Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Neuropeptide Y (NPY) is expressed in a number of brain regions and plays a key role in the regulation of fear, stress, anxiety, learning, and memory. The functional single nucleotide polymorphism in the NPY gene promoter rs16147 accounts for more than half of the variation in the expression of plasma NPY, and previous studies have found that NPY rs16147 genotype interacts with traumatic or stressful experiences to predict stress-related outcomes, with the T allele being protective under conditions of high stress. To date, however, no known study has examined whether polymorphisms in this gene may be linked to resilience to traumatic stress.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Association Between Functional Polymorphism in Neuropeptide Y Gene Promoter rs16147 and Resilience to Traumatic Stress in US Military Veterans
To the Editor: Neuropeptide Y (NPY) is expressed in a number of brain regions and plays a key role in the regulation of fear, stress, anxiety, learning, and memory.1 The functional single nucleotide polymorphism in the NPY gene promoter rs16147 accounts for more than half of the variation in the expression of plasma NPY,2 and previous studies have found that NPY rs16147 genotype interacts with traumatic or stressful experiences to predict stress-related outcomes, with the T allele being protective under conditions of high stress.3 To date, however, no known study has examined whether polymorphisms in this gene may be linked to resilience to traumatic stress.
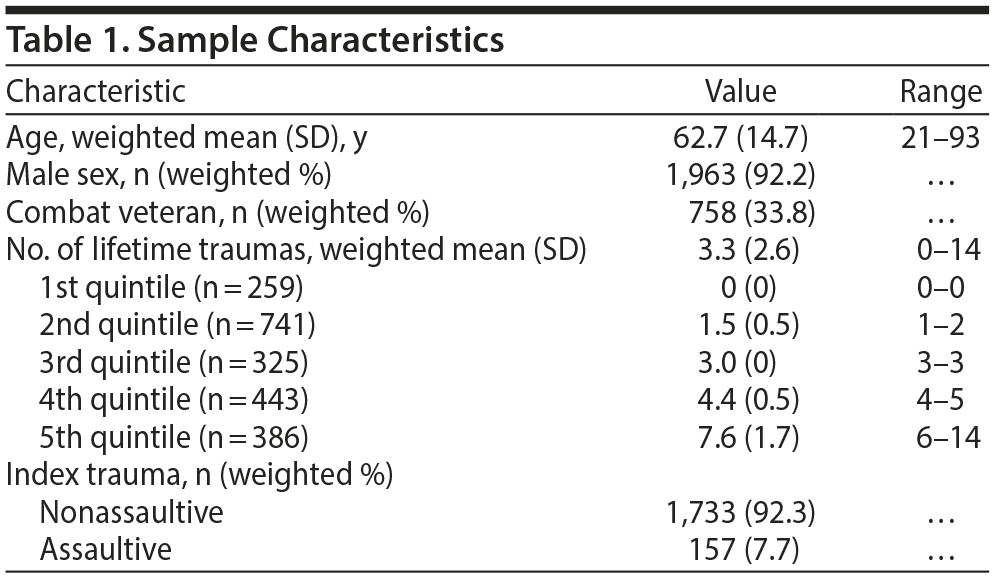
Method. To address the research gap, we analyzed data from the National Health and Resilience in Veterans Study, which surveyed a nationally representative sample of 2,162 European American US military veterans from a survey panel of more than 50,000 US households maintained by GfK, Inc; 1,585 veterans were surveyed in 2011, and 557, in 2013.4 Sample characteristics are shown in Table 1. NPY rs16147 genotype frequencies were as follows: C/C (n = 587; 27.2%), T/C (n = 1,095; 50.6%), and T/T (n = 480; 22.2%); they did not deviate from Hardy-Weinberg expectations (χ2 = 0.51, P = .47).
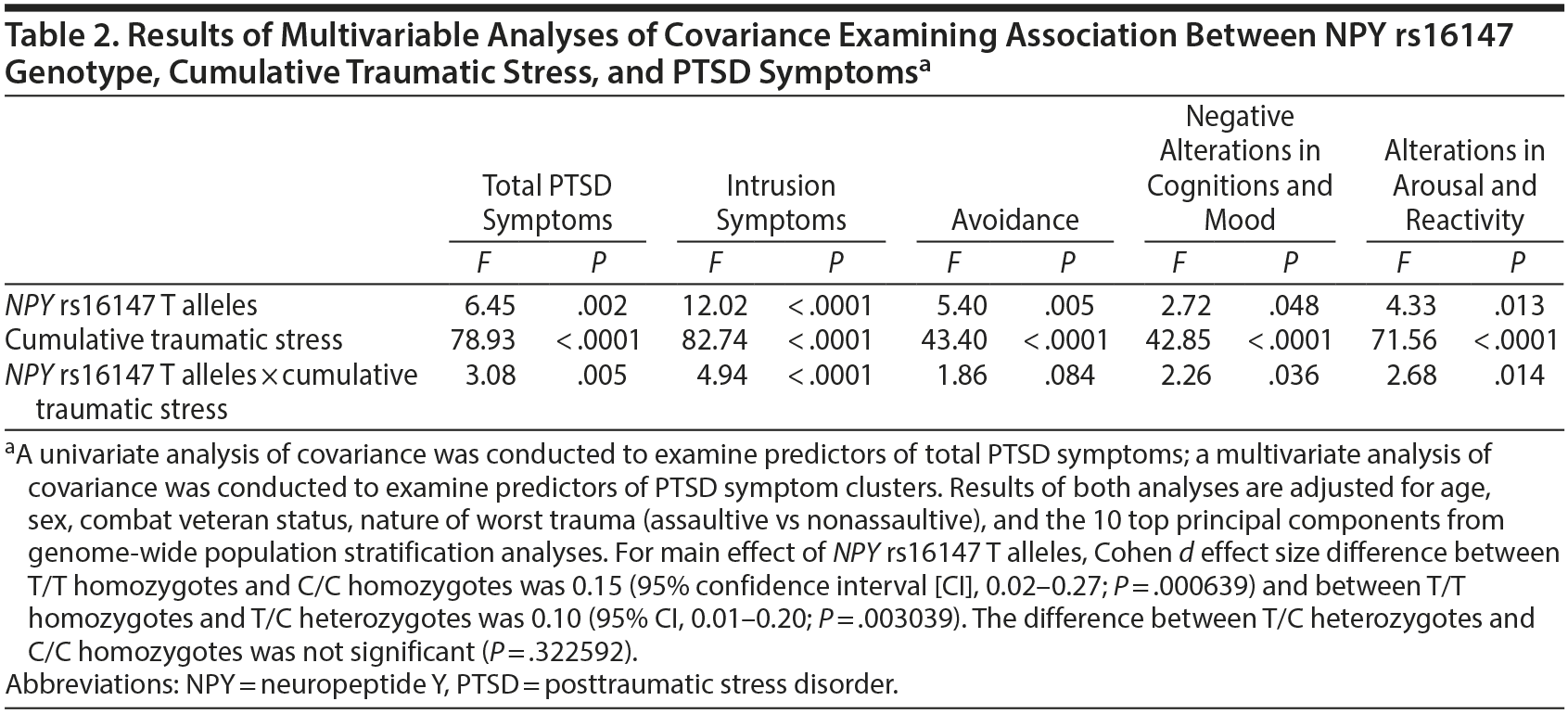
A univariate analysis of covariance (ANCOVA) and a multivariate ANCOVA (MANCOVA) were conducted to evaluate the relation between NPY rs16147 genotype, cumulative traumatic stress (ie, number of lifetime traumas assessed using the Trauma History Screen5; analyzed in quintiles due to positively skewed distribution), and the interaction of NPY rs16147 ×— cumulative traumatic stress in predicting both lifetime posttraumatic stress disorder (PTSD) symptoms (assessed using the PTSD Checklist for DSM-IV6 in the 2011 sample and the PTSD Checklist for DSM-57 in the 2013 sample) and the 4 PTSD symptom clusters—intrusion symptoms, avoidance, alterations in negative cognitions and mood, and alterations in arousal and reactivity. Covariates are listed in the Table 2 footnote. The significance threshold was set to .05 in the ANCOVA, and to control for type I error in the MANCOVA, a Bonferroni correction was applied (0.00625 significance threshold for 8 main and interactive effects of NPY genotype).
Results. Results revealed a significant main effect of NPY rs16147 and NPY rs16147 ×— cumulative traumatic stress interaction in predicting overall severity of PTSD symptoms (Table 2). T allele carriers had lower severity and prevalence of PTSD (mean ± SD and prevalence for T/T homozygotes = 25.3 ± 0.8 and 3.1% [n = 17] vs 27.3 ± 0.7 and 6.7% [n = 64] in T/C heterozygotes vs 28.0 ± 0.8 and 7.2% [n = 17] in C/C homozygotes) and had lower severity and prevalence of PTSD under conditions of high (5th quintile) cumulative traumatic stress (mean and prevalence for T/T homozygotes = 29.9 ± 1.5 and 8.3% [n = 14] vs 35.8 ± 1.0 and 25.2% [n = 54] in T/C heterozygotes vs 37.8 ± 1.2 and 27.6% [n = 27] in C/C homozygotes).
Analyses of PTSD symptom clusters revealed a Bonferroni-corrected significant main effect of NPY rs16147 and NPY rs16147 ×— traumatic stress interaction in predicting reexperiencing/intrusive symptoms. None of the other effects were significant (Table 2).
Results of the current study suggest that the T allele of NPY promoter rs16147 is associated with resilience in the context of cumulative traumatic stress, particularly resilience to intrusion symptoms of PTSD. T allele carriers may be less physiologically reactive to trauma cues, because they produce greater levels of NPY during stress.2 Greater NPY levels in plasma have been linked to lower stress reactivity,1 which may in turn be linked to greater resilience to cumulative traumatic stress. Indeed, lower concentrations of cerebrospinal fluid NPY are associated with PTSD.8 However, in light of prior studies showing heterogeneity in NPY expression with regard to NPY rs16147 genotype,2,9 further research is needed to evaluate neurobiological mechanisms linking polymorphisms in the NPY promoter gene rs16147 to resilience and whether interventions designed to enhance NPY levels1 may help promote stress resilience in trauma-affected populations.
References
1. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169. PubMed
2. Zhou Z, Zhu G, Hariri AR, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997-1001. PubMed doi:10.1038/nature06858
3. Witt SH, Buchmann AF, Blomeyer D, et al. An interaction between a neuropeptide Y gene polymorphism and early adversity modulates endocrine stress responses. Psychoneuroendocrinology. 2011;36(7):1010-1020. PubMed doi:10.1016/j.psyneuen.2010.12.015
4. Watkins LE, Han S, Harpaz-Rotem I, et al. FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology. 2016;69:98-105. PubMed doi:10.1016/j.psyneuen.2016.04.001
5. Weathers FW, Litz BT, Herman DS, et al. The PTSD Checklist: Reliability, validity, and diagnostic utility. Presented at the annual meeting of the International Society for Traumatic Stress Studies; October 1993; San Antonio, TX.
6. Weathers FW, Litz BT, Keane TM, et al. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD website. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp. Published 2013. Updated May 11, 2017.
7. Carlson EB, Smith SR, Palmieri PA, et al. Development and validation of a brief self-report measure of trauma exposure: the Trauma History Screen. Psychol Assess. 2011;23(2):463-477. PubMed doi:10.1037/a0022294
8. Sah R, Ekhator NN, Jefferson-Wilson L, et al. Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:277-283. PubMed doi:10.1016/j.psyneuen.2013.10.017
9. Sommer WH, Lidström J, Sun H, et al. Human NPY promoter variation rs16147:T>C as a moderator of prefrontal NPY gene expression and negative affect. Hum Mutat. 2010;31(8):E1594-E1608. PubMed doi:10.1002/humu.21299
aUS Department of Veterans Affairs National Center for Posttraumatic Stress Disorder, VA Connecticut Healthcare System, West Haven, Connecticut
bDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
cDepartment of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, Iowa
dDr Watkins is now affiliated with the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia
Potential conflicts of interest: Dr Krystal serves as a consultant to AbbVie (formerly Abbott), AMGEN, Astellas, AstraZeneca, Biomedisyn, Bristol-Myers Squibb, Eli Lilly, Euthymics Bioscience and Neurovance, Forum, Janssen, Lundbeck, Novartis, Otsuka, Sage Therapeutics, Sunovion, and Takeda; serves on the scientific advisory board for Lohocla Research Corporation, Mnemosyne, Naurex, Pfizer, and Synaptic; owns stock in Biohaven Medical Sciences and stock options in Mnemosyne; and has multiple patents; these relationships are not related to the subject of this letter. Dr Pietrzak serves as a scientific consultant to Cogstate for work that bears no relationship to the current study. Drs Watkins, Han, Southwick, and Gelernter report no financial disclosures or potential conflicts of interest.
Funding/support: The authors acknowledge support from the US Department of Veterans Affairs through its support of the VA National Center for PTSD and the Consortium to Alleviate PTSD. Preparation of this manuscript was also supported in part by the Department of Veterans Affairs Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, the Department of Veterans Affairs National Center for Posttraumatic Stress Disorder Clinical Neurosciences Division, and VA Connecticut Healthcare System.
J Clin Psychiatry 2017;78(8):e1058-e1059
https://doi.org/10.4088/JCP.17l11646
© Copyright 2017 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!
Save
Cite