ABSTRACT
Objective: To determine the extent that treatment with transcranial magnetic stimulation (TMS) in diverse clinical settings has anxiolytic and antidepressant effects in patients with major depressive disorder (MDD) and moderate-to-severe anxiety symptoms and to contrast anxious and nonanxious depression subgroups in antidepressant effects.
Methods: Within the NeuroStar Advanced Therapy System Clinical Outcomes Registry, 1,820 patients were identified with a diagnosis of MDD (using ICD-9, ICD-10, or DSM-IV) who completed the Patient Health Questionnaire-9 (PHQ-9) and Global Anxiety Disoder-7 scale (GAD-7) at baseline and following at least 1 TMS treatment between May 2016 and January 2021. Anxious depression was defined as a baseline GAD-7 score of 10 or greater (n = 1,514) and nonanxious depression by GAD-7 scores below this threshold (n = 306). Intent-to-treat and Completer samples were defined for patients treated with any TMS protocol and for the subgroup treated only with high-frequency left dorsolateral prefrontal cortex stimulation.
Results: Patients with anxious depression showed clinically meaningful anxiolytic and antidepressant effects, averaging approximately 50% or greater reductions in both GAD-7 and PHQ-9 scores following TMS in all samples. The anxious and nonanxious depression groups had equivalent absolute improvement in PHQ-9 scores (P values ≥ .29). However, the anxious group had higher scores both at baseline and following TMS resulting in significantly lower categorical rates of response (P values < .02) and remission (P values < .001) in depressive symptoms. Among those with anxious depression, the change in anxiety and depression symptoms strongly covaried (r1512 = 0.75, P < .001).
Conclusions: Routine TMS delivered in diverse clinical settings results in marked anxiolytic and antidepressant effects in patients with anxious depression. The extent of improvement in anxiety and depression symptoms strongly covaries.
J Clin Psychiatry 2023;84(1):22m14571
To cite: Hutton TM, Aaronson ST, Carpenter LL, et al. The anxiolytic and antidepressant effects of transcranial magnetic stimulation in patients with anxious depression. J Clin Psychiatry. 2023;84(1):22m14571.
To share: https://doi.org/10.4088/JCP.22m14571
© 2023 Physicians Postgraduate Press, Inc.
aSouthern California TMS Center, Los Angeles, California
bSheppard Pratt Health System, Baltimore, Maryland
cDepartment of Psychiatry, University of Maryland, Baltimore, Maryland
dButler Hospital, Providence, Rhode Island
eDepartment of Psychiatry and Human Behavior, Brown University, Providence, Rhode Island
fTMS of South Tampa, Tampa, Florida
gNashville NeuroCare Therapy, Nashville, Tennessee
hNAMSA, St Louis Park, Minnesota
iDepartment of Psychiatry, Columbia University, New York, New York
jDepartment of Radiology, Columbia University, New York, New York
*Corresponding author: Harold A. Sackeim, PhD, 2124 Moselem Springs Rd, Fleetwood, PA 19522 ([email protected]).
Anxiety and depression commonly co-occur.1,2 Anxious depression has been variously defined as the presentation of major depressive disorder (MDD) with a comorbid anxiety disorder, MDD with clinically significant anxiety symptoms, or MDD with the anxious distress specifier, as introduced in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).3 Regardless of definition, the prevalence of anxious depression is high.4,5 National6,7 and multinational8 epidemiologic studies have estimated that approximately 50%–80% of individuals with a lifetime history of MDD also have a history of anxiety disorder. Some studies suggest that the majority of outpatients with MDD present with comorbid generalized anxiety disorder (GAD).9,10 Similarly, the DSM-5 specifier anxious distress applies to approximately 50%–75% of patients diagnosed with MDD.11 The temporal pattern of an anxiety disorder preceding the onset of MDD is considerably more common than the reverse.7,8
Compared to MDD without significant anxiety symptoms (ie, low anxiety or nonanxious depression), anxious depression is associated with greater depression symptom severity, suicidality, chronicity, and functional impairment.7,9,12 Multiple pharmacologic studies have documented poorer MDD treatment outcome in anxious compared to nonanxious depression.5,12–16 For example, in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial,5 53.2% of patients treated with citalopram in Level 1 presented with anxious depression. Remission in this group was less likely and occurred after a longer delay compared to patients with nonanxious depression. Side effect frequency, intensity, and burden and the frequency of serious adverse effects were greater in the anxious depression group. Level 2 treatment outcomes were also significantly poorer in the anxious depression group, both for pharmacologic augmentation and for switching strategies.5
The efficacy of transcranial magnetic stimulation (TMS) in treatment-resistant depression (TRD) is well established and based on randomized sham-controlled trials,17–19 meta-analyses,20–22 and studies of real-world outcomes across diverse clinical settings.23,24 Several randomized controlled trials have reported that active TMS in patients with MDD results in larger reductions of anxiety symptoms than sham or other comparison conditions.17–19,25 A variety of open-label, naturalistic studies26–31 have also documented substantial reductions of anxiety symptoms in MDD patients treated with TMS. It has been reported that, independent of the presence of MDD, the TMS protocols used in MDD exert substantial therapeutic effects in GAD and posttraumatic stress disorder (PTSD).32 Thus, it could be that TMS, when configured for the treatment of MDD, exerts clinically meaningful anxiolytic effects in individuals with treatment-resistant anxious depression. However, traditional outcome metrics, specifically rates of response and remission of anxiety symptoms, have not been reported in a large sample of patients with anxious depression treated with TMS. Furthermore, it has been recently suggested that different anatomic TMS targets may be optimal to treat the “dysphoric” and “anxiosomatic” symptoms of MDD, with the former symptoms more responsive to dorsolateral prefrontal cortex (DLPFC) targeting and the later symptoms more responsive to dorsomedial prefrontal cortex targeting.33 This perspective predicts that, especially when using a focal TMS coil, the relative change in dysphoric versus anxiosomatic symptoms varies with coil positioning.
There is general consensus in the pharmacologic literature that the likelihoods of antidepressant response and remission are reduced in patients with anxious depression,5,12–15 although there are some reports with null results.34,35 In contrast, the sparse TMS literature is inconsistent. While a retrospective analysis25 suggested that high baseline anxiety in MDD patients is associated with marked post-TMS reductions in depression symptoms and low anxiety with minimal change, other studies29,31,36 found that patients with and without anxious depression did not differ in degree of in depressive symptom improvement. Of note, the presence of a comorbid anxiety disorder, but not the severity of anxiety symptoms, has also been associated with poorer antidepressant response to TMS.36–38
The NeuroStar Advanced Therapy System Clinical Outcomes Registry documents clinical outcomes with routine administration of TMS in largely private practice settings across the US. It constitutes the largest outcomes registry for any treatment of MDD. Findings from this registry have been reported regarding the overall antidepressant efficacy of TMS and demographic and treatment parameter efficacy correlates.24,39,40 The Patient Health Questionnaire-9 (PHQ-9)41 was completed at all registry sites, and a subset of sites also asked patients to complete the Generalized Anxiety Disorder-7 scale (GAD-7).42 The availability of serial scores on both instruments allowed for characterization of the severity of depression and anxiety symptoms at baseline and tracking TMS therapeutic outcomes in both domains.
This study addressed the following questions: (1) Do providers administer different TMS protocols to patients with anxious and nonanxious depression? (2) Does treatment with TMS for MDD have clinically significant anxiolytic effects in patients with anxious depression, as defined by high baseline GAD-7 score? (3) Are patients with anxious depression less likely to have a clinically significant antidepressant response, and does the extent of depression symptom improvement and rates of antidepressant response and remission differ in patients with anxious versus nonanxious depression at baseline? (4) To what extent does improvement in depression symptoms covary with improvement in anxiety symptoms?
METHODS
Clinical Outcomes Registry
This study involved retrospective analysis of data collected prospectively in the NeuroStar Advanced Therapy System Clinical Outcomes Registry. In particular, the classification of participants at baseline as presenting with anxious or nonanxious depression and the determination as to when the acute TMS treatment course ended were based on retrospective application of decision rules. As described previously,24,39,40 site selection for inclusion in this registry required that clinical facilities treated a minimum of 24 patients the year before joining the registry, used TrakStar Cloud software for recording deidentified patient characteristics and treatment parameters, and had a secure link for electronic data transfer. In addition, sites used the PHQ-941 and/or the Clinical Global Impressions–Severity of Illness scale (CGI-S)43 to assess the severity of depressive symptoms by self-report and clinician rating, respectively. Once a site joined the registry, all patients treated at the site were included in the database. The registry was developed and maintained by Neuronetics Inc (Malvern, Pennsylvania), the manufacturer of the NeuroStar TMS system. The registry was compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Patient data were deidentified prior to electronic transfer. Collection and analysis of clinical data in this way does not require local Institutional Review Board approval or informed consent.
Data entry in the registry started on May 5, 2016, and this report concerns all data collected until January 22, 2021. Site personnel entered patient demographic information (date of birth, gender), site identifier, primary diagnosis and diagnoses of comorbid psychiatric conditions (using ICD-9, ICD-10, or DSM-IV), and the PHQ-9 and GAD-7 scores. TMS parameters were captured passively at each session and included session date, treatment location of stimulation (ie, left DLPFC, right DLPFC, or both), motor threshold (MT), number of pulses per treatment location or session, treatment level (% device output relative to motor threshold), pulse frequency (eg, 10 Hz vs 1 Hz), duration of pulse trains, intertrain interval (ITI), and the number of treatment sessions during the acute phase treatment course. The acute phase treatment period was defined as starting with the patient’s first recorded TMS treatment and continuing until there was a period of at least 7 days without any treatment.24,39,40 There was no documentation in the registry database of DLPFC targeting method (eg, 5.5 cm rule, Beam F3, neuronavigation). While theNeuroStar system provided default coordinates for a target 5.5 cm anterior to the MT location, modifications (such as coil rotation) were often made to address patient discomfort, and other targeting methods could be used.
Sample Definitions
The registry collected data on 11,738 patients treated at 110 US sites (mean [SD] per site = 106.7 [111.6] patients) (see Table 1) during the specified time period. These registry participants were unique individuals who received at least 1 TMS treatment. The sites were almost exclusively either private practice practitioners or private practice TMS centers.
The intent-to-treat (ITT) sample was defined by inclusion and exclusion criteria summarized in Table 1. Due to the COVID-19 pandemic, the TMS course was interrupted in 164 patients, who were excluded. Other exclusions included age less than 18 years, no MDD diagnosis, or a primary diagnosis other than MDD. To ensure that the treatment objective was management of an acute episode of MDD, patients with comorbid psychiatric diagnoses other than anxiety disorders were also excluded (eg, schizophrenia, bipolar disorder, autism, attention-deficit/hyperactivity disorder). Most critically, patients were excluded who did not have PHQ-9 and GAD-7 assessments within 14 days prior to the first TMS session (n = 7,831) or who did not have at least one PHQ-9 and GAD-7 assessment after starting TMS (n = 164). Finally, individuals were excluded whose baseline PHQ-9 score was less than 10, indicating insufficient severity of baseline depressive symptoms (n = 60). The ITT sample comprised 1,820 patients treated at 43 sites. The lack of GAD-7 data was the predominant reason for exclusion, as the completion of this scale was at the discretion of the sites.
A subset of the ITT sample comprised the “Completer” sample (Table 1). To ensure a minimally adequate course of TMS,44 individuals were excluded if classified as PHQ-9 nonresponders and had ended TMS after fewer than 20 sessions. Patients were also excluded if PHQ-9 and GAD-7 assessments were not conducted near the end of acute phase treatment, ie, within ± 4 days of the final session. Thus, to be included in the ITT sample, patients had to receive at least one TMS treatment and complete the PHQ-9 and GAD-7 assessments at baseline and at any point after the start of TMS. In contrast, Completers (n = 1,429, 42 sites) received at least 20 TMS sessions before classification as PHQ-9 nonresponders and had PHQ-9 and GAD-7 assessments within 4 days of the final TMS session and within 4 days of each other.
The ITT and Completer samples were subdivided into 4 TMS protocol groups (see Table 1 for the criteria used to define each protocol): (1) patients treated with high frequency (HF; 10 Hz) left DLPFC (unilateral) stimulation throughout their treatment course (HF-LUL group), (2) patients treated with sequential bilateral (SBL) TMS in at least 90% of sessions (SBL group), (3) patients who initiated treatment with HF-LUL TMS for at least 5 sessions and then switched to SBL TMS (Switch group), and (4) patients whose treatments were not otherwise classified (Other group).
High baseline anxiety or “anxious depression” was defined by a baseline GAD-7 score ≥ 10, with nonanxious depression defined by a GAD-7 score < 10. The GAD-7 was originally developed as a screen to detect GAD in primary care settings.42 It has strong psychometric properties, including excellent internal consistency and a unidimensional factor structure,45,46 and is widely used to assess the severity of anxiety symptoms across populations and settings.47–50 Cut-offs ranging from a GAD-7 score of 8 to 10 have been recommended to identify individuals with clinically significant anxiety symptoms and likely to meet diagnostic criteria for an anxiety disorder.45,51,52 Response was defined for both the GAD-7 and the PHQ-9 as a reduction of at least 50% in final score relative to pre-TMS baseline. Remission was defined as a final score less than 5 on both the GAD-7 and the PHQ-9, corresponding to the traditional cutoffs demarking minimal or no anxiety or depressive symptoms on the respective scales.41,42,53
Statistical Analyses
The primary analyses were conducted in the total ITT sample and then repeated for confirmation in the total Completer sample and the ITT and Completer subsamples treated only with HF-LUL TMS. The anxious depression and nonanxious depression groups were compared in demographic and treatment parameters using t tests and χ2 analyses on continuous and categorical measures, respectively.
In the ITT sample, the final GAD-7 and PHQ-9 scores were the last observations obtained after baseline (last observation carried forward [LOCF]), while in the Completer sample, the final scores were obtained at the end of acute (EOA) treatment. Anxiolytic effects were defined as the change in GAD-7 scores from baseline to final observation and in terms of rates of anxiety symptom response and remission. Within the anxious and nonanxious depression groups, effect sizes (d) were calculated for the change in GAD-7 and PHQ-9 scores.54 The anxious depression and nonanxious depression groups were compared in antidepressant effects by conducting t tests on the change in PHQ-9 scores and χ2 tests on PHQ-9 response and remission rates. To confirm the findings of these bivariate analyses, analyses of covariance (ANCOVAs) and logistic regressions were conducted on the continuous change in PHQ-9 scores and the categorical outcomes, respectively. These models included as terms anxious/nonanxious depression group, baseline PHQ-9 score, age, gender, MT level, treatment level, pulse frequency, pulse train duration, interval between pulses trains (ITI), number of delivered pulses per session, and total number of treatment sessions in the acute course. In addition, to estimate the extent of covariation between anxiolytic and antidepressant effects, Pearson product-moment correlations were computed in each sample between the absolute changes in GAD-7 and PHQ-9 scores.
Descriptive statistics are reported as mean ± SD for continuous variables and frequency counts and percentages for categorical variables. For each patient, treatment parameters were averaged over all treatment sessions in their acute course. Significance values are 2-tailed with an α of .05. All P values reported are without multiplicity adjustment. Analyses were conducted using SAS v9.4 (SAS Institute Inc; Cary, North Carolina).
RESULTS
Sample Characteristics
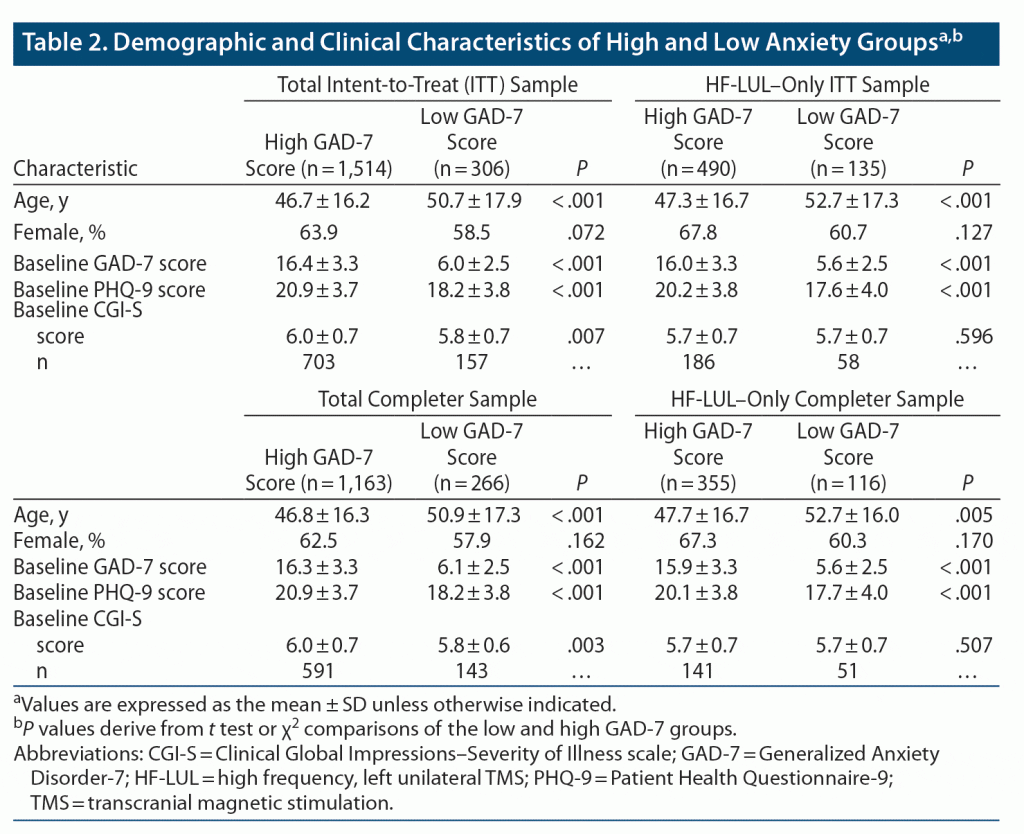
Table 2 presents demographic and clinical characteristics for the nonanxious and anxious depression groups in the total ITT and Completer samples and for the subsets treated only with HF-LUL TMS. More than 75% of patients had baseline GAD-7 scores of 10 or greater and were classified with anxious depression. The anxious and nonanxious depression groups did not differ in the distribution of gender. The anxious group was significantly younger than the nonanxious group by an average of approximately 5 years. In addition to the marked differences in baseline GAD-7 scores, the groups differed in baseline PHQ-9 scores. In line with reports that the severity of depressive symptoms is greater in anxious than in nonanxious depression,12,55–57 baseline PHQ-9 scores were approximately 2.5 points higher in the anxious depression group, a notable finding since the PHQ-9 does not contain items directly assessing anxiety.
Treatment Protocols and Parameters
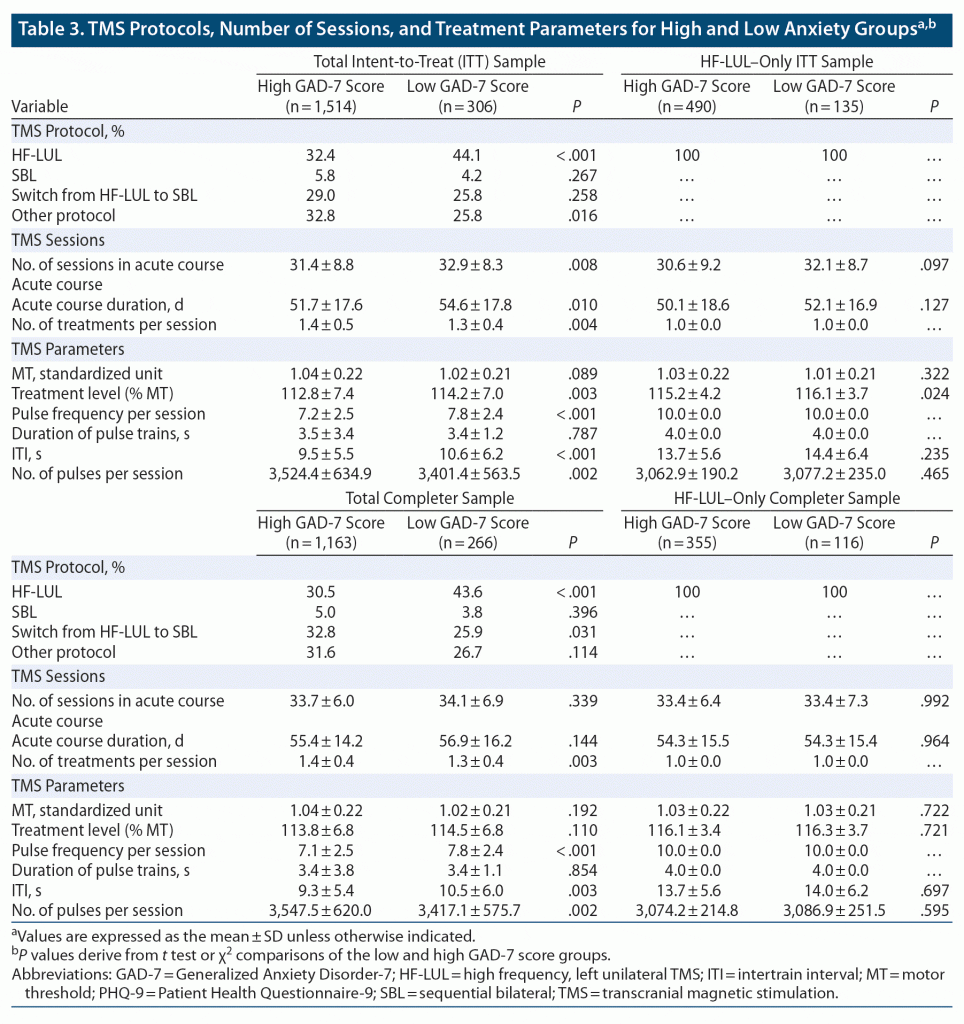
The anxious and nonanxious groups differed in the TMS protocols they were administered (ITT sample: χ23 = 16.22, P < .001; Completer sample: χ23 = 16.93, P < .001) (see Table 3). The exclusive use of the HF-LUL protocol was more common in the nonanxious depression group, while the anxious depression group was more likely to receive TMS protocols that were unclassified (eg, < 2,000 pulses per session, 1 Hz over right DLPFC) or protocols that involved SBL stimulation. Accordingly, outcomes for the two groups were evaluated both across all forms of TMS (total ITT and Completer samples) and when restricted to the HF-LUL protocol. The anxious and nonanxious depression groups treated with HF-LUL TMS did not differ in the duration of the TMS course or in virtually all TMS parameters. In the total sample, the nonanxious depression group received slightly more sessions over a slightly longer acute course duration. In the total sample, the difference between the anxious and nonanxious depression groups in treatment parameters reflected the fact that patients with anxiety were more likely to be started on or switched to SBL protocols than nonanxious patients.
Anxiolytic and antidepressant Effects
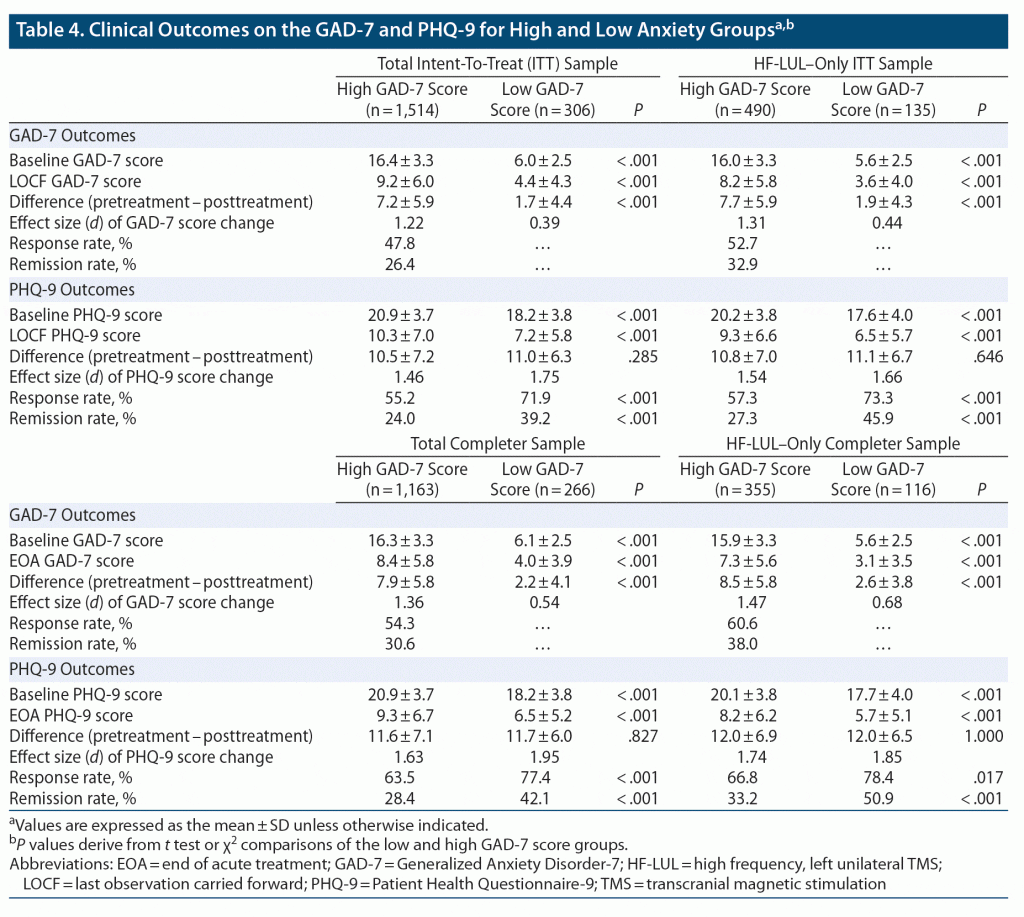
Anxiolytic and antidepressants effects were consistent across the ITT and Completer samples and patients who received any TMS protocol or only HF-LUL TMS (Table 4). GAD-7 scores decreased markedly in the anxious depression group, with the GAD-7 response rates ranging from 47.8% to 60.6% and GAD-7 remission rates ranging from 26.4% to 38.0%. GAD-7 scores also decreased significantly in the nonanxious group (all P values < .0001). The effects size for the decrease in GAD-7 scores ranged from 1.22 to 1.47 among the anxious depression samples and from 0.39 to 0.68 among the nonanxious depression samples.
The anxious depressed group scored approximately 2.5 points higher on the PHQ-9 than the nonanxious group both before TMS and at final observation, and the groups did not differ in the magnitude of change in PHQ-9 scores. The effect size for the change in PHQ-9 scores ranged from 1.46 to 1.74 in the anxious depression samples and from 1.66 to 1.95 in the nonanxious depression samples. Both groups showed marked antidepressant effects, with response rates in the anxious depression group ranging from 55.2% to 66.8% and remission rates ranging from 24.0% to 33.2%. Nonetheless, in each comparison, response and remission rates were significantly higher in the nonanxious depression group. Thus, despite the two groups’ manifesting the same degree of change in PHQ-9 scores, the higher baseline and post-TMS scores in the anxious depression group resulted in significantly lower response and remission rates. The multivariate ANCOVA and logistic regression analyses confirmed these findings. The nonanxious group had PHQ-9 outcomes superior to those of the anxious group in all comparisons (all P values ≤ .002). However, the difference in post-TMS adjusted means was small (ITT sample: 10.2 vs 8.4 in anxious and nonanxious groups), and the groups also did not differ in the absolute extent of symptom improvement after multivariate adjustment.
Correlation Between Anxiolytic and Antidepressant Effects
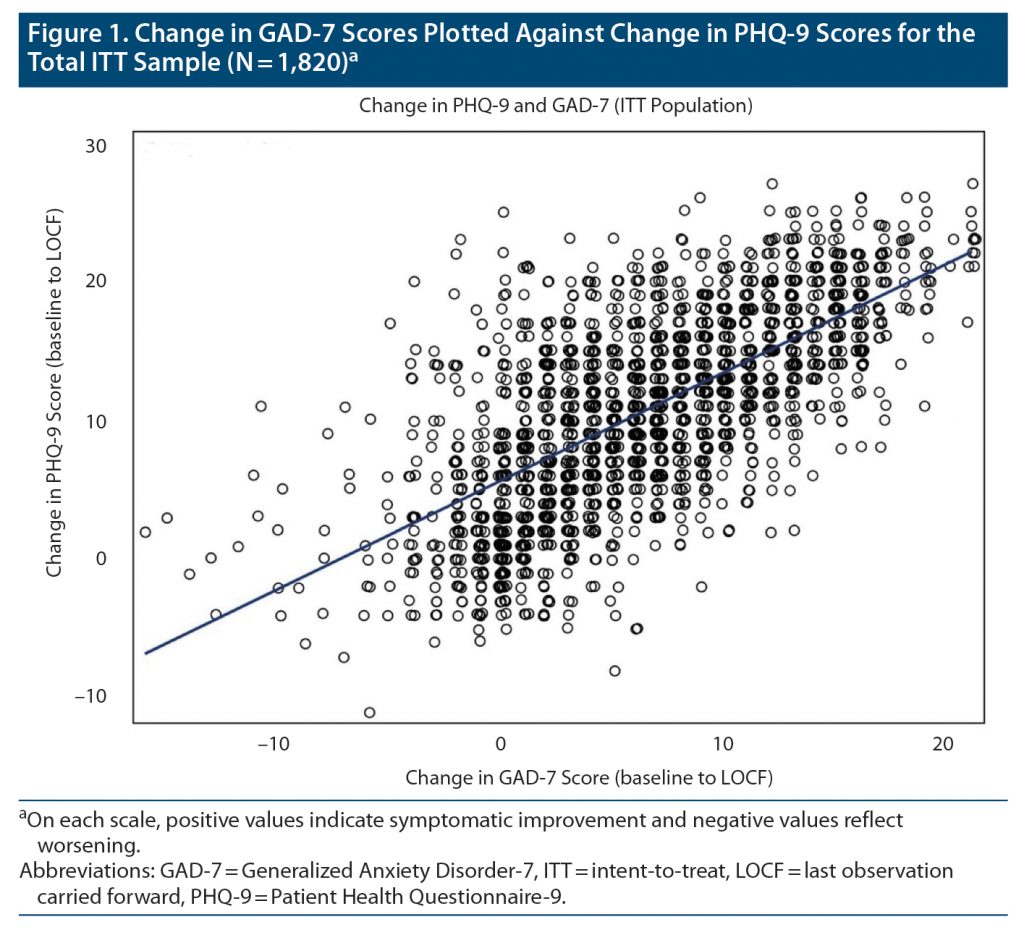
Figure 1 plots absolute change in GAD-7 scores versus change in PHQ-9 scores for the total ITT sample, r1818 = 0.69, P < .001. The relationship was robust in the anxious depression group (r1512 = 0.75, P < .001) and less robust in the nonanxious depression group (r304 = 0.50, P < .001), in which patients had much lower baseline GAD-7 scores. This pattern was consistent across all the samples. In the anxious depression group, change in GAD-7 and PHQ-9 scores shared approximately 60% variance.
DISCUSSION
At the outset, we posed 4 questions to be addressed in this study. The first was, “Do providers administer different TMS protocols to patients with anxious and nonanxious depression?” We found that exclusive use of the HF-LUL TMS protocol was more likely in the nonanxious depressed group, while the SBL TMS protocol was used more commonly in the anxious depressed group.
The second question was, “Does treatment with TMS for MDD have clinically significant anxiolytic effects in patients with anxious depression?” In by far the largest sample collected to date of patients with anxious depression treated with TMS, there were marked and clinically meaningful anxiolytic and antidepressant effects in self-report measures. In this group, on average, GAD-7 scores were approximately halved following TMS compared to baseline, and approximately 50% of patients were classified as anxiety symptom responders and 30% as remitters.
The third question was, “Are patients with anxious depression less likely to have a clinically significant antidepressant response?” The anxious depressed group had comparable reductions in PHQ-9 scores over the TMS course when compared to a similar nonanxious depression group treated at the same sites. Since the anxious depression group also had higher PHQ-9 scores at baseline, they were less likely to meet traditional categorical thresholds for antidepressant response and remission despite the same change in depression severity scores. In this respect, the findings are consistent with multiple reports that severity of depressive symptoms is greater in anxious depression12,55–57 and a large pharmacologic literature that documents reduced antidepressant effects.5,12–16 The differences between the anxious and nonanxious groups in depression efficacy measures were relatively minor compared to the magnitude of the antidepressant effects observed in both groups and the anxiolytic effects observed in the anxious depression group.
The fourth question was, “To what extent does improvement in depression symptoms covary with improvement in anxiety symptoms?” The findings also indicted that the extent of improvement in depression severity scores over the TMS course was highly correlated with the change in anxiety severity scores. Providers can generally expect that if there is improvement in one domain, there will be improvement in the other domain. This information may be useful when educating patients about the potential benefits of TMS, gauging treatment progress, and in managing concomitant pharmacotherapy during TMS.
At the mechanistic level, the strong concordance between improvement in anxiety and depression symptoms among patients treated with the HF-LUL protocol suggests that TMS delivered to the left DLPFC with a relatively focal figure-8 coil58 modulates in a similar manner the circuitry subserving therapeutic effects in these symptom domains. Nonetheless, it is also possible that spatially disparate targets or other variation in TMS protocols may be optimal in treating different depression subtypes or symptom constellations, especially since the correlation between anxiolytic and antidepressant effects, while robust, was imperfect. Indeed, in this study the anxious and nonanxious depression groups differed in the TMS protocols they were administered, with less utilization of HF-LUL treatment in the anxious depression group. While the method used to position the coil over the DLPFC target was not documented, the spatial coordinates of the coil position were routinely recorded, and future research may test whether coil positioning and other protocol variations are systematically associated with the magnitude of anxiolytic and/or antidepressant effects.
Although the clinical data examined in this study were prospectively collected, a limitation is the retrospective nature of the analyses, as key design considerations involved retrospective application of decision rules. The classification of participants as anxious or nonanxious was based on a threshold retrospectively applied to the baseline GAD-7 score, with a single point on this scale defining the boundary between these two groups. The determination of when the acute TMS course ended was based on a minimum gap of 7 days without receiving TMS, as opposed to prospective documentation of when the acute course was terminated.
Another limitation of this study is the fact that the GAD-7 was administered at only a minority of the registry sites and inconsistently among patients treated at individual sites. Undoubtedly, the desire to track anxiety symptoms in symptomatic patients drove use of the GAD-7, and the rates of anxious and nonanxious depression in this study may not be representative of the total registry. Nonetheless, such a selection bias enriched this sample with patients presenting with anxious depression. It is noteworthy that the antidepressant effects observed in the anxious depression group were similar in magnitude to those reported for the much larger registry sample.24,39 The findings indicate that, in this subgroup, routine TMS in diverse clinical settings results in marked anxiolytic and antidepressant effects. Furthermore, the extents of improvement in anxiety and depression symptoms are strongly linked.
Submitted: June 27, 2022; accepted October 17, 2022.
Published online: January 11, 2023.
Relevant financial relationships: Drs Hutton, Pages, and West serve as consultants to Neuronetics Inc. Dr Aaronson serves as a scientific adviser to Genomind Inc, LivaNova PLC, Neuronetics Inc, Janssen Pharmaceuticals Inc, and Sage Therapeutics and has received research support from Compass Pathways Inc and Neuronetics Inc. Dr Carpenter serves as a scientific advisor to or received consulting income from Neuronetics Inc, Nexstim PLC, Affect Neuro Inc, Neurolief LTD, Sage Therapeutics, Otsuka, Sunovion, and Janssen Pharmaceuticals Inc and has received research support (to Butler Hospital) from Neuronetics Inc, Neosync Inc, Nexstim PLC, Affect Neuro Inc, Neurolief, and Janssen Pharmaceuticals Inc. Ms Kraemer reports no financial relationship with commercial interests. Dr Sackeim serves as a scientific adviser to and receives consulting fees from Cerebral Therapeutics Inc, Holmusk Inc, LivaNova PLC, MECTA Corporation, Neurolief LTD, Neuronetics Inc, Parow Entheobiosciences LLC, and SigmaStim LLC. He receives honoraria and royalties from Elsevier Inc and Oxford University Press. He is the inventor on non-remunerative US patents for Focal Electrically-Administered Seizure Therapy (FEAST), titration in the current domain in electroconvulsive therapy (ECT), and the adjustment of current in ECT devices, each held by SigmaStim LLC. He is also the originator of magnetic seizure therapy (MST).
Funding/support: The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc (Malvern, Pennsylvania).
Role of the sponsor: While staff at Neuronetics Inc provided comments on the manuscript, final approval of its content and the decision to submit were solely determined by the authors.
Previous presentation: Presented at the 4th International Brain Stimulation Conference; Charleston, SC; December 9, 2021.
Acknowledgments: The authors thank Cory S. Anderson, MS, and Steve Erickson, BA, who facilitated the conduct of the statistical analyses and commented on the manuscript. Messrs Anderson and Erickson are employees of Neuronetics Inc and report no financial relationship with other commercial interests.
Clinical Points
- Anxious depression is common and is associated with diminished response to antidepressant medications, increased suicidality, and increased chronicity.
- Highly anxious depressed patients treated with transcranial magnetic stimulation (TMS) began with higher Patient Health Questionnaire-9 (PHQ-9) scores but showed strong antidepressant responses, obtaining decreases in PHQ-9 scores comparable to those of non-anxious patients.
- TMS improves both depression and anxiety, and the effects strongly covary.
References (58)

- Kalin NH. The critical relationship between anxiety and depression. Am J Psychiatry. 2020;177(5):365–367. PubMed CrossRef
- Nutt D. Treatment of depression and concomitant anxiety. Eur Neuropsychopharmacol. 2000;10(suppl 4):S433–S437. PubMed CrossRef
- Ionescu DF, Niciu MJ, Henter ID, et al. Defining anxious depression: a review of the literature. CNS Spectr. 2013;18(5):252–260. PubMed CrossRef
- Gaspersz R, Nawijn L, Lamers F, et al. Patients with anxious depression: overview of prevalence, pathophysiology and impact on course and treatment outcome. Curr Opin Psychiatry. 2018;31(1):17–25. PubMed CrossRef
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. PubMed CrossRef
- Kessler RC, DuPont RL, Berglund P, et al. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry. 1999;156(12):1915–1923. PubMed CrossRef
- Lamers F, van Oppen P, Comijs HC, et al. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 2011;72(3):341–348. PubMed CrossRef
- Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210–226. PubMed CrossRef
- Zhou Y, Cao Z, Yang M, et al. Comorbid generalized anxiety disorder and its association with quality of life in patients with major depressive disorder. Sci Rep. 2017;7(1):40511. PubMed CrossRef
- Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585–599. PubMed CrossRef
- Zimmerman M, Martin J, McGonigal P, et al. Validity of the DSM-5 anxious distress specifier for major depressive disorder. Depress Anxiety. 2019;36(1):31–38. PubMed CrossRef
- Dold M, Bartova L, Souery D, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders - results from a European multicenter study. J Psychiatr Res. 2017;91:1–13. PubMed CrossRef
- Fava M, Uebelacker LA, Alpert JE, et al. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42(7):568–576. PubMed CrossRef
- Davidson JR, Meoni P, Haudiquet V, et al. Achieving remission with venlafaxine and fluoxetine in major depression: its relationship to anxiety symptoms. Depress Anxiety. 2002;16(1):4–13. PubMed CrossRef
- Flint AJ, Rifat SL. Anxious depression in elderly patients: response to antidepressant treatment. Am J Geriatr Psychiatry. 1997;5(2):107–115. PubMed CrossRef
- Clayton PJ, Grove WM, Coryell W, et al. Follow-up and family study of anxious depression. Am J Psychiatry. 1991;148(11):1512–1517. PubMed CrossRef
- O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. PubMed CrossRef
- George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. PubMed CrossRef
- Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64–73. PubMed CrossRef
- Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336–346. PubMed CrossRef
- Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143–152. PubMed CrossRef
- Mutz J, Vipulananthan V, Carter B, et al. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:l1079. PubMed CrossRef
- Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. PubMed CrossRef
- Sackeim HA, Aaronson ST, Carpenter LL, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J Affect Disord. 2020;277:65–74. PubMed CrossRef
- Pell GS, Harmelech T, Zibman S, et al. Efficacy of deep TMS with the H1 coil for anxious depression. J Clin Med. 2022;11(4):1015. PubMed CrossRef
- Griffiths C, O’Neill-Kerr A, De Vai R, et al. Impact of repetitive transcranial magnetic stimulation on generalized anxiety disorder in treatment-resistant depression. Ann Clin Psychiatry. 2019;31(4):236–241. PubMed
- White D, Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann Clin Psychiatry. 2015;27(3):192–196. PubMed
- Clarke E, Clarke P, Gill S, et al. Efficacy of repetitive transcranial magnetic stimulation in the treatment of depression with comorbid anxiety disorders. J Affect Disord. 2019;252:435–439. PubMed CrossRef
- Diefenbach GJ, Bragdon L, Goethe JW. Treating anxious depression using repetitive transcranial magnetic stimulation. J Affect Disord. 2013;151(1):365–368. PubMed CrossRef
- Zhang L, Zhu J, Zhang T, et al. Comparative efficacy of add-on rTMS in treating the somatic and psychic anxiety symptoms of depression comorbid with anxiety in adolescents, adults, and elderly patients: a real-world clinical application. J Affect Disord. 2020;276:305–311. PubMed CrossRef
- Tuinstra D, Percifield C, Stilwell K, et al. Treatment of anxiety symptoms in patients receiving rTMS for treatment resistant depression. Psychiatry Res Commun. 2022;2(1):100014. CrossRef
- Cirillo P, Gold AK, Nardi AE, et al. Transcranial magnetic stimulation in anxiety and trauma-related disorders: a systematic review and meta-analysis. Brain Behav. 2019;9(6):e01284. PubMed CrossRef
- Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435–446. PubMed CrossRef
- Nelson JC, Delucchi K, Schneider LS. Anxiety does not predict response to antidepressant treatment in late life depression: results of a meta-analysis. Int J Geriatr Psychiatry. 2009;24(5):539–544. PubMed CrossRef
- Fava M, Martinez JM, Greist J, et al. The efficacy and tolerability of duloxetine in the treatment of anxious versus non-anxious depression: a post-hoc analysis of an open-label outpatient study. Ann Clin Psychiatry. 2007;19(3):187–195. PubMed CrossRef
- Trevizol AP, Downar J, Vila-Rodriguez F, et al. Effect of repetitive transcranial magnetic stimulation on anxiety symptoms in patients with major depression: an analysis from the THREE-D trial. Depress Anxiety. 2021;38(3):262–271. PubMed CrossRef
- Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–534. PubMed CrossRef
- Brakemeier EL, Wilbertz G, Rodax S, et al. Patterns of response to repetitive transcranial magnetic stimulation (rTMS) in major depression: replication study in drug-free patients. J Affect Disord. 2008;108(1-2):59–70. PubMed CrossRef
- Aaronson ST, Carpenter LL, Hutton TM, et al. Comparison of clinical outcomes with left unilateral and sequential bilateral Transcranial Magnetic Stimulation (TMS) treatment of major depressive disorder in a large patient registry. Brain Stimul. 2022;15(2):326–336. PubMed CrossRef
- Carpenter L, Aaronson S, Hutton TM, et al. Comparison of clinical outcomes with two Transcranial Magnetic Stimulation treatment protocols for major depressive disorder. Brain Stimul. 2021;14(1):173–180. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Superintendent of Documents, US Government Printing Office, US Department of Health, Education, and Welfare Publication No. 76–338; 1976.
- Sackeim HA, Aaronson ST, Bunker MT, et al. The assessment of resistance to antidepressant treatment: rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res. 2019;113:125–136. PubMed CrossRef
- Johnson SU, Ulvenes PG, Øktedalen T, et al. Psychometric properties of the General Anxiety Disorder 7-Item (GAD-7) scale in a heterogeneous psychiatric sample. Front Psychol. 2019;10:1713. PubMed CrossRef
- Jordan P, Shedden-Mora MC, Löwe B. Psychometric analysis of the Generalized Anxiety Disorder scale (GAD-7) in primary care using modern item response theory. PLoS One. 2017;12(8):e0182162. PubMed CrossRef
- Kertz S, Bigda-Peyton J, Bjorgvinsson T. Validity of the Generalized Anxiety Disorder-7 scale in an acute psychiatric sample. Clin Psychol Psychother. 2013;20(5):456–464. PubMed
- Rutter LA, Brown TA. Psychometric properties of the Generalized Anxiety Disorder Scale-7 (GAD-7) in outpatients with anxiety and mood disorders. J Psychopathol Behav Assess. 2017;39(1):140–146. PubMed CrossRef
- Löwe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. PubMed CrossRef
- Hinz A, Klein AM, Brähler E, et al. Psychometric evaluation of the Generalized Anxiety Disorder Screener GAD-7, based on a large German general population sample. J Affect Disord. 2017;210:338–344. PubMed CrossRef
- Plummer F, Manea L, Trepel D, et al. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. PubMed CrossRef
- Schueller SM, Kwasny MJ, Dear BF, et al. Cut points on the Patient Health Questionnaire (PHQ-9) that predict response to cognitive-behavioral treatments for depression. Gen Hosp Psychiatry. 2015;37(5):470–475. PubMed CrossRef
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
- Fava M, Alpert JE, Carmin CN, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34(7):1299–1308. PubMed CrossRef
- Howland RH, Rush AJ, Wisniewski SR, et al. Concurrent anxiety and substance use disorders among outpatients with major depression: clinical features and effect on treatment outcome. Drug Alcohol Depend. 2009;99(1–3):248–260. PubMed CrossRef
- Wiethoff K, Bauer M, Baghai TC, et al. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German Algorithm Project. J Clin Psychiatry. 2010;71(8):1047–1054. PubMed CrossRef
- Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top