not promote weight loss in obese, antipsychotic-treated patients with schizophrenia compared to placebo.
Amantadine, a dopamine agonist approved for the treatment of extrapyramidal side effects of medications, idiopathic parkinsonism, and influenza-A infection, was investigated for weight management in SMI in 2 RCTs.59,60 The mechanism by which amantadine stabilizes weight is unknown, but it is postulated to be related to its ability to decrease prolactin and thereby influence gonadal and adrenal steroids.91 In 2 short-term RCTs, amantadine appeared to attenuate weight gain or promote weight loss in some patients who had gained weight during olanzapine therapy.59,60
Atypical antipsychotics, such as aripiprazole, have a relatively low potential for weight gain.92 Some long-term studies in schizophrenia have suggested that aripiprazole treatment is not associated with an increase in body weight93 and may promote some weight loss.94 We found 1 RCT61 that evaluated if adding aripiprazole to clozapine can prevent the weight gain observed with this agent. Fleischhacker et al61 studied aripiprazole used as an add-on medication in a sample of patients with schizophrenia using a stable dose of clozapine and showed a statistically significant weight loss compared to placebo.
Atomoxetine is an SNRI associated with appetite suppression.62 It was evaluated in 1 RCT62 and failed to demonstrate a difference compared to placebo in weight loss.
Betahistine, an antivertigo agent, may be useful to partially counteract the antipsychotic-associated weight gain that might be caused by H1 blockade.63 Recent studies in rats showed that both subchronic95 and chronic96 betahistine cotreatment prevented olanzapine-induced weight gain. In 1 trial performed by Barak et al,63 betahistine was not superior to placebo on weight parameters.
Melatonin, a hormone that regulates the suprachiasmatic nucleus, has been associated with prevention of weight gain induced by antipsychotics in an experimental study of animals treated with this hormone.97 In humans, 2 studies65,66 were performed using melatonin to treat weight gain in patients with SMI. Although both studies65,66 failed to demonstrate weight loss, patients in the melatonin groups gained significantly less weight than patients in the placebo groups.
Naltrexone is an opioid receptor blocker that has been studied for weight loss based on findings suggesting that orexigenic opioid pathways are critically involved in the regulation of food intake98 enhancing food pleasantness.99 The fixed combination of naltrexone with bupropion is currently approved in many countries for the treatment of obesity. We found 2 studies67,68 that tested naltrexone to counteract weight gain induced by antipsychotic agents in patients with SMI, which had different results. While Taveira et al67 found no significant difference between the groups, Tek et al68 found that naltrexone-treated patients had significant weight loss compared to placebo.
Ramelteon is a MT1 and MT2 melatonin receptor selective agonist100 used for insomnia treatment and sleep cycle disturbances that might be able to improve metabolic abnormalities in the schizophrenia population.69 It was tested by Borba et al69 in a small sample size, short-term RCT, and no statistically significant difference in body weight was found between the ramelteon and placebo groups.
Quality Assessment
A modified version of the Delphi List20 was used to assess the methodological quality of the RCTs included in this review. Overall, the studies presented a good quality (scores ≥ 7), which indicates a low risk of bias. Major weaknesses found in the studies include lack of sample size calculation and nonblinding of the care provider. It is important to consider that the sample size calculation is one of the most important items in quality assessment once it supports an external validity of the findings. Of note, these negative points were less observed in metformin studies, which had an overall better quality assessment.
Meta-Analysis
There were enough studies focusing on our primary outcome to analyze pooled effect sizes for metformin, sibutramine, topiramate, and nizatidine. Additional analyses for subgroups focusing on both study quality and study duration were only possible for metformin and sibutramine. For nizatidine and topiramate, we did not perform a subgroup analysis for study duration.
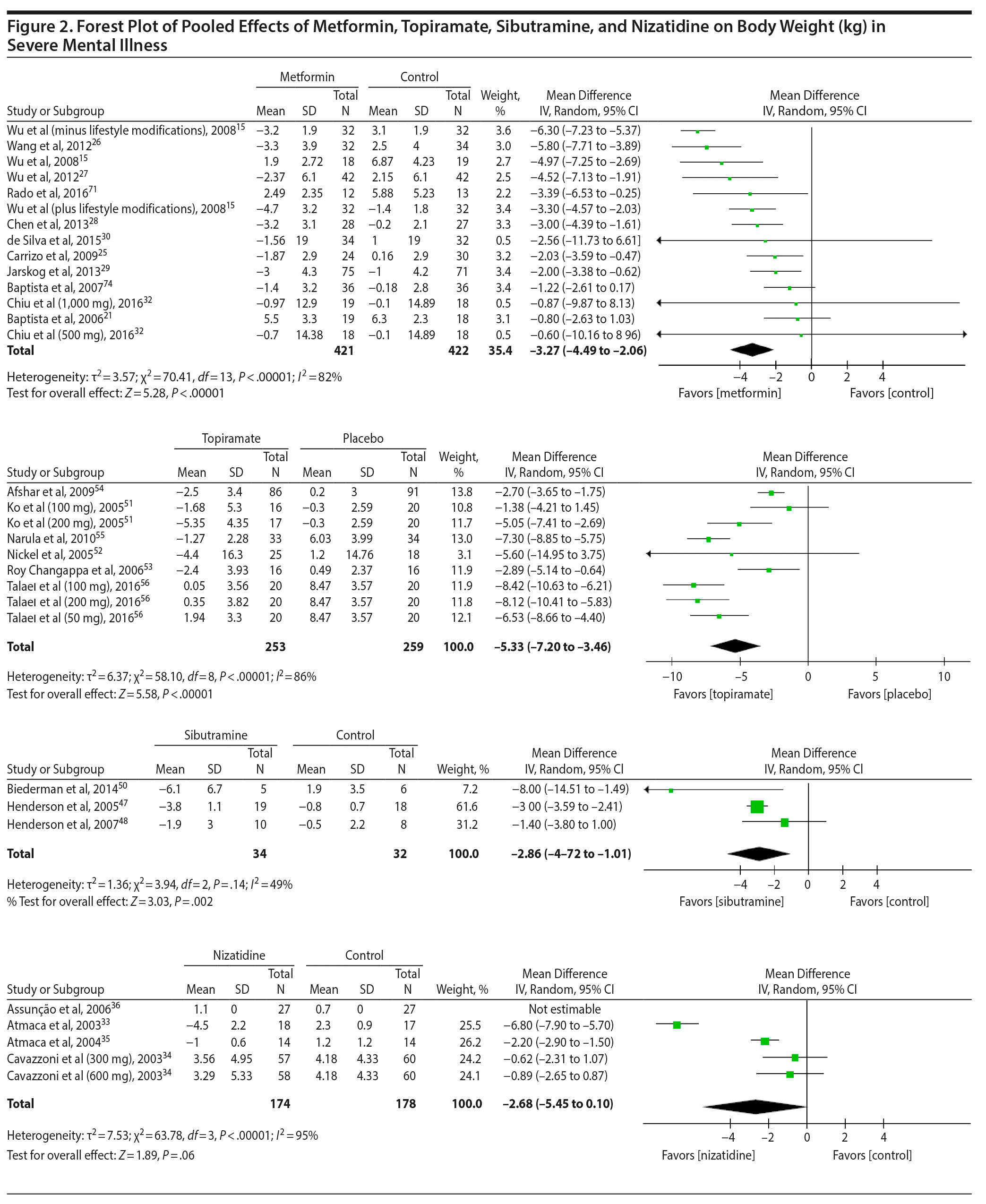
Metformin. Data were available for 10 metformin studies,15,21,22,24-29,71 which included 704 individuals and yielded a pooled significant mean difference of −3.36 kg (95% CI, −4.63 to −2.1) favoring metformin. There was a significantly high heterogeneity (χ2 = 69.63, P < .00001, I2 = 86%) among studies. A second analysis imputed SD values for 2 studies30,32 reaching a total of 12 studies15,21,22,24-30,32,71 with 843 individuals and a pooled effect size of −3.27 kg (95% CI, −4.49 to −2.06) favoring metformin. Interestingly, heterogeneity decreased but remained significant (χ2 = 70.41, P < .00001, I2 = 82%) (Figure 2). Regarding subgroups, there was no significant difference between the pooled mean differences of long-duration (N = 3; mean difference: −3.34 kg; 95% CI, −4.49 to −2.20)27,28,30,71 versus short-duration trials (N = 8; mean difference: −3.34 kg; 95% CI, −5.01 to −1.67).15,21,22,24-26,29,32 A significant difference between all high-quality studies15,21,24-30,32,71 (−3.60 kg; 95% CI, −4.89 to −2.31) and the only low-quality study22 emerged, but as the latter group had only 1 observation, this result is probably not generalizable.
Sibutramine. A total of 3 studies47,48,50 provided data for 66 subjects using sibutramine. There was a significant mean difference of −2.86 kg (95% CI, −4.72 to −1.01) favoring sibutramine. Heterogeneity was nonsignificant and moderate (χ2 = 3.94, P < .14, I2 = 49%) (see Figure 2). Removal of the only high-quality study47 changed the pooled effect size to −3.98 kg (95% CI, −10.29 to 2.33) and removing the only long-duration study50 changed it to −2.64 kg (95% CI, −3.95 to −1.33), both with no significant difference from the overall effect size for sibutramine.
Topiramate. Six studies were available with a total of 469 subjects tested using topiramate.51-56 There was a significant mean difference of −5.33 kg (95% CI, −7.20 to −3.46) favoring the topiramate group. Heterogeneity was high and significant (χ2 = 58.10, P < .00001, I2 = 86%). Furthermore, we imputed SD values for 1 study52 and reached a total of 512 studied individuals and a pooled effect size of −5.33 kg (95% CI, −7.20 to −3.46) favoring topiramate (χ2 = 58.10, P < .00001, I2 = 86%) (see Figure 2). Removing the only low-quality study55 changed the effect size to −5.03 kg (95% CI, −7.02 to −3.04), which did not significantly differ from the pooled effect size for all topiramate studies.
Nizatidine. Four studies33-36 provided data for 298 individuals participating in research with this drug. A mean weight loss of −2.68 kg (95% CI, −5.45 to 0.10) favoring nizatidine was found (χ2 = 63.78, I2 = 95%) (see Figure 2); however, this result was not statistically significant (P = .06). The removal of 1 low-quality study35 yielded an effect size of −2.81 kg (95% CI, −7.23 to 1.61), which was not significantly different from the pooled effect size for nizatidine.
DISCUSSION
The pharmacologic management of weight gain in SMI is an evolving area of research. This study, to the best of our knowledge, expands previous reviews on the subject by including at least 12 RCTs not previously analyzed.30-32,39,50,56,63,65-68,71 Our meta-analysis uncovered that metformin, topiramate, and sibutramine induced significantly more weight loss than placebo in such patients. Metformin was the most-studied compound for the treatment of weight gain in SMI and was evaluated in 14 RCTs,15,21-32,71 followed by topiramate (6 RCTs51-56), nizatidine (4 RCTs33-36), and sibutramine (3 RCTs47,48,50). The other agents (famotidine,37 ranitidine,38,39 fluoxetine,40,41 fluvoxamine,43,46 reboxetine,42,44 orlistat,49 zonizamide,57,58 amantadine,59,60 aripiprazole,61 betahistine,63 ramelteon,69 melatonin,65,66 naltrexone,67,68 rosiglitazone70) were investigated in only 1 or 2 isolated studies. Metformin showed significant results in 10 out of 12 trials, including 4 trials with longer duration (24/26 weeks).27,28,30,71 A meta-analytical procedure demonstrated a pooled weight decrease of −3.27 kg (95% CI, −4.49 to −2.06) favoring metformin. For topiramate, the second most-studied agent for weight gain in SMI, the pooled effect was even higher: −5.33 kg (95% CI, −7.20 to −3.46). The last agent with a positive effect on weight management was sibutramine, with a pooled effect of −2.86 kg (95% CI, −4.72 to −1.01). However, as sibutramine was removed from the market in several countries, it will not be included in our discussion. Finally, the meta-analytical procedure performed with nizatidine RCTs33-36 showed a result of −2.68 kg (95% CI, −5.45 to 0.10), which was not statistically different compared to placebo. Regarding the agents with positive effect on the quantitative analysis (metformin and topiramate), the results did not differ significantly between groups.
The current meta-analysis confirms the findings of previous metformin reviews101,102 and supports its potential role in the pharmacologic treatment of weight gain in patients with SMI. Proposed for the first time in 1999 for patients with drug-treated SMI,91 it was tested 2 years later103 and since then has been studied using different SMI populations104 and designs. In a meta-analysis, de Silva and colleagues102 assessed the effects of metformin on weight in SMI in 12 RCTs (including 2 studies with adolescents) and found a similar pooled weight change compared to our data (mean change in weight: −3.27 kg; 95% CI, −4.66 to −1.89). These findings were considered not only statistically significant but also clinically meaningful. Use of metformin resulted in clinically significant weight loss (more than 5%) in about half the patients in several trials.24,26 The authors102 also demonstrated a significant effect of this agent in secondary outcomes such as BMI and insulin resistance index, but not in fasting blood glucose. Moreover, the antiobesity properties of metformin appear to be more pronounced in first-episode patients than in chronic patients who have already gained weight.24,27 Taken together, this cumulative evidence appears to support the use of metformin in patients with weight gain in SMI, mostly in early stages of the illness. Additionally, as metformin does not act on the central nervous system, it can be considered safer than other agents in patients with SMI. However, it is important to note that a recent report105 suggests that metformin might alter mitochondrial processes involved in fitness and energy. This possibility should be properly assessed before recommending its use to treat weight gain in patients with SMI, since not only weight control but also cardiovascular and metabolic health are important issues to be considered.
Topiramate is a promising agent to treat weight gain in SMI, considering its effect size on weight reduction. This reduction appears to be clinically meaningful, with more than 50% of the topiramate-treated patients showing a reduction of 10% of initial body weight.51 Two trials54,55 demonstrated a concomitant reduction in BMI, fasting blood glucose, total cholesterol, low-density lipoprotein cholesterol, and waist circumference, as well as an increase in high-density lipoprotein cholesterol. As suggested in 2 other RCTs51,56 included in our review, higher doses of topiramate are associated with a higher effect on body weight. Overall, topiramate was considered safe in this population; adverse events were generally mild to moderate and were tolerated or resolved over time.53-55 However, in contrast to metformin, topiramate was evaluated only in a few short-duration trials, and more studies with different designs and long-term duration are needed to confirm its usefulness in SMI patients with weight gain.
As observed with nizatidine, trials with other H2 antagonists (famotidine37 and ranitidine38,39) did not show consistent results on weight outcomes in SMI. In the class of antidepressants, the SSRI subgroup (fluvoxamine43,46 and fluoxetine40,41) did not show positive results in weight outcomes in this population. Despite these findings, the SNRI reboxetine was shown to prevent weight gain in 3 trials.42,44,45 Orlistat, an antiobesity agent, failed to demonstrate effectiveness in SMI.49 Other compounds tested in the management of weight gain observed in patients with SMI showed negative (betahistine,63 ramelteon,69 and rosiglitazone70) or conflicting results (amantadine,59,60 melatonin,65,66 aripiprazole,61 naltrexone67,68), and more studies with enough power and longer duration are needed to gain a better understanding of their potential role.
A recent study106 with liraglutide, a glucagon-like peptide-1 receptor agonist, showed some promise in promoting weigh loss in patients with SMI. The mean difference in body weight in the liraglutide group was significant compared with placebo (−5.3 kg; 95% CI, −7.0 to −3.7). Since body weight was not a primary outcome, this study106 was not included in our review.
Overall, studies evaluating the effects of pharmacologic agents in the management of weight gain in SMI showed several methodological flaws, such as small sample size, lack of sample size calculation, short duration, and lack or incomplete assessment of metabolic parameters. To overcome these limitations, the US Food and Drug Administration Guidelines for Developing Products for Weight Management107 provide a good reference for future studies in the area, which includes a specific topic about medication-induced weight gain. The inclusion of categorical measures (proportion of subjects losing at least 5% of baseline body weight) as primary outcomes and the assessment minimum secondary outcomes (blood pressure and heart rate, lipids, fasting glucose and insulin, HbA1c in patients with type 2 diabetes, and waist circumference) are mandatory for further trials in the field. Another important point is the recommendation of a minimum duration of 1 year for an obesity trial. Therefore, even metformin—our most-studied agent for weight management in SMI—still needs to be evaluated in longer studies and is not approved as an antiobesity agent. In conclusion, we suggest that future studies involving the pharmacologic treatment of weight gain in patients with SMI should be more aligned with the methodological standards of the general obesity field.
Weight gain in individuals with SMI has a multifactorial etiology10-12 with a marked interplay between the underlying disease and medication-related factors. For such complex conditions, the better option usually is a multimodal treatment with a combination of interventions to address all those components. Due to the paucity of information regarding this issue, it is recommended to explore in future studies the effectiveness of combining pharmacologic, nutritional, and psychological interventions to deal with weight gain in SMI.
Limitations of this review were mainly characterized by the limitations of the trials already mentioned. Other limitations are that we did not search for unpublished materials or contact pharmaceutical companies that are potentially interested in the development of agents to manage weight gain in SMI, and other databases were not included. Despite these limitations, this systematic review and meta-analysis was comprehensive, including several studies not covered by previous reviews.
In conclusion, this review provides additional evidence to support a potential role of metformin and topiramate in the management of weight gain in patients with SMI. The higher current level of evidence available for metformin may support its utilization early in the treatment schedule for patients with SMI presenting a significant increase in body weight. Furthermore, topiramate also showed positive weight reduction properties in this population and may represent an alternative to metformin. Overall, trials assessing the effectiveness of pharmacologic agents in the area are characterized by some flaws and need to be more aligned with the methodological standards of the general obesity field.
<!–
–>
Submitted: May 17, 2019; accepted July 15, 2019.
Published online: December 19, 2019.
Author contributions: Dr Hiluy: bibliographic search and review and manuscript preparation and review. Dr Nazar: manuscript preparation and review. Dr Gonçalves: bibliographic search and review and manuscript review. Dr Coutinho: manuscript review. Dr Appolinario: bibliographic review of data and manuscript preparation and review.
Potential conflicts of interest: Dr Nazar has received honoraria from Shire and Lundbeck for academic work and lecturing. Dr Coutinho has received lecture fees from Abbott Diabetes Care, Janssen, Germed, and Novo Nordisk; served on advisory boards for Abbott Diabetes Care, Germed, EMS, and Novo Nordisk; and received travel reimbursement from Abbott Diabetes Care, Janssen, Novo Nordisk, Germed, and EMS. Dr Appolinario has received research grants, consultancy fees, and advisory board fees from Shire, royalties/honoraria from Artmed Panamericana Editora, and a research grant from the Brazilian National Research Council. Drs Hiluy and Gonçalves report no conflicts of interest related to the subject of this article.
Funding/support: None.
Previous presentation: Presented at the XXXV Brazilian Psychiatry Congress; October 25, 2017; São Paulo, Brazil.
REFERENCES
1.Rössner S. Obesity: the disease of the twenty-first century. Int J Obes Relat Metab Disord. 2002;26(suppl 4):S2-S4. PubMed CrossRef
2.Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215-220. PubMed CrossRef
3.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293(20):2528-2530. PubMed CrossRef
4.Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36(4):341-350. PubMed CrossRef
5.Baptista T, Serrano A, Uzcátegui E, et al. The metabolic syndrome and its constituting variables in atypical antipsychotic-treated subjects: comparison with other drug treatments, drug-free psychiatric patients, first-degree relatives and the general population in Venezuela. Schizophr Res. 2011;126(1-3):93-102. PubMed CrossRef
6.Janssen EM, McGinty EE, Azrin ST, et al. Review of the evidence: prevalence of medical conditions in the United States population with serious mental illness. Gen Hosp Psychiatry. 2015;37(3):199-222. PubMed CrossRef
7.Health US. With Special Feature on Prescription Drugs. National Center for Health Statistics website. https://www.cdc.gov/nchs/data/hus/hus13.pdf. 2013.
8.Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850. PubMed CrossRef
9.Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484. PubMed CrossRef
10.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(suppl 4):477S-480S. PubMed CrossRef
11.Brewerton TD. Toward a unified theory of serotonin dysregulation in eating and related disorders. Psychoneuroendocrinology. 1995;20(6):561-590. PubMed CrossRef
12.Jimerson DC, Lesem MD, Kaye WH, et al. Eating disorders and depression: is there a serotonin connection? Biol Psychiatry. 1990;28(5):443-454. PubMed CrossRef
13.Callaghan P. Exercise: a neglected intervention in mental health care? J Psychiatr Ment Health Nurs. 2004;11(4):476-483. PubMed CrossRef
14.Fernández-San-Mart×n MI, Mart×n-López LM, Masa-Font R, et al. The effectiveness of lifestyle interventions to reduce cardiovascular risk in patients with severe mental disorders: meta-analysis of intervention studies. Community Ment Health J. 2014;50(1):81-95. PubMed CrossRef
15.Wu R-R, Zhao J-P, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193. PubMed CrossRef
16.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530. PubMed CrossRef
17.Fiedorowicz JG, Miller DD, Bishop JR, et al. systematic review and meta-analysis of pharmacological interventions for weight gain from antipsychotics and mood stabilizers. Curr Psychiatry Rev. 2012;8(1):25-36. PubMed CrossRef
18.Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403. PubMed CrossRef
19.Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. PubMed CrossRef
20.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235-1241. PubMed CrossRef
21.Baptista T, Mart×nez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51(3):192-196. PubMed CrossRef
22.Baptista T, Rangel N, Fernández V, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. 2007;93(1-3):99-108. PubMed CrossRef
23.Baptista T, Uzcátegui E, Rangel N, et al. Metformin plus sibutramine for olanzapine-associated weight gain and metabolic dysfunction in schizophrenia: a 12-week double-blind, placebo-controlled pilot study. Psychiatry Res. 2008;159(1-2):250-253. PubMed CrossRef
24.Wu R-RR, Zhao J-PP, Guo X-FF, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165(3):352-358. PubMed CrossRef
25.Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26. PubMed CrossRef
26.Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57. PubMed CrossRef
27.Wu R-R, Jin H, Gao K, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2012;169(8):813-821. PubMed CrossRef
28.Chen C-H, Huang M-C, Kao C-F, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430. PubMed CrossRef
29.Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040. PubMed CrossRef
30.de Silva VA, Dayabandara M, Wijesundara H, et al. Metformin for treatment of antipsychotic-induced weight gain in a South Asian population with schizophrenia or schizoaffective disorder: a double blind, randomized, placebo controlled study. J Psychopharmacol. 2015;29(12):1255-1261. PubMed CrossRef
31.Peng P-J, Ho P-S, Tsai C-K, et al. A pilot study of randomized, head-to-head of metformin versus topiramate in obese people with schizophrenia. Clin Neuropharmacol. 2016;39(6):306-310. PubMed CrossRef
32.Chiu C-CC, Lu M-LL, Huang M-CC, et al. Effects of low dose metformin on metabolic traits in clozapine-treated schizophrenia patients: an exploratory twelve-week randomized, double-blind, placebo-controlled study. PLoS One. 2016;11(12):e0168347. PubMed CrossRef
33.Atmaca M, Kuloglu M, Tezcan E, et al. Nizatidine treatment and its relationship with leptin levels in patients with olanzapine-induced weight gain. Hum Psychopharmacol. 2003;18(6):457-461. PubMed CrossRef
34.Cavazzoni P, Tanaka Y, Roychowdhury SM, et al. Nizatidine for prevention of weight gain with olanzapine: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(2):81-85. PubMed CrossRef
35.Atmaca M, Kuloglu M, Tezcan E, et al. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Hum Psychopharmacol. 2004;19(1):37-40. PubMed CrossRef
36.Assunç×£o SSM, Ruschel SII, Rosa L de CRCR, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Br J Psychiatry. 2006;28(4):270-276. PubMed CrossRef
37.Poyurovsky M, Tal V, Maayan R, et al. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. Eur Neuropsychopharmacol. 2004;14(4):332-336. PubMed CrossRef
38.Ranjbar F, Ghanepour A, Sadeghi-Bazargani H, et al. The effect of ranitidine on olanzapine-induced weight gain. BioMed Res Int. 2013;2013:639391. PubMed CrossRef
39.Mehta VS, Ram D. Efficacy of ranitidine in olanzapine-induced weight gain: a dose-response study. Early Interv Psychiatry. 2016;10(6):522-527. PubMed CrossRef
40.Poyurovsky M, Pashinian A, Gil-Ad I, et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry. 2002;159(6):1058-1060. PubMed CrossRef
41.Bustillo JR, Lauriello J, Parker K, et al. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology. 2003;28(3):527-529. PubMed CrossRef
42.Poyurovsky M, Isaacs I, Fuchs C, et al. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. Am J Psychiatry. 2003;160(2):297-302. PubMed CrossRef
43.Lu M-L, Lane H-Y, Lin S-K, et al. Adjunctive fluvoxamine inhibits clozapine-related weight gain and metabolic disturbances. J Clin Psychiatry. 2004;65(6):766-771. PubMed CrossRef
44.Poyurovsky M, Fuchs C, Pashinian A, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology (Berl). 2007;192(3):441-448. PubMed CrossRef
45.Poyurovsky M, Fuchs C, Pashinian A, et al. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology (Berl). 2013;226(3):615-622. PubMed CrossRef
46.Lu M-L, Chen T-T, Kuo P-H, et al. Effects of adjunctive fluvoxamine on metabolic parameters and psychopathology in clozapine-treated patients with schizophrenia: A 12-week, randomized, double-blind, placebo-controlled study. Schizophr Res. 2018;193:126-133. PubMed CrossRef
47.Henderson DC, Copeland PM, Daley TB, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry. 2005;162(5):954-962. PubMed CrossRef
48.Henderson DC, Fan X, Copeland PM, et al. A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta Psychiatr Scand. 2007;115(2):101-105. PubMed CrossRef
49.Joffe G, Takala P, Tchoukhine E, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):706-711. PubMed CrossRef
50.Biedermann F, Fleischhacker WW, Kemmler G, et al. Sibutramine in the treatment of antipsychotic-induced weight gain: a pilot study in patients with schizophrenia. Int Clin Psychopharmacol. 2014;29(3):181-184. PubMed CrossRef
51.Ko Y-HH, Joe S-HH, Jung I-KK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175. PubMed CrossRef
52.Nickel MK, Nickel C, Muehlbacher M, et al. Influence of topiramate on olanzapine-related adiposity in women: a random, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2005;25(3):211-217. PubMed CrossRef
53.Roy Chengappa KN, Schwarzman LK, Hulihan JF, et al; Clinical Affairs Product Support Study-168 Investigators. Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry. 2006;67(11):1698-1706. PubMed CrossRef
54.Afshar H, Roohafza H, Mousavi G, et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol. 2009;23(2):157-162. PubMed CrossRef
55.Narula PK, Rehan HS, Unni KES, et al. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophr Res. 2010;118(1-3):218-223. PubMed CrossRef
56.Talaeı A, Farıdhosseını F, Kazemı H, et al. Effect of topiramate on drug associated weight gain of patients with schizophrenia and bipolar I disorders: a dose ranging randomized trial [in Turkish]. Turk Psikiyatr Derg. 2016;27(2):0. PubMed
57.McElroy SL, Winstanley E, Mori N, et al. A randomized, placebo-controlled study of zonisamide to prevent olanzapine-associated weight gain. J Clin Psychopharmacol. 2012;32(2):165-172. PubMed CrossRef
58.Ghanizadeh A, Nikseresht MS, Sahraian A. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, double-blind, placebo-controlled clinical trial. Schizophr Res. 2013;147(1):110-115. PubMed CrossRef
59.Deberdt W, Winokur A, Cavazzoni PA, et al. Amantadine for weight gain associated with olanzapine treatment. Eur Neuropsychopharmacol. 2005;15(1):13-21. PubMed CrossRef
60.Graham KA, Gu H, Lieberman JA, et al. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. Am J Psychiatry. 2005;162(9):1744-1746. PubMed CrossRef
61.Fleischhacker WW, Heikkinen ME, Olié J-P, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13(8):1115-1125. PubMed CrossRef
62.Ball MP, Warren KR, Feldman S, et al. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clin Schizophr Relat Psychoses. 2011;5(1):17-25. PubMed CrossRef
63.Barak N, Beck Y, Albeck JHA. A randomized, double-blind, placebo-controlled pilot study of betahistine to counteract olanzapine-associated weight gain. J Clin Psychopharmacol. 2016;36(3):253-256. PubMed CrossRef
64.Ish׸y PL, Knop FK, Broberg BV, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(2):162-171. PubMed CrossRef
65.Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140. PubMed CrossRef
66.Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421. PubMed CrossRef
67.Taveira TH, Wu W-C, Tschibelu E, et al. The effect of naltrexone on body fat mass in olanzapine-treated schizophrenic or schizoaffective patients: a randomized double-blind placebo-controlled pilot study. J Psychopharmacol. 2014;28(4):395-400. PubMed CrossRef
68.Tek C, Ratliff J, Reutenauer E, et al. A randomized, double-blind, placebo-controlled pilot study of naltrexone to counteract antipsychotic-associated weight gain: proof of concept. J Clin Psychopharmacol. 2014;34(5):608-612. PubMed CrossRef
69.Borba CPCC, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658. PubMed
70.Baptista T, Rangel N, El Fakih Y, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry. 2009;42(1):14-19. PubMed CrossRef
71.Rado J, von Ammon Cavanaugh S. A naturalistic randomized placebo-controlled trial of extended-release metformin to prevent weight gain associated with olanzapine in a US community-dwelling population. J Clin Psychopharmacol. 2016;36(2):163-168. PubMed CrossRef
72.Rasouli N, Kern PA, Reece EA, et al. Effects of pioglitazone and metformin on beta-cell function in nondiabetic subjects at high risk for type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(1):E359-E365. PubMed CrossRef
73.Kitabchi AE, Temprosa M, Knowler WC, et al; Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404-2414. PubMed CrossRef
74.Baptista T, Sandia I, Lacruz A, et al. Insulin counter-regulatory factors, fibrinogen and C-reactive protein during olanzapine administration: effects of the antidiabetic metformin. Int Clin Psychopharmacol. 2007;22(2):69-76. PubMed CrossRef
75.Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes [online ahead of print February 4, 2019]. Hormones (Athens). PubMed
76.St׸a-Birketvedt G, L׸vhaug N, Vonen B, et al. H2-receptor antagonist reduces food intake and weight gain in rats by non-gastric acid secretory mechanisms. Acta Physiol Scand. 1997;161(4):489-494. PubMed CrossRef
77.St׸a-Birketvedt G, Paus PN, Ganss R, et al. Cimetidine reduces weight and improves metabolic control in overweight patients with type 2 diabetes. Int J Obes Relat Metab Disord. 1998;22(11):1041-1045. PubMed CrossRef
78.Appolinario JC, Bueno JR, Coutinho W. Psychotropic drugs in the treatment of obesity: what promise? CNS Drugs. 2004;18(10):629-651. PubMed CrossRef
79.Stahl SM. How to appease the appetite of psychotropic drugs. J Clin Psychiatry. 1998;59(10):500-501. PubMed CrossRef
80.Gutiérrez A, Sarac×bar G, Casis L, et al. Effects of fluoxetine administration on neuropeptide y and orexins in obese Zucker rat hypothalamus. Obes Res. 2002;10(6):532-540. PubMed CrossRef
81.Wieczorek I, Schulz C, Jarry H, et al. The effects of the selective serotonin reuptake-inhibitor fluvoxamine on body weight in Zucker rats are mediated by corticotropin-releasing hormone. Int J Obes Relat Metab Disord. 2001;25(10):1566-1569. PubMed CrossRef
82.Lu TY-T, Kupa A, Easterbrook G, et al. Profound weight loss associated with reboxetine use in a 44-year-old woman. Br J Clin Pharmacol. 2005;60(2):218-220. PubMed CrossRef
83.Silveira RO, Zanatto V, Appolinário JC, et al. An open trial of reboxetine in obese patients with binge eating disorder. Eat Weight Disord. 2005;10(4):e93-e96. PubMed CrossRef
84.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM study group. sibutramine trial of obesity reduction and maintenance. Lancet. 2000;356(9248):2119-2125. PubMed CrossRef
85.Sjöström L, Rissanen A, Andersen T, et al; European Multicentre Orlistat Study Group. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167-172. PubMed CrossRef
86.Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord. 1997;21(suppl 3):S12-S23. PubMed
87.Richard D, Ferland J, Lalonde J, et al. Influence of topiramate in the regulation of energy balance. Nutrition. 2000;16(10):961-966. PubMed CrossRef
88.Oommen KJ, Mathews S. Zonisamide: a new antiepileptic drug. Clin Neuropharmacol. 1999;22(4):192-200. PubMed
89.Okada M, Hirano T, Kawata Y, et al. Biphasic effects of zonisamide on serotonergic system in rat hippocampus. Epilepsy Res. 1999;34(2-3):187-197. PubMed CrossRef
90.Gadde KM, Franciscy DM, Wagner HR 2nd, et al. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820-1825. PubMed CrossRef
91.Baptista T. Body weight gain induced by antipsychotic drugs: mechanisms and management. Acta Psychiatr Scand. 1999;100(1):3-16. PubMed CrossRef
92.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. PubMed CrossRef
93.Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adolesc Psychopharmacol. 2003;13(3):319-327. PubMed CrossRef
94.Pigott TA, Carson WH, Saha AR, et al; Aripiprazole Study Group. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048-1056. PubMed CrossRef
95.Lian J, Huang X-F, Pai N, et al. Preventing olanzapine-induced weight gain using betahistine: a study in a rat model with chronic olanzapine treatment. PLoS One. 2014;9(8):e104160. PubMed CrossRef
96.Lian J, Huang X-F, Pai N, et al. Betahistine ameliorates olanzapine-induced weight gain through modulation of histaminergic, NPY and AMPK pathways. Psychoneuroendocrinology. 2014;48:77-86. PubMed CrossRef
97.Raskind MA, Burke BL, Crites NJ, et al. Olanzapine-induced weight gain and increased visceral adiposity is blocked by melatonin replacement therapy in rats. Neuropsychopharmacology. 2007;32(2):284-288. PubMed CrossRef
98.Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31(10):2091-2120. PubMed CrossRef
99.Peci×±a S. Opioid reward ‘ liking’ and ‘ wanting’ in the nucleus accumbens. Physiol Behav. 2008;94(5):675-680. PubMed CrossRef
100.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63(10):1149-1157. PubMed CrossRef
101.Hendrick V, Dasher R, Gitlin M, et al. Minimizing weight gain for patients taking antipsychotic medications: the potential role for early use of metformin. Ann Clin Psychiatry. 2017;29(2):120-124. PubMed
102.de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341. PubMed CrossRef
103.Baptista T, Hern× ndez L, Prieto LA, et al. Metformin in obesity associated with antipsychotic drug administration: a pilot study. J Clin Psychiatry. 2001;62(8):653-655. PubMed CrossRef
104.Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry. 2002;159(4):655-657. PubMed CrossRef
105.Konopka AR, Laurin JL, Schoenberg HM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18(1):e12880. PubMed
106.Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728. PubMed CrossRef
107.Guidance for Industry. Developing products for weight management. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/developing-products-weight-management-revision-1.
Please sign in or purchase this PDF for $40.00.
Save
Cite