Objective: Anosognosia, or impaired illness awareness, is a common feature of Alzheimer’s disease (AD) and less so of mild cognitive impairment (MCI). Importantly, anosognosia negatively influences clinical outcomes for patients and their caregivers and may predict the conversion from MCI to AD. This study aimed to examine (1) the relationship between brain glucose metabolism as measured by fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) and anosognosia in patients with MCI and AD and (2) the predictive utility of anosognosia in patients with MCI for later conversion to AD, even when controlling for other factors, including gender, education, apolipoprotein E ε4 carrier status, dementia severity, and cognitive dysfunction.
Methods: Data for 1,062 participants from the Alzheimer’s Disease Neuroimaging Initiative database (2003 to August 2015) classified as having AD (n = 191) or MCI (n = 499) or as healthy comparison (HC) subjects (n = 372) were analyzed. HC participants had Mini-Mental State Examination (MMSE) scores from 24 to 30 and a Clinical Dementia Rating (CDR) of 0. MCI participants had MMSE scores from 24 to 30, a memory complaint, objective memory loss, a CDR of 0.5, absence of significant levels of impairment in other cognitive domains, and essentially preserved activities of daily living. AD participants had MMSE scores ≤ 26 and a CDR of ≥ 0.5, and met National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable AD. Anosognosia was measured with the composite discrepancy score of the study partner and participants’ scores on the Everyday Cognition scale (ECog). Bivariate correlations and multiple regression analyses were performed to assess the relationship between anosognosia and FDG-PET findings in each group. Lastly, logistic regression and receiver operating characteristic curve analyses were performed in the MCI sample to determine if anosognosia was predictive of conversion from MCI to AD.
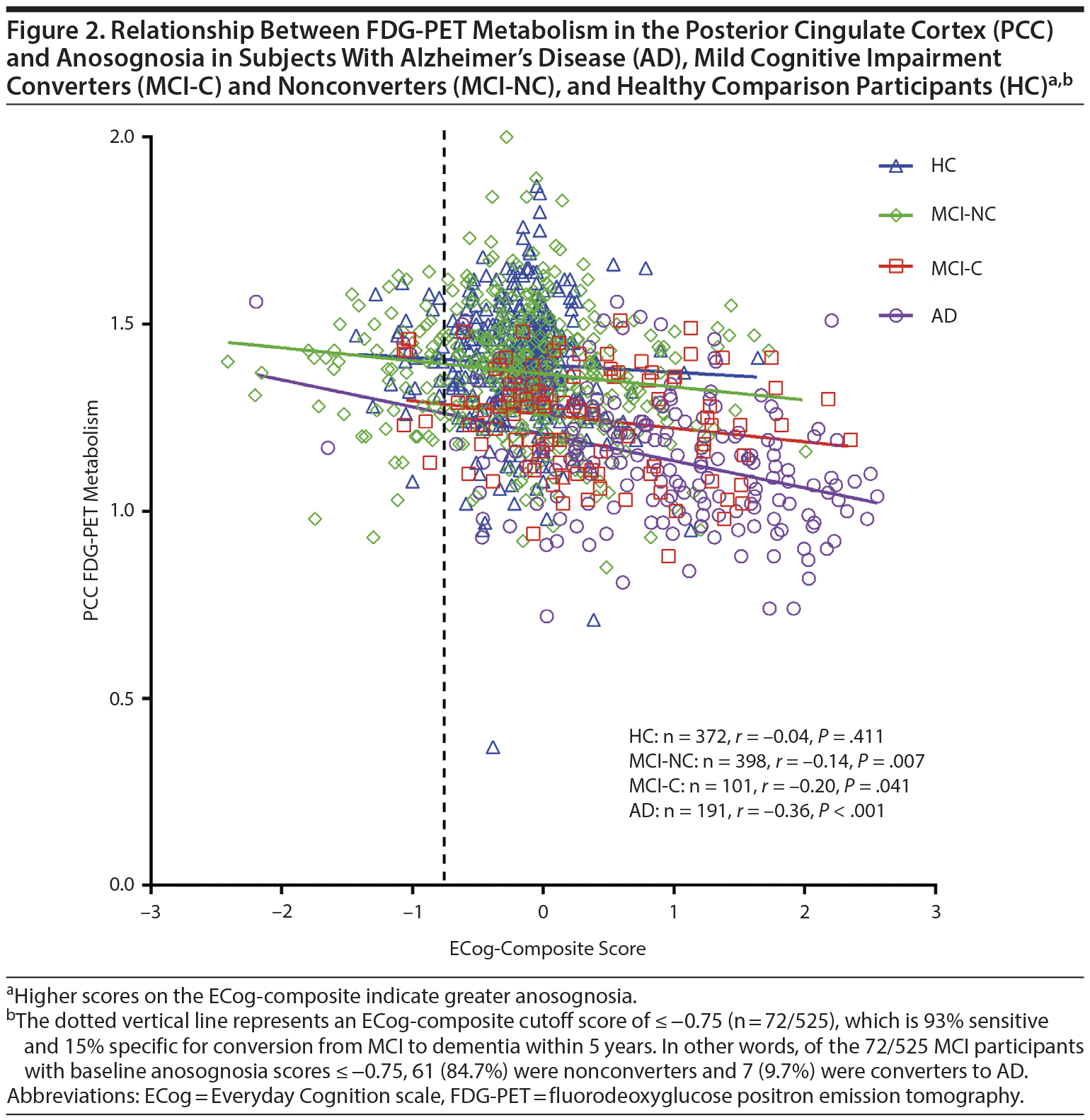
Results: Hypometabolism was independently associated with anosognosia in AD, particularly in the posterior cingulate cortex and right angular gyrus. Anosognosia was associated with conversion from MCI to AD within 5 years (OR = 2.74 [95% CI, 1.95 to 3.85], χ21 = 33.65, P < .001), even after including covariates (OR = 1.64 [95% CI, 1.12 to 2.40], χ21 = 6.43, P = .011). ECog-composite scores ≤ −0.75 were 93% sensitive and 15% specific for conversion from MCI to AD.
Conclusions: Anosognosia in AD is related to brain glucose hypometabolism. Further, anosognosia independently predicts conversion from MCI to AD. The absence of anosognosia may be clinically useful to identify those patients that are unlikely to convert from MCI to AD.
Anosognosia Is an Independent Predictor of Conversion From Mild Cognitive Impairment to Alzheimer’s Disease and Is Associated With Reduced Brain Metabolism

ABSTRACT
Objective: Anosognosia, or impaired illness awareness, is a common feature of Alzheimer’s disease (AD) and less so of mild cognitive impairment (MCI). Importantly, anosognosia negatively influences clinical outcomes for patients and their caregivers and may predict the conversion from MCI to AD. This study aimed to examine (1) the relationship between brain glucose metabolism as measured by fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) and anosognosia in patients with MCI and AD and (2) the predictive utility of anosognosia in patients with MCI for later conversion to AD, even when controlling for other factors, including gender, education, apolipoprotein E ε4 carrier status, dementia severity, and cognitive dysfunction.
Methods: Data for 1,062 participants from the Alzheimer’s Disease Neuroimaging Initiative database (2003 to August 2015) classified as having AD (n = 191) or MCI (n = 499) or as healthy comparison (HC) subjects (n = 372) were analyzed. HC participants had Mini-Mental State Examination (MMSE) scores from 24 to 30 and a Clinical Dementia Rating (CDR) of 0. MCI participants had MMSE scores from 24 to 30, a memory complaint, objective memory loss, a CDR of 0.5, absence of significant levels of impairment in other cognitive domains, and essentially preserved activities of daily living. AD participants had MMSE scores ≤ 26 and a CDR of ≥ 0.5, and met National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable AD. Anosognosia was measured with the composite discrepancy score of the study partner and participants’ scores on the Everyday Cognition scale (ECog). Bivariate correlations and multiple regression analyses were performed to assess the relationship between anosognosia and FDG-PET findings in each group. Lastly, logistic regression and receiver operating characteristic curve analyses were performed in the MCI sample to determine if anosognosia was predictive of conversion from MCI to AD.
Results: Hypometabolism was independently associated with anosognosia in AD, particularly in the posterior cingulate cortex and right angular gyrus. Anosognosia was associated with conversion from MCI to AD within 5 years (OR = 2.74 [95% CI, 1.95 to 3.85], χ21 = 33.65, P < .001), even after including covariates (OR = 1.64 [95% CI, 1.12 to 2.40], χ21 = 6.43, P = .011). ECog-composite scores ≤ −0.75 were 93% sensitive and 15% specific for conversion from MCI to AD.
Conclusions: Anosognosia in AD is related to brain glucose hypometabolism. Further, anosognosia independently predicts conversion from MCI to AD. The absence of anosognosia may be clinically useful to identify those patients that are unlikely to convert from MCI to AD.
J Clin Psychiatry 2017;78(9):e1187-e1196
https://doi.org/10.4088/JCP.16m11367
© Copyright 2017 Physicians Postgraduate Press, Inc.
aMultimodal Imaging Group, Research Imaging Centre, Centre for Addiction and Mental Health, Toronto, Canada
bCampbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, Canada
cInstitute of Medical Science, University of Toronto, Toronto, Canada
dDepartment of Psychiatry, University of Toronto, Toronto, Canada
eGeriatric Psychiatry Division, Centre for Addiction and Mental Health, Toronto, Canada
*Corresponding author: Philip Gerretsen, MSW, MD, PhD, FRCPC, Centre for Addiction and Mental Health, 80 Workman Way, 6th Floor, Toronto, ON M6J1H4 Canada ([email protected]).
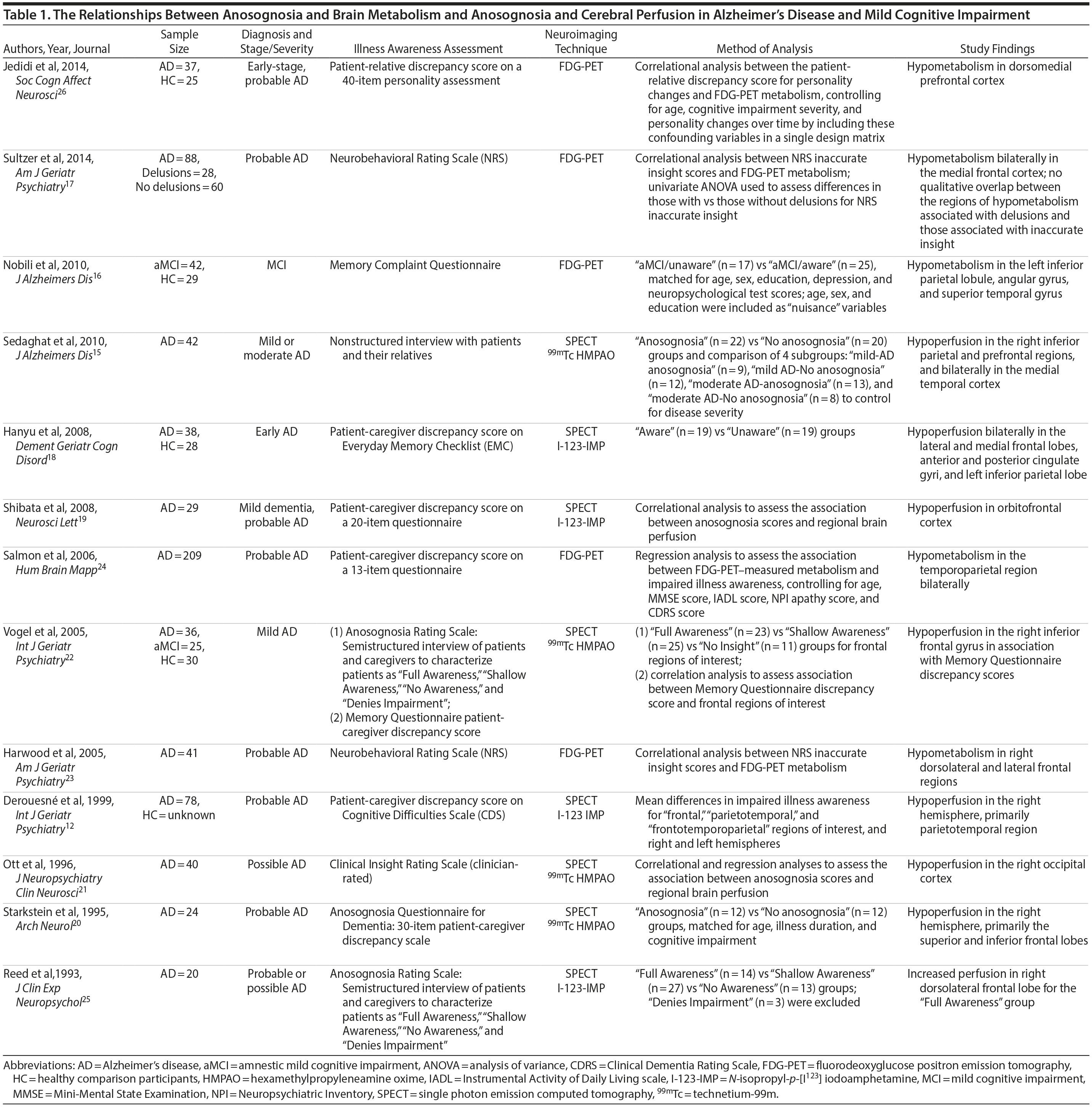
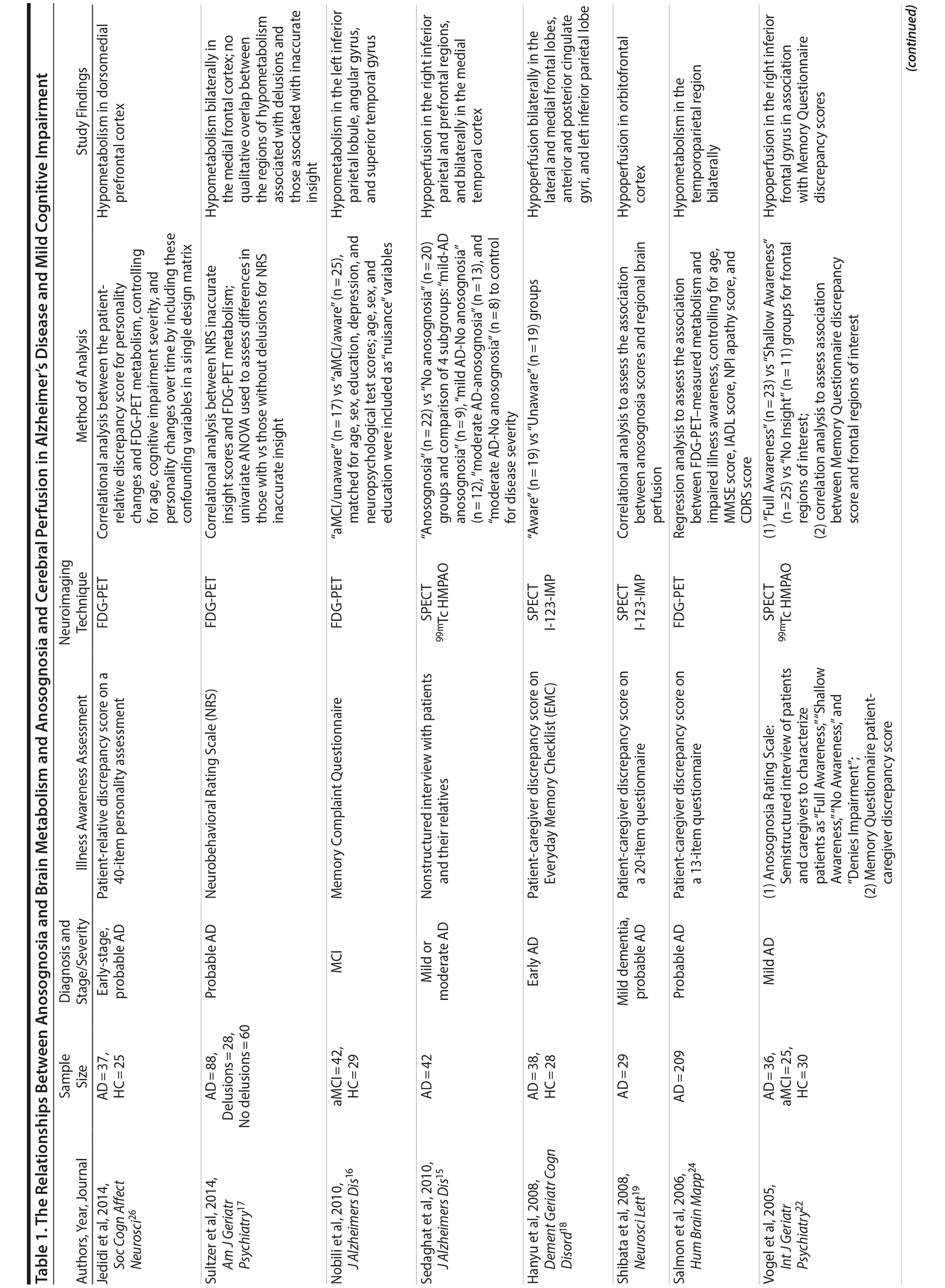
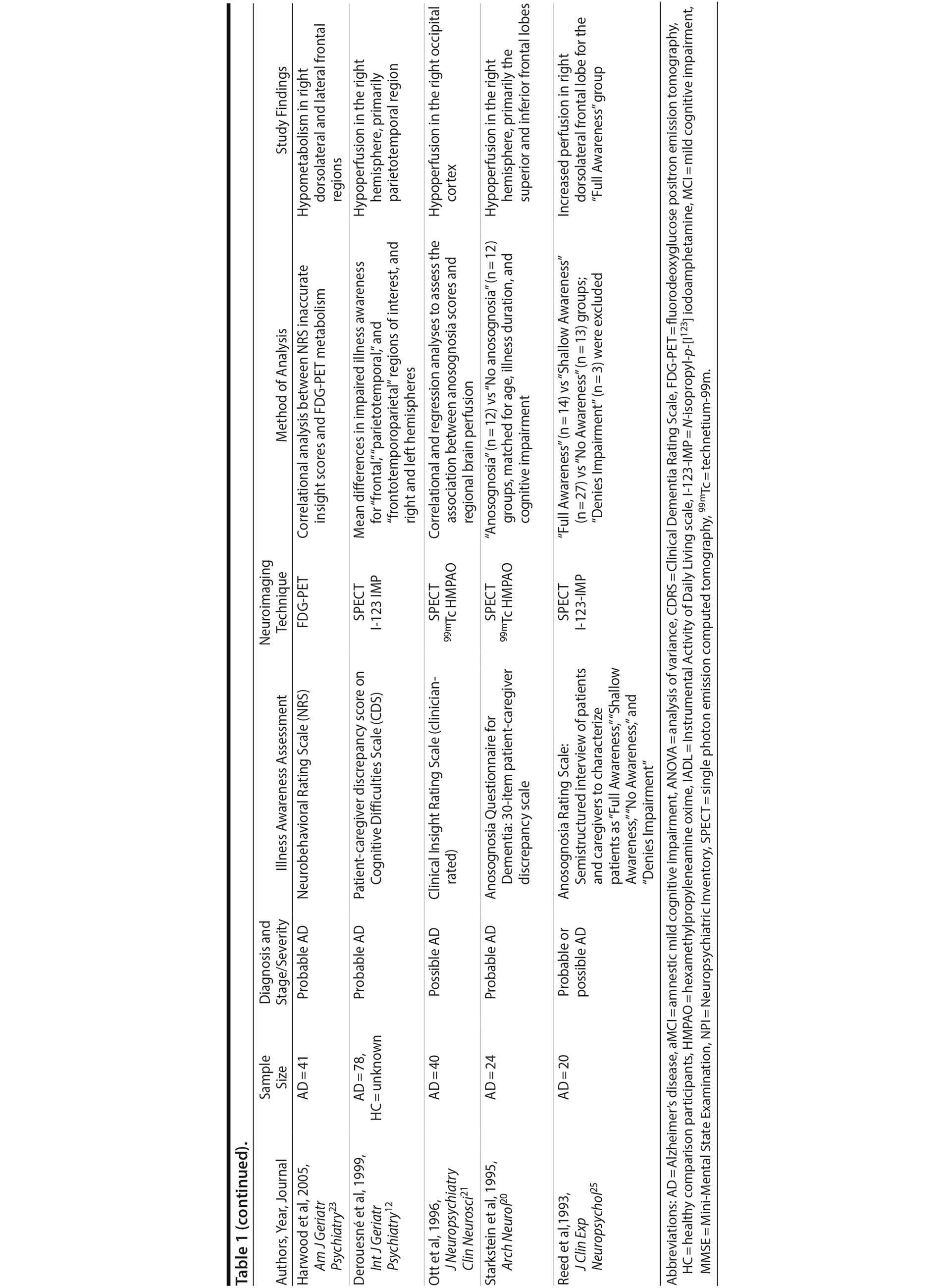
Anosognosia, or impaired illness awareness, is common in Alzheimer’s disease (AD) and to a lesser degree in mild cognitive impairment (MCI), becoming more frequent with progressive cognitive decline.1 Anosognosia has implications for clinical, functional, and quality-of-life outcomes for patients and their caregivers and is a factor associated with treatment nonadherence.2-4 Whereas intact illness awareness may contribute to depressed mood and reduced quality of life in patients with moderate-to-severe dementia, anosognosia is associated with disinhibition, dangerous behaviors, and caregiver burden.1,5,6 Less is known about the prevalence and clinical impact of anosognosia in MCI.3,7,8 Intriguingly, in comparison with patients with mild AD, those with MCI tend to overestimate cognitive impairment in relation to their caregiver’s assessment,9,10 which suggests anosognosia may be a predictor of conversion from MCI to AD.2,11 For example, anosognosia may distinguish MCI patients whose awareness of cognitive symptoms reflects hypervigilance versus MCI patients who are pathologically unaware due to neurodegeneration in brain regions implicated in anosognosia.12 In support of this hypothesis, a longitudinal study found that baseline informant data were a better predictor of later conversion to dementia than were participants’ subjective memory complaints.13
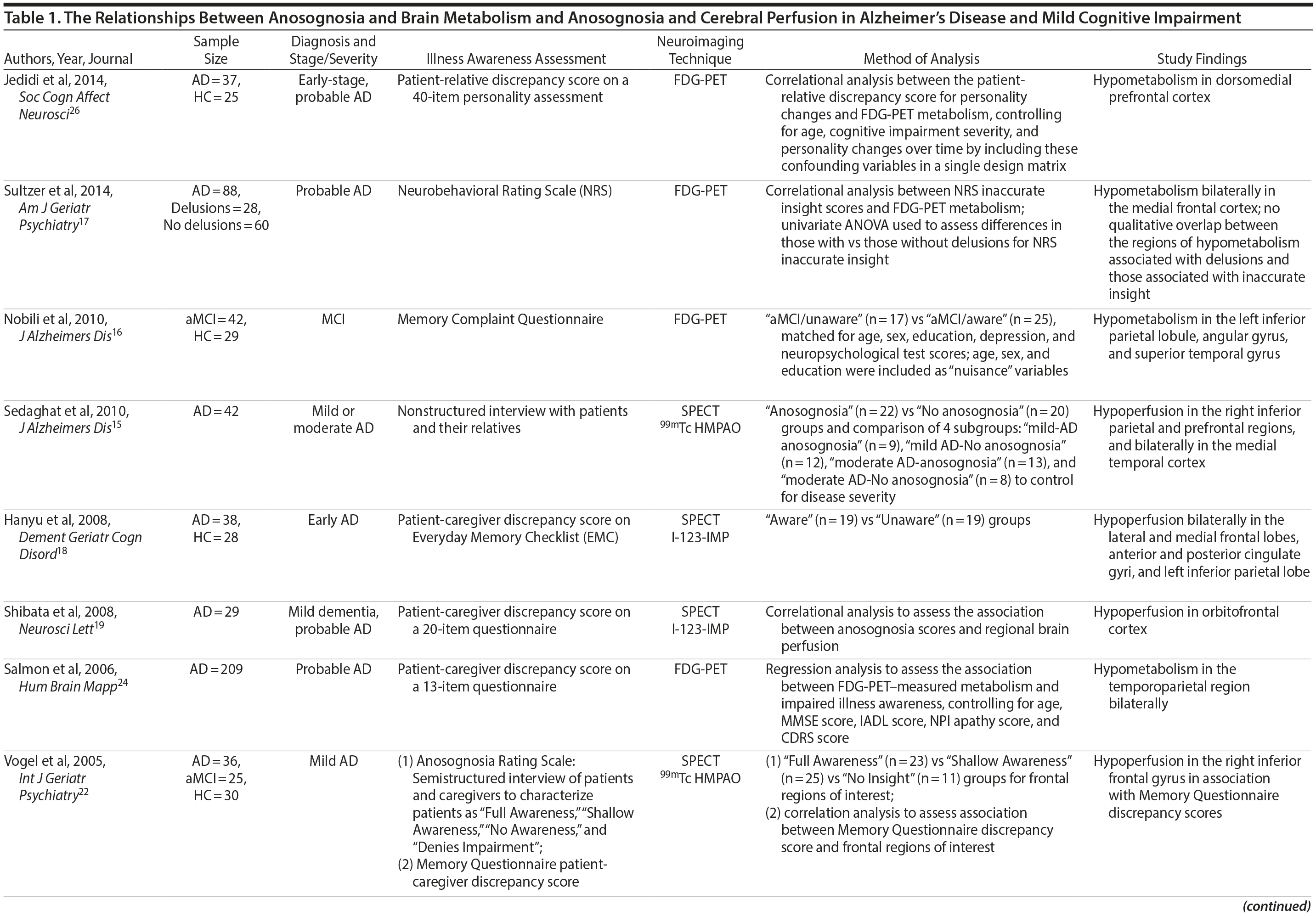
Abnormal brain hypometabolism, particularly within the temporal, parietal, and posterior cingulate cortices, is one of the few biomarkers that can aid in the diagnosis of AD and MCI due to AD and identify those in the preclinical stages of the disease.14 Imaging studies investigating the neurologic correlates of anosognosia in AD have generally found an association with brain metabolism or cerebral perfusion, as measured by positron emission tomography (PET) or single photon emission computerized tomography (SPECT), respectively. These investigations implicate a number of regions within the cingulate cortex and frontal, parietal, and temporal lobes.12,15-24 However, few studies to date have included patients with MCI and healthy comparison (HC) groups16,22 (Table 1). Lesion studies of stroke patients suggest anosognosia is related to right cerebral hemisphere hypometabolism or perfusion, particularly within the parietal lobe and typically in association with visuospatial hemineglect.27 Similarly, studies of neurodegenerative disorders indicate an association between anosognosia and brain hypometabolism or perfusion in the right hemisphere, but possibly with less specificity for hemispheric lateralization than in patients with stroke.15,18,27
With a large sample, the aims of the current study were to examine (1) the relationship between brain glucose metabolism as measured by fluorine-18 fluorodeoxyglucose PET (FDG-PET) and anosognosia in patients with MCI and AD when controlling for other factors that may be related to brain glucose metabolism or anosognosia in AD, such as age, gender, education, apolipoprotein E ε4 (ApoE4) carrier status, dementia severity, and cognitive dysfunction; and (2) the predictive utility of anosognosia in patients with MCI for later conversion to AD when controlling for brain glucose metabolism and the other aforementioned factors.
METHODS
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). ADNI was launched in 2003 with the primary goal of combining serial MRI, PET, other biological markers, and clinical and neuropsychological assessments to measure the progression of MCI and early AD. The entire dataset was downloaded from ADNI-1, ADNI-2, and ADNI Grand Opportunity (ADNI-GO) databases on August 7, 2015.28-30 The region-of-interest (ROI)-based FDG-PET dataset that we used was updated as of July 30, 2015. Data for 1,062 participants were analyzed and consisted of the following classifications: AD (n = 191), MCI (n = 499), and HC (n = 372) (Figure 1). Eligibility criteria for ADNI, ADNI-2, and ADNI-GO are identical.29,30 Each subject was either an English or a Spanish speaker, was between 55 and 90 years of age, and had a study partner able to provide an independent evaluation of functioning.29 For our current study, we included only participants with an assessment of anosognosia and an FDG-PET scan. Additionally, MCI participants were included if they had at least 1 follow-up diagnostic assessment within 5 years to determine their conversion status (Figure 1). The diagnostic inclusion criteria for MCI were early MCI, late MCI, normal-to-MCI, and dementia-to-MCI, and the diagnostic inclusion criteria of AD were dementia, MCI-to-dementia, and normal-to-dementia, as defined by the ADNI group. Full details on the inclusion criteria are available in ADNI study protocols at the aforementioned website. In summary, HC participants had Mini-Mental State Examination (MMSE) scores between 24 and 30 (inclusive) and a Clinical Dementia Rating (CDR) of 0 and were nondepressed, non-MCI, and nondemented. MCI participants had MMSE scores between 24 and 30 (inclusive), a memory complaint, objective memory loss measured by education adjusted scores on Wechsler Memory Scale Logical Memory II, a CDR of 0.5, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. Lastly, mild AD participants had MMSE scores between 20 and 26 and a CDR of 0.5 or 1.0 and met NINCDS/ADRDA (National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association) criteria for probable AD. To meet inclusion criteria for follow-up, participants must have been originally diagnosed with either MCI or as HC ("Cognitively Normal") and have been willing and able to continue to participate. Participants were asked to continue the trial even if a diagnostic conversion occurred or they were no longer willing and able to continue with neuroimaging or lumbar puncture procedures.
Study Assessments
Assessments were obtained from ADNI-1, ADNI-2, and ADNI-GO databases. Anosognosia was assessed using a caregiver-patient discrepancy strategy,31 a validated approach to assessing anosognosia in dementia in which the information provided by the caregiver is the standard against which the patient’s report is compared.32 Although this approach is limited by the accuracy of the caregivers’ report, clinicians’ assessments are correlated with caregivers’ reports and do not appear to provide meaningful additional information.32,33 Study partner-patient discrepancy scores consisted of the composite score (ECog-composite) of the Everyday Cognition-Study Partner Report (ECog-PR) and the Everyday Cognition-Participant Self-Report (ECog-SR).34 The ECog scales cover 6 cognitive domains that consist of "Everyday" memory, language, visuospatial abilities, planning, organization, and divided attention. Item ratings are made on a 4-point scale: 1 = better or no change compared to 10 years earlier, 2 = questionable/occasionally worse, 3 = consistently a little worse, 4 = consistently much worse. A total ECog score is calculated separately for the study partner report and the participant self-report and consists of the sum of all completed items divided by the number of items completed. Thus, total scores range from 1 to 4. ECog-composite scores were calculated as follows: total ECog-PR minus total ECog-SR (ie, ECog-composite = ECog-PR – Ecog-SR). Higher positive scores represent greater anosognosia, whereas lower negative scores indicate greater self-perceived illness.

- Anosognosia or impaired illness awareness is a common feature of Alzheimer’s disease (AD) and less so of mild cognitive impairment (MCI) that negatively influences clinical outcomes for patients and their caregivers and may predict the conversion from MCI to AD.
- Brain glucose hypometabolism as measured by fluorine-18 fluorodeoxyglucose positron emission tomography was independently associated with anosognosia in AD, particularly in the posterior cingulate cortex and right angular gyrus.
- Anosognosia was an independent predictor of conversion from MCI to AD within 5 years. The absence of anosognosia may be clinically useful to identify those patients that are unlikely to convert from MCI to AD.
AD severity was assessed using the Alzheimer’s Disease Assessment Scale (ADAS) 11- and 13-item versions35 and the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), which is a summation of disease severity and functional domain scores.36 Global cognition was assessed using the MMSE and the Montreal Cognitive Assessment (MoCA).37 Verbal memory performance was assessed using the Rey Auditory Verbal Learning Test (RAVLT),38 including the immediate recall, learning, forgetting, and percentage of forgetting scores.39 Higher scores for immediate recall and learning and lower scores for forgetting and percentage of forgetting indicate better verbal memory. Overall function was assessed using the Functional Activities Questionnaire (FAQ).40 PET scans were performed within approximately 2 weeks of the clinical testing sessions.41
FDG-PET Scans and ROI Generation
PET images were acquired from multiple centers using a standardized approach. Details of the ADNI PET data acquisition protocol and image preprocessing are publicly available on the ADNI website (http://adni.loni.usc.edu/) and are described by Landau and colleagues.41 Predefined ROIs were generated by the ADNI group by identifying regions cited frequently in FDG-PET studies of AD and MCI using a meta-analytic approach. The 5 individual FDG-PET ROI volumes consist of the right and left angular gyri, bilateral posterior cingulate cortex (PCC), and left and right middle/inferior temporal gyrus as well as a combined region created from all 5 subregions.41
Statistical Analysis
Demographic, clinical, cognitive, and FDG-PET data. Statistical analyses of demographic, baseline clinical, and FDG-PET ROI variables were carried out with PASW software (PASW Statistics for Windows, Version 18.0. Released 2009. Chicago, Illinois: SPSS Inc). Means and standard deviations were calculated for each variable for HC participants and patients with AD or MCI. Bivariate Pearson correlations were performed between anosognosia (ECog-composite) scores and relevant demographic, clinical, cognitive, and FDG-PET variables. Tests of mean differences and analyses of variance (ANOVAs) were used for group analyses when appropriate. The significance level for tests was established at P ≤ .05 with Bonferroni correction for multiple testing (P < .001).
Relationship of FDG-PET-measured metabolism to anosognosia in MCI and AD. To determine the relationship between FDG-PET-measured metabolism and anosognosia, a series of multiple linear regression analyses were performed after confirming the data met the necessary assumptions. Anosognosia (ECog-composite score) was used as the dependent variable. FDG-PET ROI values were separately entered as the predictor variable. Age, education, gender, ApoE4 carrier status, dementia severity (CDR-SB score), and cognitive impairment (MoCA score) were included as covariates in a stepwise manner.
Anosognosia as a predictor of conversion from MCI to AD. A logistic regression analysis was performed in the MCI sample to determine the odds that anosognosia (ECog-composite score) predicts conversion from MCI to AD after confirming the data met the necessary assumptions. Follow-up data were available for 499 of the 525 MCI participants up to 5 years after participants’ baseline assessment. At the time of this analysis, the mean (SD) duration of follow-up or conversion to AD was 2.5 (1.0) years (range, 0.5 to 5 years). The last reported diagnosis was used to determine participants’ conversion status. The aforementioned covariates, in addition to the duration of follow-up and FDG-PET combined ROI values, were added to the analysis.
A receiver operating characteristic (ROC) curve analysis was performed to determine the specificity and sensitivity of anosognosia scores for predicting conversion from MCI to AD.
RESULTS
Demographic and Clinical Data
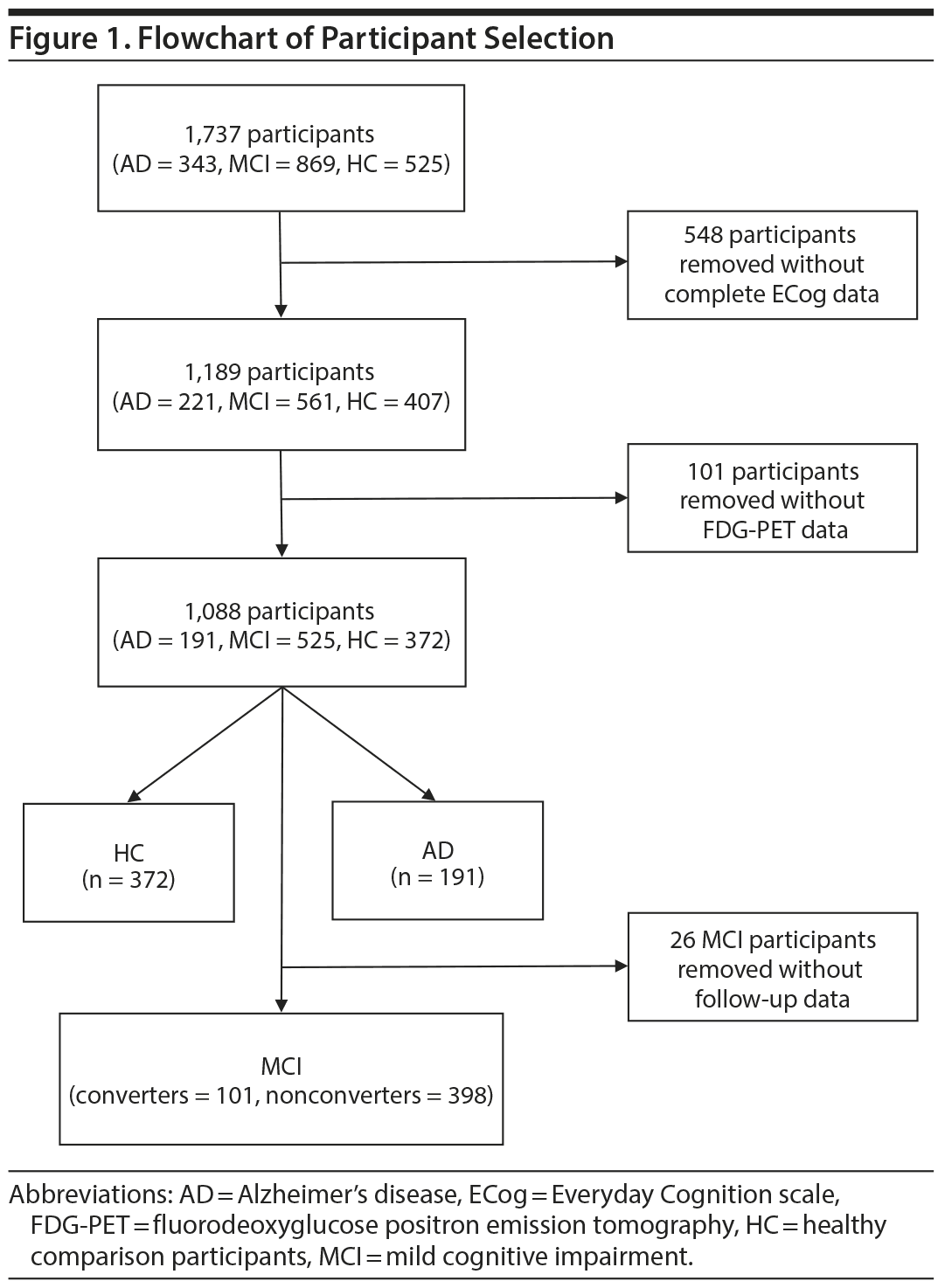
The demographic and clinical data are presented in Table 2. Within 5 years of follow-up, 73.1% of participants (n = 384) remained classified as having MCI, 19.2% (n = 101) converted to "dementia," and 2.7% (n = 14) reverted to "normal." Therefore, 75.8% (n = 398) were identified as MCI-Nonconverters and 19.2% (n = 101) as MCI-Converters.
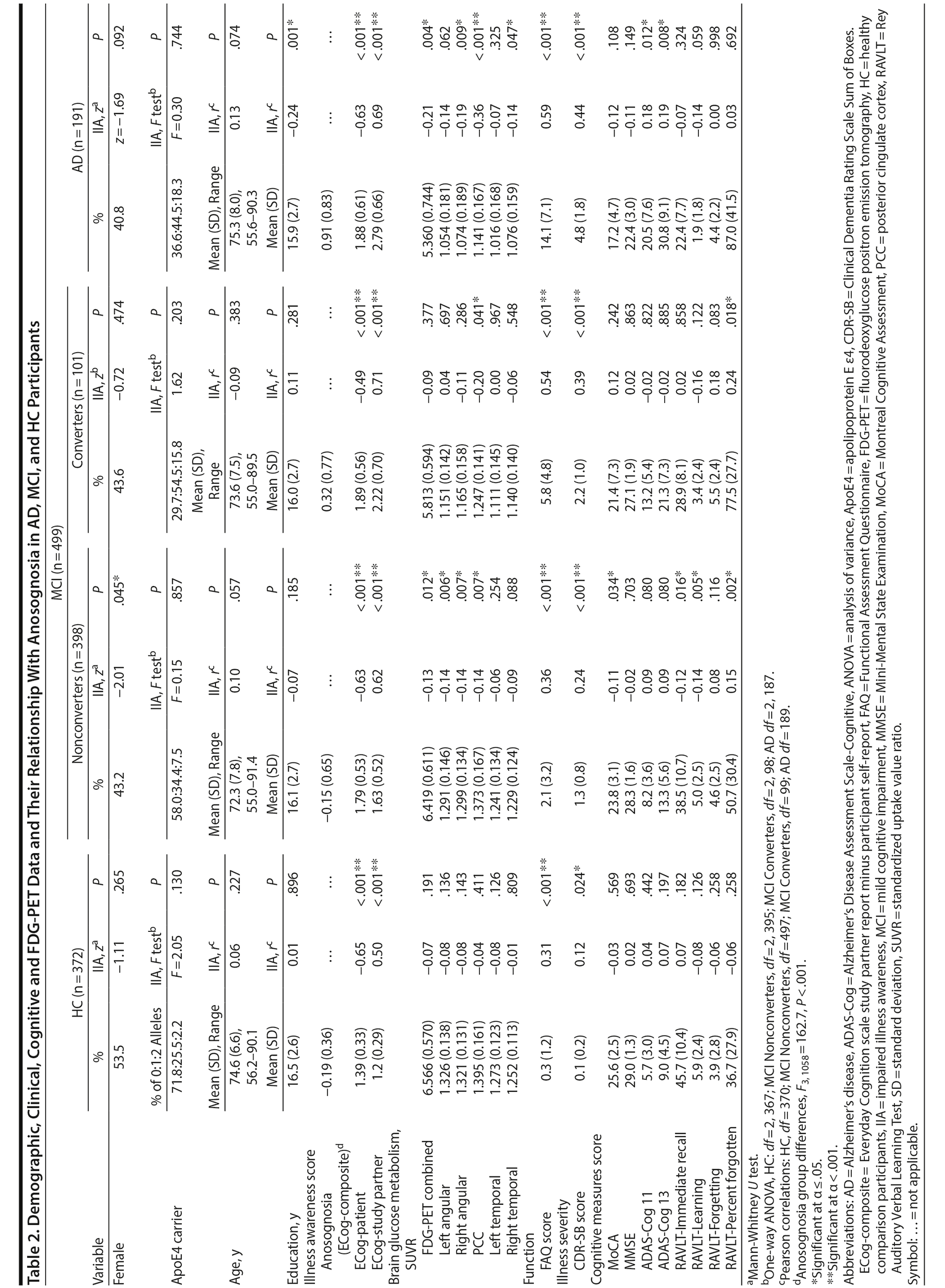
There were mean group differences for anosognosia scores (F3,1058 = 162.7, P < .001). Anosognosia was higher in AD subjects than in MCI-Converters (0.60 [95% CI, 0.39 to 0.80], P < .001), MCI-Nonconverters (1.07 [95% CI, 0.93 to 1.22], P < .001), and HC participants (1.11 [95% CI, 0.96 to 1.25], P < .001). Anosognosia was also higher in MCI-Converters than in MCI-Nonconverters (0.47 [95% CI, 0.29 to 0.66], P < .001) and HC participants (0.51 [95% CI, 0.33 to 0.70], P < .001). Lastly, there was no difference in anosognosia scores between MCI-Nonconverters and healthy participants (0.04 [95% CI, −0.08 to 0.16], P = 1.000).
In HC participants, there was no association between anosognosia and demographic, clinical, and cognitive measures (Table 2). Higher ECog-composite scores were associated with worse overall function (ie, FAQ). Similarly, higher ECog-composite scores were modestly associated with dementia severity (ie, CDR-SB); however, this did not survive Bonferroni correction for multiple testing.
In both MCI-Converters and MCI-Nonconverters, higher anosognosia scores were associated with worse overall function (ie, FAQ scores) and dementia severity (ie, CDR-SB scores). Anosognosia was also associated with cognitive impairment, including MoCA, RAVLT-Immediate recall, RAVLT-Learning, and RAVLT-Percent forgotten scores in MCI-Nonconverters, but not after Bonferroni correction for multiple testing.
In AD, similar to MCI, higher ECog-composite scores were associated with worse overall function (ie, FAQ) and dementia severity (ie, CDR-SB). Anosognosia was also associated with cognitive impairment, specifically ADAS-Cog scores, and negatively associated with education; however, these associations did not survive Bonferroni correction for multiple testing.
Relationship Between FDG-PET Metabolism and Anosognosia in AD and MCI
As expected, in the HC group, FDG-PET-measured metabolism was not associated with ECog-composite scores in the combined or any other ROI (Table 2, Supplementary eTable 1, and Figure 2).
In MCI-Nonconverters, anosognosia was associated with FDG-PET-measured hypometabolism in the combined, PCC, and angular ROIs, while in the MCI-Converters, anosognosia was associated with FDG-PET-measured hypometabolism only in the PCC ROI (Table 2 and Figure 2). However, none of these associations remained after including covariates (Supplementary eTable 1).
Lastly, in the AD group, anosognosia was associated with FDG-PET-measured hypometabolism in the combined, PCC (Figure 2), right angular, and right temporal ROIs (Table 2) and in the combined and PCC ROIs after including covariates (Supplementary eTable 1).
Anosognosia as a Predictor of Conversion From MCI to AD
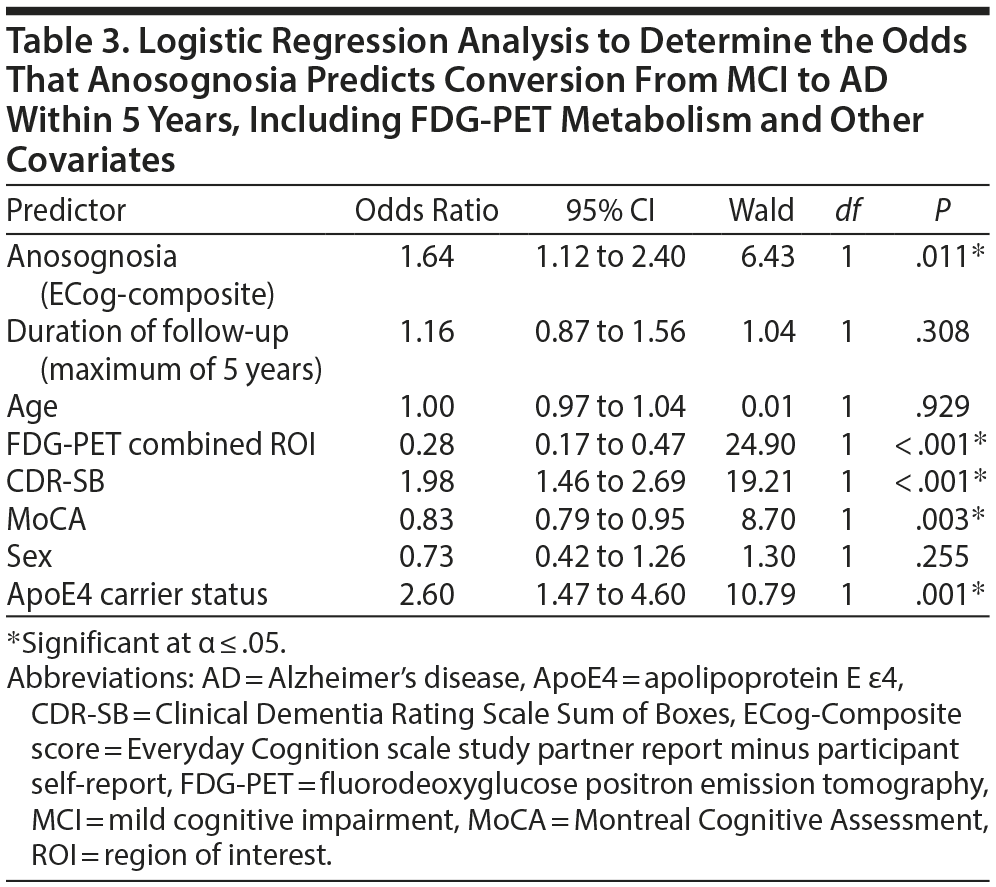
For the logistic regression analyses, anosognosia (ECog-composite scores) was associated with increased odds of converting from MCI to AD within 5 years (OR = 2.74 [95% CI, 1.95 to 3.85], χ21 = 33.65, P < .001). In the initial model, anosognosia as the sole independent variable explained 11.4% (Nagelkerke R2) of the variance in MCI-Converters versus MCI-Nonconverters and correctly classified 80.8% of cases. Sensitivity was 8.9%, specificity was 99.0%, positive predictive value was 69.2%, and negative predictive value was 81.1% for this model. In the second model, anosognosia remained associated with increased odds of converting from MCI to AD after including the covariates (OR = 1.64 [95% CI, 1.12 to 2.40], χ21 = 6.43, P = .011) (Table 3). The model explained 40.4% of the variance in MCI-Converters versus MCI-Nonconverters and correctly classified 83.7% of cases. In addition to anosognosia, ApoE4 carrier status, FDG-PET combined ROI, CDR-SB score, and MoCA score were predictors of converting from MCI to AD.
The area under the curve for the ROC analysis of the ECog-composite score’s ability to predict progression from MCI to AD was 0.668 (SE = 0.31 [95% CI, 0.606 to 0.729]). The coordinates of the curve are presented in Supplementary eTable 2. A single cutoff score of 0 is 61% sensitive and 64% specific for conversion from MCI to AD within 5 years, which is of little clinical utility. By comparison, ECog-composite scores ≤ −0.75 were 93% sensitive and 15% specific for conversion from MCI to AD. In other words, of the 72 of 525 MCI participants with baseline anosognosia scores ≤ −0.75, 61 were MCI-Nonconverters (84.7%) and 7 (9.7%) were MCI-Converters to AD within 5 years of follow-up. Interestingly, of the 23 of 525 MCI participants with baseline anosognosia scores ≤ −1.25, 22 (95.7%) were MCI-Nonconverters.
DISCUSSION
To the best of our knowledge, this study is the largest to date investigating the association between anosognosia and FDG-PET-measured brain metabolism in AD, MCI, and HC participants and the predictive utility of anosognosia for identifying patients with MCI that will progress to AD. Our study revealed 3 main results. First, FDG-PET-measured brain hypometabolism was associated with anosognosia in AD even when controlling for variables commonly associated with reduced brain metabolism and anosognosia. Second, anosognosia was an independent predictor of conversion from MCI to AD within 5 years. Third, anosognosia was associated with greater cognitive impairment, dementia severity, and functional impairment in MCI and AD.8,42,43 Intriguingly, functional impairment and dementia severity were also associated with the degree of anosognosia in HC participants.
FDG-PET-measured hypometabolism is one of the few biomarkers available to substantiate the diagnosis of AD and differentiate it from other neurodegenerative disorders and brain lesions.41 Our results suggest FDG-PET-measured hypometabolism is related to anosognosia in AD, particularly in the PCC, and to a lesser degree, the right angular gyrus (Supplementary eTable 1). This finding is consistent with prior literature that suggests anosognosia or impaired illness awareness is associated with brain hypometabolism or hypoperfusion, even when controlling for the confounders of cognitive dysfunction or dementia severity.15,18,20,22-26 In both SPECT and FDG-PET studies of AD, anosognosia is associated with hypoperfusion or hypometabolism in multiple brain regions within the frontal,15,18,22-25 parietal,15,18,24 cingulate,18 and temporal cortices15,24 (Table 1). Contrary to our finding, the only prior FDG-PET study of anosognosia in amnestic MCI (n = 42) found that anosognosia was associated with reduced brain metabolism in the left angular region.16 However, our results, in a much larger sample of MCI participants, indicate anosognosia is more generally associated with brain hypometabolism in the PCC and, to a lesser degree, both angular gyri (Table 1), but not after inclusion of covariates (Supplementary eTable 1). We suspect that anosognosia was not independently associated with brain hypometabolism in MCI participants as it was likely overshadowed by the degree of cognitive dysfunction, but later emerged in AD independent of cognitive dysfunction with the progression of neurodegeneration.
Based on the diversity of brain regions associated with anosognosia in MCI and AD, it is difficult to come to any definitive conclusions about the underlying neural correlates. Our results of lower FDG-PET-measured metabolism in the PCC and right hemisphere, particularly the right angular gyrus, in association with anosognosia in AD is consistent with existing structural brain lesion and functional imaging studies attributing impaired illness awareness to right hemisphere dysfunction relative to the left, ultimately resulting in left hemisphere dominance.27 While the posterior parietal cortex, which includes the angular gyrus, is primarily associated with visuospatial reasoning and attention, it is also related to a range of other cognitive functions that may be implicated in illness awareness44,45 and establishing a coherent sense of self or self-awareness.46-50
Anosognosia was an independent predictor of conversion from MCI to AD within 5 years, with an odds ratio of 2.74 (1.64 after controlling for confounding variables) (Table 3). Moreover, ECog-composite scores ≤ −0.75 were 93% sensitive and 15% specific for conversion from MCI to AD, which suggests this cutoff could be used clinically to identify those patients who subjectively report cognitive impairment (eg, in the context of anxiety and mood disorders), but who are unlikely to convert from MCI to AD in 5 years (Supplementary eTable 2 and Figure 2). Even lower anosognosia scores demonstrated greater confidence in ruling out conversion from MCI to AD. These results are consistent with prior studies that identified discrepancy between caregiver and self-reported cognitive and functional impairment to be a predictor of conversion from MCI to AD.11,51 Relatedly, a longitudinal study found that baseline informant data better predicted later conversion to AD than participants’ subjective cognitive complaints.13
The results of the present study also substantiate prior findings of the association of anosognosia with cognitive dysfunction, dementia severity, and functional impairment.8,42,43 In the literature, illness awareness is associated with depressed mood and reduced quality of life.1 Intriguingly, any protective effects of anosognosia in preventing reduced quality of life and symptoms of depression appear to come at the expense of greater functional impairment and increased caregiver burden.1,6,42,43
Our study is limited by a few factors. First, as with other ADNI studies, our investigation relies on clinical diagnoses of MCI and AD for group classification of participants. As a result, the prediction models of conversion from MCI to AD are limited by the accuracy of the clinical classifications. However, although imperfect, clinical assessments are often more predictive of progression of cognitive decline than available biomarkers.52 Second, although a caregiver-patient discrepancy strategy is a consistently used approach for the assessment of anosognosia in dementia (Table 1),31 there are inherent flaws in relying on a caregiver’s report as a single proxy, including lack of knowledge, denial of the patient’s dysfunction, or exaggeration due to caregiver distress. However, a clinician’s assessment is similarly biased and may be influenced by a lack of adequate exposure to the patient’s deficits and behavior, a propensity to pathologize behavior, or a lack of knowledge of dementia. Interestingly, although a caregiver-patient discrepancy strategy is limited by the accuracy of the caregivers’ report, clinicians’ assessments are correlated with caregivers’ reports and do not appear to provide meaningful additional information.32,33 Third, our study lacked the inclusion of variables that may possibly influence conversion rates, anosognosia scores, or the relationship between anosognosia and FDG-PET-measured metabolism, such as metabolic factors, mood symptoms, and history of other neuropsychiatric illness. That being said, the influence of these variables is most likely accounted for by measures of cognitive impairment and dementia severity. Future studies should consider incorporating these possible confounding variables. Fourth, our FDG-PET analysis was restricted to the ADNI group’s predefined FDG-PET ROIs, which consist of right and left angular gyri, bilateral PCC, and left and right middle/inferior temporal gyri, as well as a combined region created from all 5 subregions.41 The ADNI group selected these ROIs as they are the regions most frequently cited as determined by a meta-analytic approach of FDG-PET studies in AD and MCI.41 Notably absent were frontal regions, which are regularly implicated in brain metabolic and perfusion studies of anosognosia (Table 1). Future neuroimaging biomarker studies of FDG-PET in AD and MCI using large samples should consider including frontal ROIs or adopting a whole brain analytic approach to identify subregions associated with anosognosia.
In summary, anosognosia is related to reduced brain metabolism in AD, particularly in the PCC and, to a lesser degree, the right angular gyrus. Clinically, anosognosia, as measured by the discrepancy between caregiver and patient reports, independently predicts conversion from MCI to AD. Using a prospective longitudinal design, future studies should test the predictive utility of anosognosia scores to facilitate clinical decision-making in MCI, and also HC participants, for whom we found an association between anosognosia and functional impairment and, to a lesser degree, dementia severity. Specifically, anosognosia scores may be beneficial in identifying those MCI patients who may have another etiology for their cognitive impairment or require careful monitoring.
Submitted: November 29, 2016; accepted March 6, 2017.
Published online: October 10, 2017.
Potential conflicts of interest: Dr Gerretsen has received fellowship awards from Canadian Institutes of Health Research (CIHR), Ontario Mental Health Foundation (OMHF), and Centre for Addiction and Mental Health (CAMH). Dr Nakajima has received fellowship grants from the Canadian CIHR, Japan Society for the Promotion of Science, and Nakatomi Foundation and has received manuscript fees from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin. Dr Pollock has received research support from the National Institutes of Health (NIH) and CIHR. Dr Graff-Guerrero has received support from Brain Canada, Canadian Foundation for Innovation, CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US NIH, OMHF, Consejo Nacional de Ciencia y Tecnologia (CONACyT), Instituto de Ciencia y Tecnolog×a del DF (ICyTDF), and Brain & Behavior Research Foundation and reports no competing interests. Drs Iwata and Caravaggio, Ms Shah, Mr Chung, and Mr Plitman report no conflicts of interest.
Funding/support: The research was partially supported by OMHF -Type A Grant (Dr Graff-Guerrero); NIH RO1MH084886-01A2 (Dr Graff-Guerrero); and by CIHR, OMHF, and CAMH fellowship awards (Dr Gerretsen).
Role of the sponsor: The listed funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and or preparation, review, or approval of the manuscript.
Acknowledgments: Data collection and sharing for this project were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) NIH Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica; Biogen; Bristol-Myers Squibb; CereSpir; Cogstate; Eisai; Elan Pharmaceuticals; Eli Lilly; EuroImmun; F. Hoffmann-La Roche and its affiliated company Genentech; Fujirebio; GE Healthcare; IXICO; Janssen Alzheimer Immunotherapy Research & Development; Johnson & Johnson Pharmaceutical Research & Development; Lumosity; Lundbeck; Merck; Meso Scale Diagnostics; NeuroRx Research; Neurotrack Technologies; Novartis; Pfizer; Piramal Imaging; Servier; Takeda; and Transition Therapeutics. CIHR is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data used in preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Supplementary material: See accompanying pages.
REFERENCES
1. Kashiwa Y, Kitabayashi Y, Narumoto J, et al. Anosognosia in Alzheimer’s disease: association with patient characteristics, psychiatric symptoms and cognitive deficits. Psychiatry Clin Neurosci. 2005;59(6):697-704. PubMed doi:10.1111/j.1440-1819.2005.01439.x
2. Weston A, Barton C, Lesselyong J, et al. Functional deficits among patients with mild cognitive impairment. Alzheimers Dement. 2011;7(6):611-614. PubMed doi:10.1016/j.jalz.2010.12.011
3. Mak E, Chin R, Ng LT, et al. Clinical associations of anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2015;30(12):1207-1214. PubMed doi:10.1002/gps.4275
4. Hurt CS, Banerjee S, Tunnard C, et al; AddNeuroMed Consortium. Insight, cognition and quality of life in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2010;81(3):331-336. PubMed doi:10.1136/jnnp.2009.184598
5. Starkstein SE, Jorge R, Mizrahi R, et al. Insight and danger in Alzheimer’s disease. Eur J Neurol. 2007;14(4):455-460. PubMed
6. Turró-Garriga O, Garre-Olmo J, Vilalta-Franch J, et al. Burden associated with the presence of anosognosia in Alzheimer’s disease. Int J Geriatr Psychiatry. 2013;28(3):291-297. PubMed doi:10.1002/gps.3824
7. Spalletta G, Girardi P, Caltagirone C, et al. Anosognosia and neuropsychiatric symptoms and disorders in mild Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2012;29(4):761-772. PubMed
8. Orfei MD, Varsi AE, Blundo C, et al. Anosognosia in mild cognitive impairment and mild Alzheimer’s disease: frequency and neuropsychological correlates. Am J Geriatr Psychiatry. 2010;18(12):1133-1140. PubMed doi:10.1097/JGP.0b013e3181dd1c50
9. Kalbe E, Salmon E, Perani D, et al. Anosognosia in very mild Alzheimer’s disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord. 2005;19(5-6):349-356. PubMed doi:10.1159/000084704
10. Albert SM, Michaels K, Padilla M, et al. Functional significance of mild cognitive impairment in elderly patients without a dementia diagnosis. Am J Geriatr Psychiatry. 1999;7(3):213-220. PubMed doi:10.1097/00019442-199908000-00005
11. Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758-764. PubMed doi:10.1212/WNL.58.5.758
12. Derouesné C, Thibault S, Lagha-Pierucci S, et al. Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. Int J Geriatr Psychiatry. 1999;14(12):1019-1030. PubMed doi:10.1002/(SICI)1099-1166(199912)14:12<1019::AID-GPS61>3.0.CO;2-F
13. Slavin MJ, Sachdev PS, Kochan NA, et al. Predicting cognitive, functional, and diagnostic change over 4 years using baseline subjective cognitive complaints in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2015;23(9):906-914. PubMed doi:10.1016/j.jagp.2014.09.001
14. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed doi:10.1016/j.jalz.2011.03.005
15. Sedaghat F, Dedousi E, Baloyannis I, et al. Brain SPECT findings of anosognosia in Alzheimer’s disease. J Alzheimers Dis. 2010;21(2):641-647. PubMed
16. Nobili F, Mazzei D, Dessi B, et al. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J Alzheimers Dis. 2010;22(3):993-1003. PubMed doi:10.3233/JAD-2010-100423
17. Sultzer DL, Leskin LP, Melrose RJ, et al. Neurobiology of delusions, memory, and insight in Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(11):1346-1355. PubMed doi:10.1016/j.jagp.2013.06.005
18. Hanyu H, Sato T, Akai T, et al. Neuroanatomical correlates of unawareness of memory deficits in early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(4):347-353. PubMed doi:10.1159/000119594
19. Shibata K, Narumoto J, Kitabayashi Y, et al. Correlation between anosognosia and regional cerebral blood flow in Alzheimer’s disease. Neurosci Lett. 2008;435(1):7-10. PubMed doi:10.1016/j.neulet.2008.01.065
20. Starkstein SE, Vázquez S, Migliorelli R, et al. A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Arch Neurol. 1995;52(4):415-420. PubMed
21. Ott BR, Noto RB, Fogel BS. Apathy and loss of insight in Alzheimer’s disease: a SPECT imaging study. J Neuropsychiatry Clin Neurosci. 1996;8(1):41-46. PubMed doi:10.1176/jnp.8.1.41
22. Vogel A, Hasselbalch SG, Gade A, et al. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20(3):238-246. PubMed doi:10.1002/gps.1272
23. Harwood DG, Sultzer DL, Feil D, et al. Frontal lobe hypometabolism and impaired insight in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(11):934-941. PubMed doi:10.1097/00019442-200511000-00003
24. Salmon E, Perani D, Herholz K, et al. Neural correlates of anosognosia for cognitive impairment in Alzheimer’s disease. Hum Brain Mapp. 2006;27(7):588-597. PubMed doi:10.1002/hbm.20203
25. Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer’s disease: relationships to depression, cognitive function, and cerebral perfusion. J Clin Exp Neuropsychol. 1993;15(2):231-244. PubMed doi:10.1080/01688639308402560
26. Jedidi H, Feyers D, Collette F, et al. Dorsomedial prefrontal metabolism and unawareness of current characteristics of personality traits in Alzheimer’s disease. Soc Cogn Affect Neurosci. 2014;9(10):1458-1463. PubMed doi:10.1093/scan/nst132
27. Orfei MD, Robinson RG, Bria P, et al. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14(2):203-222. PubMed doi:10.1177/1073858407309995
28. Alzheimer’s Disease Neuroimaging I. ADNI Website. 2014. http://adni.loni.usc.edu/methods/documents/. Accessed September 19, 2017.
29. Alzheimer’s Disease Neuroimaging I. ADNI General Procedures Manual. 2010. http://adni.loni.usc.edu/methods/documents/. Accessed September 19, 2017.
30. Alzheimer’s Disease Neuroimaging I. ADNI GO Procedures Manual. 2008. http://adni.loni.usc.edu/methods/documents/. Accessed September 19, 2017.
31. Starkstein SE. Anosognosia in Alzheimer’s disease: diagnosis, frequency, mechanism and clinical correlates. Cortex. 2014;61:64-73. PubMed doi:10.1016/j.cortex.2014.07.019
32. Starkstein SE, Jorge R, Mizrahi R, et al. A diagnostic formulation for anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(6):719-725. PubMed doi:10.1136/jnnp.2005.085373
33. Snow AL, Norris MP, Doody R, et al. Dementia Deficits Scale: rating self-awareness of deficits. Alzheimer Dis Assoc Disord. 2004;18(1):22-31. PubMed doi:10.1097/00002093-200401000-00005
34. Tomaszewski Farias S, Mungas D, Harvey DJ, et al. The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement. 2011;7(6):593-601. PubMed doi:10.1016/j.jalz.2011.02.007
35. Kolibas E, Korinkova V, Novotny V, et al. ADAS-cog (Alzheimer’s Disease Assessment Scale-cognitive subscale)—validation of the Slovak version. Bratisl Lek Listy (Tlacene Vyd). 2000;101(11):598-602. PubMed
36. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173-176, discussion 177-178. PubMed doi:10.1017/S1041610297004870
37. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
38. Rey A. L’ examen clinique en psychologie. Paris, France: Presses Universitaires de France; 1964. [Clinical tests in psychology].
39. Estévez-González A, Kulisevsky J, Boltes A, et al. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr Psychiatry. 2003;18(11):1021-1028. PubMed doi:10.1002/gps.1010
40. Pfeffer RI, Kurosaki TT, Harrah CH Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323-329. PubMed doi:10.1093/geronj/37.3.323
41. Landau SM, Harvey D, Madison CM, et al; Alzheimer’s Disease Neuroimaging Initiative. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207-1218. PubMed
42. Kelleher M, Tolea MI, Galvin JE. Anosognosia increases caregiver burden in mild cognitive impairment. Int J Geriatr Psychiatry. 2016;31(7):799-808. PubMed doi:10.1002/gps.4394
43. Conde-Sala JL, Reñé-Ramírez R, Turró-Garriga O, et al. Severity of dementia, anosognosia, and depression in relation to the quality of life of patients with Alzheimer disease: discrepancies between patients and caregivers. Am J Geriatr Psychiatry. 2014;22(2):138-147. PubMed doi:10.1016/j.jagp.2012.07.001
44. Constantinidis C, Bucci DJ, Rugg MD. Cognitive functions of the posterior parietal cortex. Front Integr Neurosci. 2013;7:35. PubMed
45. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225. PubMed doi:10.1016/j.schres.2007.08.023
46. van der Meer L, Costafreda S, Aleman A, et al. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34(6):935-946. PubMed
47. Vogeley K, Bussfeld P, Newen A, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 pt 1):170-181. PubMed doi:10.1006/nimg.2001.0789
48. Zahn R, Talazko J, Ebert D. Loss of the sense of self-ownership for perceptions of objects in a case of right inferior temporal, parieto-occipital and precentral hypometabolism. Psychopathology. 2008;41(6):397-402. PubMed
49. David N, Bewernick BH, Cohen MX, et al. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. J Cogn Neurosci. 2006;18(6):898-910. PubMed doi:10.1162/jocn.2006.18.6.898
50. Corradi-Dell’ acqua C, Ueno K, Ogawa A, et al. Effects of shifting perspective of the self: an fMRI study. Neuroimage. 2008;40(4):1902-1911. PubMed doi:10.1016/j.neuroimage.2007.12.062
51. Tierney MC, Szalai JP, Snow WG, et al. The prediction of Alzheimer disease: the role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53(5):423-427. PubMed doi:10.1001/archneur.1996.00550050053023
52. Korolev IO, Symonds LL, Bozoki AC; Alzheimer’s Disease Neuroimaging Initiative. Predicting progression from mild cognitive impairment to Alzheimer’s dementia using clinical, mri, and plasma biomarkers via probabilistic pattern classification. PLoS One. 2016;11(2):e0138866. PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite