Depression: Addressing Partial Response After First-Line Antidepressant Treatment
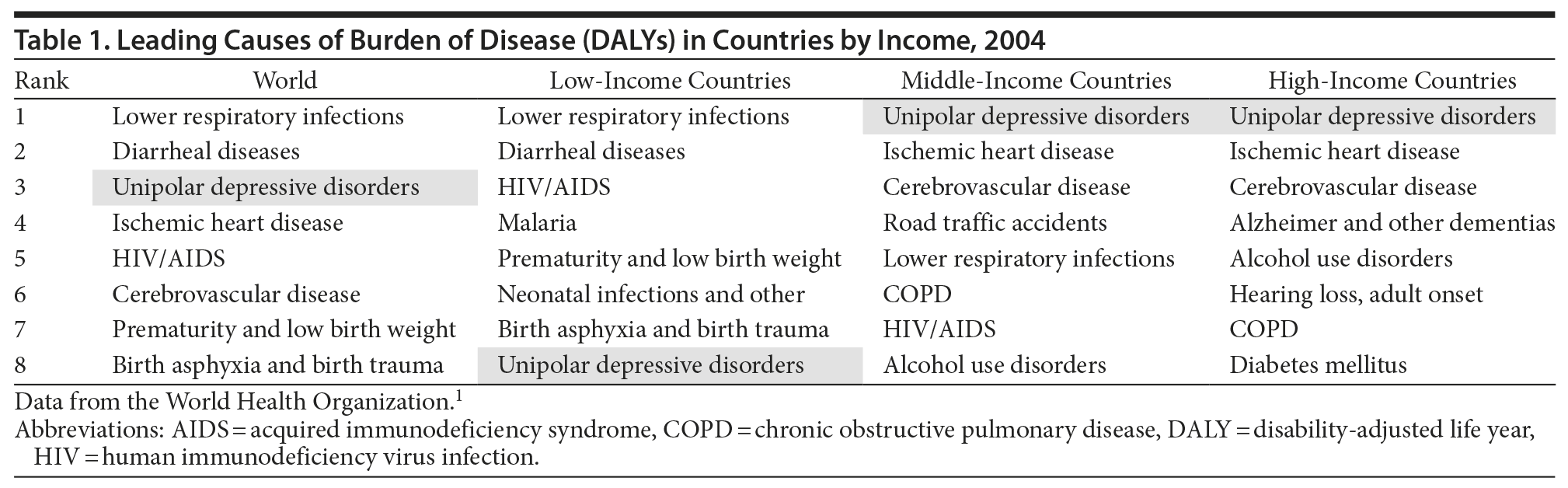
Major depressive disorder (MDD) remains a leading cause of disability worldwide (Table 1).1 Despite the range of available first-line treatments for MDD, many patients do not achieve full remission with initial antidepressant therapy and continue to experience depressive symptoms. Clinicians must be prepared to assess and treat these residual symptoms to reduce patients’ risk of relapse. The online CME series “Depression: Addressing Partial Response After First-Line Antidepressant Treatment” (visit PSYCHIATRIST.COM and enter keyword “wellness”) features 3 activities that provide assessment tools and treatment strategies to help clinicians monitor and treat partial response and residual symptoms in their patients with MDD. Following a summary of these activities, Drs Thase, Fava, and Nierenberg review the results and feedback from participants who completed one or more of the activities in this series and discuss areas for future education.
Summary of Activities
Dr Thase’s activity “Residual Symptoms and the Risk of Relapse in Major Depression” states that a considerable proportion of patients will not achieve asymptomatic remission after initial MDD treatment and that residual symptoms increase the risk of relapse.2 Clinicians should regularly assess patients’ symptoms and treatment response using scales such as the 9-item Patient Health Questionnaire, Quick Inventory of Depressive Symptomatology-Self-Report, or Beck Depression Inventory. For patients with inadequate response, clinicians should address barriers, such as inadequate dosage or patient nonadherence, and decide whether augmenting therapy or switching medications is warranted.
In his activity “Assessing Response to Treatment and Recognizing Residual Depression Symptoms,” Dr Fava recommends that clinicians use both categorical and dimensional approaches to assess treatment response. A categorical approach assesses the overall degree of change that a patient experiences during treatment (eg, partial response), while a dimensional approach uses measurement-based assessment tools to determine the degree of change in symptoms. While rating scales can measure symptoms over time, no single scale is ideal for measuring every symptom. A combination of patient-rated and clinician-rated scales is best. Clinicians must consider whether patients who have what appear to be residual symptoms actually have comorbidities or treatment-emergent side effects. For patients experiencing true residual symptoms, clinicians must decide whether more time on the current treatment is needed or if the patient would benefit from switching or augmenting options.
Strategies for improving partial response, which are given in Dr Nierenberg’s activity “Strategies for Achieving Full Remission When First-Line Antidepressants Are Not Enough,” include starting focused psychotherapy (such as cognitive-behavioral therapy or interpersonal therapy), switching antidepressants (within or outside of the same class), or augmenting current treatment with another antidepressant, lithium, thyroid hormone, or an atypical antipsychotic. Adjunctive agents can be used to target specific symptoms, such as fatigue or insomnia. As with other treatment decisions, clinicians should consider cost, tolerability, adherence, and patient preference.
Summary of Series Outcomes
Drs Thase, Fava, and Nierenberg discussed the educational outcomes for the activity series, including participants’ satisfaction with the online activities as well as the results from posttest questions that are designed to measure knowledge, competence, and performance. The objectives for this series were for clinicians to assess patients for residual depressive symptoms and adjust treatment for patients who respond to treatment but do not reach full symptom remission. A follow-up e-mail, sent to participants 2 months after they complete an online activity, asked clinicians if they have implemented the clinical strategies and what barriers they encountered. Participants mentioned barriers such as a lack of staff/resources and a need for more information.
Dr Thase: The majority of activity participants were office-based psychiatrists. Satisfaction ratings for the series were above average, at 4.2 out of 5.
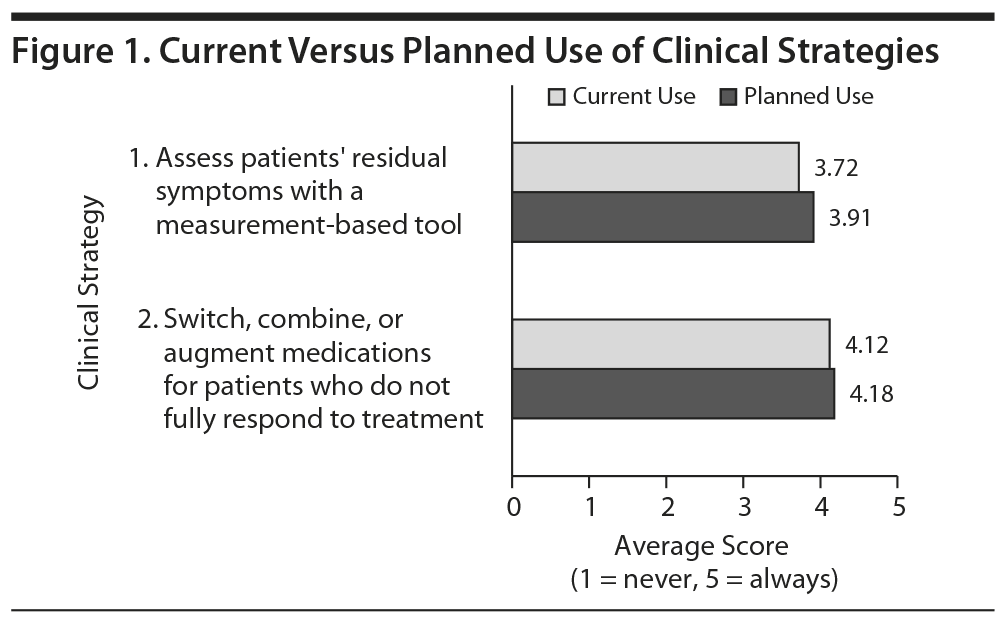
Each activity has a posttest question. Comparing the answers to the questions between a control group and the activity completers revealed that 2 of the 3 activities produced statistically significant improvement in knowledge and competence (P = .0002 and P = .02). For the other activity, 75% of the control group answered correctly, leaving little room for improvement; however, a greater percentage of activity completers (83%) answered correctly. The clinical strategies for this series were to assess patients’ residual symptoms with a measurement-based tool and to switch, combine, or augment medications for patients who do not fully respond to treatment. Participants were asked how often they currently used and planned to use these strategies. Comparing the current and planned use for each strategy revealed a nominal change (Figure 1).

- Assess patients for residual depressive symptoms.
- Use a measurement-based tool to track treatment response.
- Switch or augment treatment for patients who respond to treatment but do not reach full symptom remission.
Dr Nierenberg: It looks like there was a ceiling effect.
Dr Thase: Yes. The people who responded were already doing quite well, but there was a small increase in planned use.
Future Educational Needs
Dr Thase: What topics could we target in follow-up activities to support further change in physicians’ practices concerning the use of measurement-based care to address residual symptoms of depression?
Dr Nierenberg: I would imagine we should try to help physicians look at their workflow to find out what about these activities helps and what makes it a challenge to implement the clinical strategies. For example, links to measurement tools are provided in the activities, which should help readers, but a lack of staff may impede implementation of measurement-based care. A question for future activities is whether the use of tools can save time overall.
Dr Thase: Concerning residual symptoms and incomplete remission, we could explore topics related to cognition, which may be targeted by new agents like vortioxetine. Vortioxetine significantly improved measures of cognition compared with placebo in an 8-week study (P < .0001).3 This agent could be an option when switching medication.
Dr Fava: Anecdotally, I have been using vortioxetine for augmentation at a low dose of 5 mg/d. In my experience, it seems to address residual symptoms such as lack of motivation and impaired cognition. However, it has not been studied as an adjunctive agent yet.
Dr Thase: Adjunctive agents, such as aripiprazole and the investigational drug brexpiprazole, may resolve residual symptoms in patients with MDD.4,5 Another antidepressant agent recently approved by the US Food and Drug Administration for MDD is the serotonin-norepinephrine reuptake inhibitor levomilnacipran, which could be explored among subsets of depressed patients, such as those with fatigue, anergia, or functional impairments.6
Comorbid conditions are another area we could focus on in the future to help physicians resolve depression. Of the approximately 30% of our patients who meet remission criteria, whom we consider treatment successes, the majority have at least 1 residual symptom (median = 3), so they are statistically less likely to remain remitted over time.2 Incomplete remission remains an important unmet need.
Drug names: aripiprazole (Abilify), levomilnacipran (Fetzima), vortioxetine (Brintellix).
Disclosure of off-label usage: Dr Thase has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
Financial disclosure: Dr Thase has been an advisor/consultant for Alkermes, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Dey, Forest, Gerson Lehman, GlaxoSmithKline, Guidepoint Global, Lundbeck, MedAvante, Merck, Neuronetics, Ortho-McNeil, Otsuka, Pamlab, Pfizer, Roche, Shire, Sunovion, Takeda, and Transcept; has received grant/research support from AHRQ, Eli Lilly, Forest, NIMH, and Otsuka; has received honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck, and Pfizer; has equity holdings in MedAvante; and has received royalties from the American Psychiatric Foundation, Guilford Publications, Herald House, and W.W. Norton & Company; and his spouse/partner is employed by Peloton Advantage. Dr Fava has received research support from Abbott, Alkermes, Aspect Medical Systems, AstraZeneca, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clintara, Covance, Covidien, Eli Lilly, ElMindaA, EnVivo, Euthymics Bioscience, Forest, Ganeden Biotech, GlaxoSmithKline, Harvard Clinical Research Institute, Icon Clinical Research, i3 Innovus/Ingenix, Janssen, Johnson & Johnson, Lichtwer, Lorex, MedAvante, NARSAD, NCCAM, NIDA, NIMH, Neuralstem, Novartis, Organon, Pamlab, Pfizer, Pharmaceutical Research Associates, Pharmavite, PharmoRx Therapeutics, Photothera, Roche, RCT Logic, Sanofi-Aventis, Shire, Solvay, Synthelabo, and Wyeth-Ayerst; has been an advisor/consultant for Abbott, Affectis, Alkermes, Amarin, Aspect Medical Systems, AstraZeneca, Auspex, Bayer, Best Practice Project Management, BioMarin, Biovail, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, CNS Response, Compellis, Cypress, DiagnoSearch Life Sciences, Dainippon Sumitomo, DOV, Edgemont, Eisai, Eli Lilly, EnVivo, ePharmaSolutions, EPIX, Euthymics Bioscience, Fabre-Kramer, Forest, GenOmind, GlaxoSmithKline, Grunenthal GmbH, i3 Innovus/Ingenix, Janssen, Jazz, Johnson & Johnson, Knoll, Labopharm, Lorex, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, Naurex, Neuralstem, Neuronetics, NextWave, Novartis, NuPathe, Nutrition 21, Orexigen Therapeutics, Organon, Otsuka, Pamlab, Pfizer, PharmaStar, Pharmavite, PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa, Puretech Ventures, PsychoGenics, Psylin Neurosciences, Rexahn, Ridge Diagnostics, Roche, Sanofi-Aventis, Sepracor, Servier, Schering-Plough, Solvay, Somaxon, Somerset, Sunovion, Supernus, Synthelabo, Takeda, Tal Medical, Tetragenex, Teva, TransForm, Transcept, and Vanda; has spoken for/been published by Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis, Organon, Pfizer, PharmaStar, United BioSource, and Wyeth-Ayerst; has equity holdings in Compellis and PsyBrain; and has received income from a patent for Sequential Parallel Comparison Design (SPCD; licensed by MGH and RCT Logic), a patent application for a combination of azapirones and bupropion in MDD, and copyright royalties from Lippincott, Williams and Wilkins; Wolters Kluwer; World Scientific Publishing; and for the MGH Cognitive and Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs and Symptoms (DESS), and SAFER. Dr Nierenberg is a consultant for Abbott, AstraZeneca, Basilea, Brain Cells, Brandeis University, Bristol-Myers Squibb, Cephalon, Corcept, Eli Lilly, Forest, Genaissance, GlaxoSmithKline, Hoffman-La Roche Innapharma, Janssen, Jazz, Lundbeck, Merck, Novartis, PamLab, PGx Health, Pfizer, Ridge Diagnostics, Sepracor, Schering-Plough, Shire, Somerset, Sunovion, Takeda, Targacept, and Teva; has received research support from AHRQ, Bristol-Myers Squibb, Cederroth, Cyberonics, Elan, Forest, GlaxoSmithKline, Janssen, Lichtwer Pharma, Eli Lilly, Mylan, NARSAD, NIMH, Pamlab, Pfizer, Shire, Stanley Foundation, and Wyeth-Ayerst; has received honoraria from MGH Psychiatry Academy, ADURS, American Society for Clinical Psychopharmacology, Zucker Hillside Hospital, Forest, Janssen, Biomedical Development, Boston Center for the Arts, University of Pisa, University of Wisconsin at Madison, University of Texas Southwest at Dallas, Health New England, Harold Grinspoon Charitable Foundation, Eli Lilly, AstraZeneca, Brandeis University, International Society for Bipolar Disorder, 2nd East Asian Bipolar Forum, Mid-Atlantic Permanente Research Institute, and Up-to-Date; is a stock/shareholder in Appliance Computing, Brain Cells, and InfoMed; and has copyright joint ownership with MGH for Structured Clinical Interview for MADRS and Clinical Positive Affect Scale.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
References
1. World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: WHO Press; 2008. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. Accessed August 6, 2014.
2. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50. PubMed doi:10.1017/S0033291709006011
3. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults [published online ahead of print April 30, 2014]. Int J Neuropsychopharmacol.
4. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14(4):197-206. PubMed
5. Otsuka. Investigational compound brexpiprazole met primary and secondary study endpoints in phase III clinical trial in major depressive disorder (MDD). www.otsuka.com/en/hd_release/release/pdf.php?news=880. March 3, 2014. Accessed August 8, 2014.
6. Thase ME. Levomilnacipran: a brief overview. Medscape. July 26, 2013. http://www.medscape.com/viewarticle/808378.