Objective: To evaluate remission and recovery, safety, and tolerability for up to 12 months of open-label adjunctive l-methylfolate calcium 15 mg.
Method: Subjects in this analysis were adult outpatients (18-65 years) enrolled from 2 acute, double-blind, placebo-controlled trials comparing adjunctive l-methylfolate and placebo for DSM-IV major depressive disorder (MDD) with an inadequate response to monotherapy selective serotonin reuptake inhibitor (SSRI). Subjects who completed the acute trial were offered to enroll in a 12-month, open-label treatment phase with l-methylfolate and continued SSRI treatment, with scheduled visits for efficacy, safety, and tolerability every 12 weeks. Subjects were enrolled between September 2006 and February 2010. Efficacy outcomes included predefined criteria for response, remission, recovery, relapse, and recurrence. Subjects treated with adjunctive l-methylfolate 15 mg were included in the efficacy analysis.
Results: Of 68 subjects who met criteria for the 12-month open-label phase, 38% (n = 26) achieved full recovery, and none experienced a recurrence of MDD. For subjects entering the open-label phase in remission (n = 11), 91% (n = 10) achieved full recovery with l-methylfolate 15 mg, and none experienced a relapse or recurrence. Among 57 subjects who entered the open-label phase as nonremitted, 61% (n = 35) achieved remission. Of subjects who entered the open-label phase with a response without remission (n = 4), 50% (n = 2) had full recovery, and of subjects entering the open-label phase with no response (n = 53), 26% (n = 14) met recovery criteria.
Conclusions: Adjunctive l-methylfolate 15 mg/d may be an early option in patients who fail to adequately respond to antidepressant monotherapy, with preliminary evidence demonstrating sustained remission and sustained recovery.
Trial Registration: ClinicalTrials.gov identifier: NCT00321152
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Long-Term Efficacy, Safety, and Tolerability of l-Methylfolate Calcium 15 mg as Adjunctive Therapy With Selective Serotonin Reuptake Inhibitors:
A 12-Month, Open-Label Study Following a Placebo-Controlled Acute Study

ABSTRACT
Objective: To evaluate remission and recovery, safety, and tolerability for up to 12 months of open-label adjunctive l-methylfolate calcium 15 mg.
Method: Subjects in this analysis were adult outpatients (18-65 years) enrolled from 2 acute, double-blind, placebo-controlled trials comparing adjunctive l-methylfolate and placebo for DSM-IV major depressive disorder (MDD) with an inadequate response to monotherapy selective serotonin reuptake inhibitor (SSRI). Subjects who completed the acute trial were offered to enroll in a 12-month, open-label treatment phase with l-methylfolate and continued SSRI treatment, with scheduled visits for efficacy, safety, and tolerability every 12 weeks. Subjects were enrolled between September 2006 and February 2010. Efficacy outcomes included predefined criteria for response, remission, recovery, relapse, and recurrence. Subjects treated with adjunctive l-methylfolate 15 mg were included in the efficacy analysis.
Results: Of 68 subjects who met criteria for the 12-month open-label phase, 38% (n = 26) achieved full recovery, and none experienced a recurrence of MDD. For subjects entering the open-label phase in remission (n = 11), 91% (n = 10) achieved full recovery with l-methylfolate 15 mg, and none experienced a relapse or recurrence. Among 57 subjects who entered the open-label phase as nonremitted, 61% (n = 35) achieved remission. Of subjects who entered the open-label phase with a response without remission (n = 4), 50% (n = 2) had full recovery, and of subjects entering the open-label phase with no response (n = 53), 26% (n = 14) met recovery criteria.
Conclusions: Adjunctive l-methylfolate 15 mg/d may be an early option in patients who fail to adequately respond to antidepressant monotherapy, with preliminary evidence demonstrating sustained remission and sustained recovery.
Trial Registration: ClinicalTrials.gov identifier: NCT00321152
J Clin Psychiatry 2016;77(5):654-660
dx.doi.org/10.4088/JCP.15m10181
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Rush University Medical Center, Chicago, Illinois
bDepartment of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston
cDepartment of Psychiatry, University of Alabama at Birmingham, Birmingham
dResearch & Development, Nestlé Health Science-Pamlab, Inc, Covington, Louisiana
*Corresponding author: John M. Zajecka, MD, 1700 Van Buren St, 5th Fl, Chicago, IL 60612 ([email protected]).
The goal of treating major depressive disorder (MDD) is to achieve remission and recovery.1 Failure to achieve and sustain remission can be associated with high risk of relapse or recurrence, continued psychosocial impairments, an increase in the use of medical services, potential worsening of comorbid medical illness, ongoing risk of suicide, and greater difficulty in achieving remission over time.2,3,4 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial,5 among those who remitted with selective serotonin reuptake inhibitor (SSRI) monotherapy, 40.1% had a relapse or recurrence over the subsequent 12 months. Additionally, 40.1% of patients who remitted during monotherapy with an SSRI relapsed over the subsequent 12 months, with relapse rates up to 71.7% by the fourth intervention.6,7 Use of adjunctive treatment is an acceptable and potentially effective strategy in the acute management of MDD for an inadequate response to monotherapy.8,9,10
Although antidepressants are effective for relapse prevention, relapse rates vary between 25%-71.7%.7,11,12,13,14 Common causes for relapse and recurrence in long-term MDD treatment include side effects and lack of adherence to treatment.15 One study16 reported the rate of persistence to treatment with an SSRI decreased from 95% at 1 month to 20% at 6 months. The importance of selecting appropriate and tolerable treatment strategies including adjunctive therapy early in the course of treatment may help to increase the likelihood of acute remission and long-term recovery.
Folic acid and its biologically active form, l-methylfolate, have been widely studied for the treatment of major depression.17 However, conversion of folic acid is highly variable due to genetic polymorphism, and only the active form, l-methylfolate, crosses the blood-brain barrier.17 l-Methylfolate calcium (Deplin: Nestlé Health Science-Pamlab, Inc; Covington, Louisiana) is indicated for adjunctive management of MDD. The efficacy and tolerability of l-methylfolate 15 mg was demonstrated in 2 randomized, placebo-controlled trials.18,19 Preliminary data on long-term l-methylfolate use was reported in a 2-year, retrospective study20 comparing antidepressant monotherapy to l-methylfolate combined with an antidepressant in 242 subjects with MDD. Significantly (P = .03) more rapid improvement was demonstrated with combination therapy. Additionally, the discontinuation rate for adverse events was significantly lower with combination therapy (17.9%) compared to antidepressant monotherapy (34%) (P = .0078).20
The objective of this analysis was to evaluate efficacy (remission and recovery, sustained recovery), safety, and tolerability for up to 12 months of open-label adjunctive l-methylfolate 15 mg following an acute controlled study.
METHODS
Subjects (n = 223) were enrolled from 1 of 2 separate multicenter, acute (60-day), double-blind, sequential parallel comparison design,21 placebo-controlled trials to evaluate the efficacy of l-methylfolate as an adjunctive treatment for MDD in subjects with an inadequate response to an SSRI (Figure 1).18 The study design was approved by an institutional review board, and written informed consent was obtained from subjects entering the open-label 12-month study. Inclusion criteria for the acute studies included adult outpatients (18-65 years), current MDD (defined by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), Quick Inventory of Depressive Symptomatology-Self-Rated (QIDS-SR)22 score ≥12 at screening and baseline, and treatment with an adequate dose of an SSRI ≥ 8 weeks (stable dose for the past 4 weeks); subjects with > 2 adequate antidepressant trials in the current episode were excluded. All subjects who completed the double-blind phase were offered open-label treatment with adjunctive l-methylfolate 15 mg for 12 months. This study was registered at ClinicalTrials.gov (identifier: NCT00321152).

- The short-term efficacy of l-methylfolate as adjunctive treatment for depression has been demonstrated in randomized controlled trials, but prospective studies of the long-term effects of l-methylfolate are lacking.
- This study demonstrates the safety, tolerability, and high retention rates and potential benefits of adjunctive l-methylfolate 15 mg over 12 months for achieving high rates of response, remission, and recovery.
The analysis included 165 subjects who completed the 2 double-blind studies and who entered the open-label active treatment phase (n = 116 from study 1, and n = 49 from study 2) (Supplementary eFigure 1 at PSYCHIATRIST.COM). Study 1 allowed flexible dosing (7.5 or 15 mg of adjunctive l-methylfolate); all subjects from study 2 received 15 mg/day throughout the study. The safety and tolerability analyses included subjects taking both doses during the 12-month open-label phase. Data from the double-blind study showed that 7.5 mg of l-methylfolate was not superior to placebo. Therefore, the efficacy analyses for the long-term study included only subjects who received 15-mg l-methylfolate for most of the open-label phase, including all subjects from study 2 and subjects from study 1 who received 15-mg l-methylfolate throughout most of the 12 months. The remaining 68 subjects were included in the long-term efficacy analyses. All subjects remained on a therapeutic dose of their SSRI throughout the 12-month open-label phase.
Outcome criteria during the long-term phase included response (≥ 50% improvement on the 17-item Hamilton Depression Rating Scale [HDRS-17]23 total score from the start of the double-blind study), remission (total HDRS-17 score ≤ 7); recovery (≥ 6 months of remission from the start of the open-label phase), relapse (HDRS-17 score > 15 within 6 months of achieving remission), and recurrence (HDRS score > 15 after 6 months of remission at the time of recovery). Sustained recovery was defined as recovery that was sustained through the final study visit among completed subjects. The outcome measures were obtained every 3 months at scheduled visits.
RESULTS
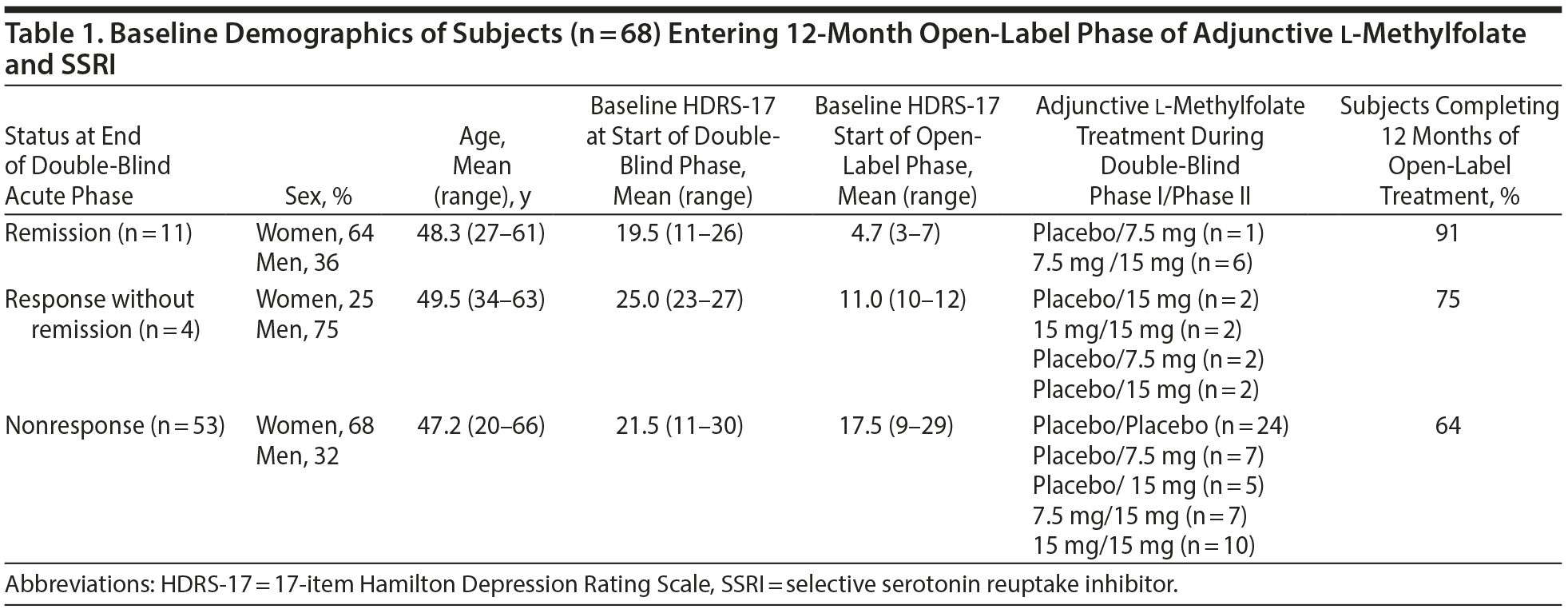
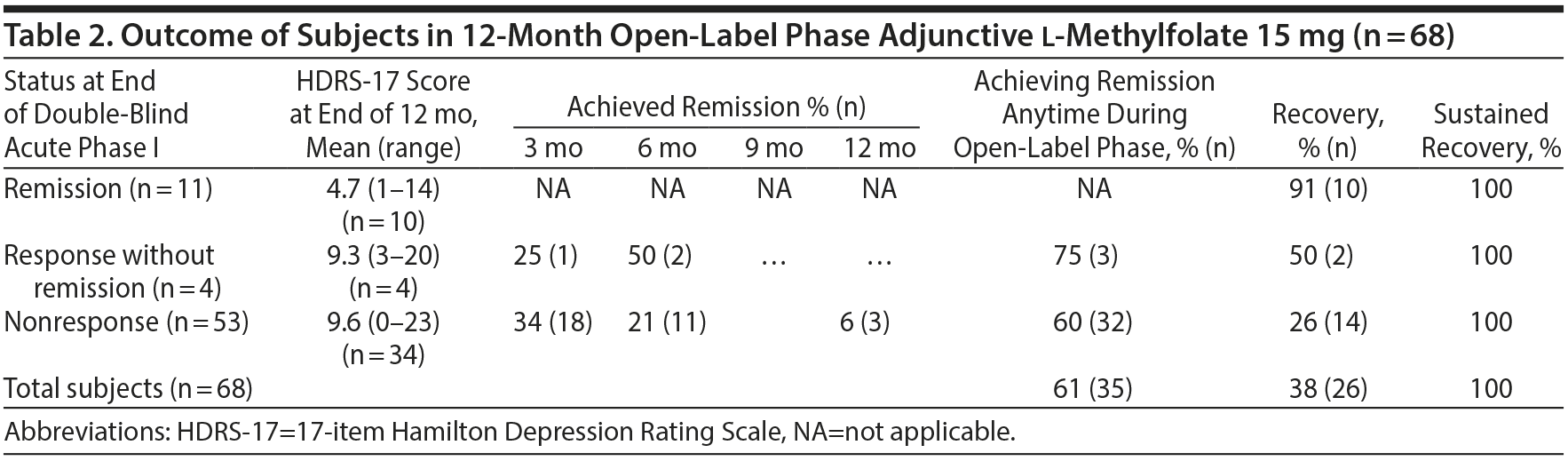
Subjects were enrolled between September 2006 and February 2010. Baseline demographics for the subjects who entered the 12-month open-label phase are reported in Table 1, and outcome measures are reported in Table 2. Sixty-eight subjects entered the 12-month open-label phase, 38% (n = 26) achieved full recovery, and none who met recovery criteria experienced a recurrence of their MDD.
Subjects Entering 12-Month Open-Label Phase in Remission
For subjects entering the open-label phase already in remission (n = 11), 91% went on to full recovery with 15 mg l-methylfolate, and none demonstrated a relapse or recurrence. Among the 36 unremitted subjects who entered the open-label phase and who remitted, 16 (53.3%) met recovery criteria. Further analyses were performed to ascertain factors associated with time to remission and recovery for the 3 groups (remitted, response without remission, and no response at the end of the double-blind treatment phase) entering the open-label phase defined by the various factors that may have impacted outcome during the long-term phase.
Subjects who entered the open-label phase in remission were observed for sustained remission and recovery. Ten of 11 subjects (91%) were taking l-methylfolate 15 mg prior to starting the open-label phase, 1 subject was taking 7.5 mg. Ten subjects were on the same dose of the same SSRI throughout the open-label phase, and 1 subject had the fluoxetine dose increased from 40 mg to 60 mg. Ten of 11 subjects (91%) completed the full 12 months of therapy; 1 subject was lost to follow-up after the 9-month assessment.
The individual outcome of each of the 11 subjects entering the 12-month open-label phase in remission is shown in Figure 1. The mean ± SD HDRS-17 score at the end of the 12-month open-label phase (n = 10) was 4.7 ± 5.4, and at the last visit for all subjects (n = 11) was 5.1 ± 5.2. Eight of 10 subjects (80%) who completed the 12-month open-label phase had sustained remission at 12 months; and 9 of the 11 subjects (82%) had sustained remission at the end of therapy (last observation carried forward). Six of 11 subjects (54.5%) maintained remission status at every visit during the 12-month open-label phase. Ten of 11 subjects (91%) met recovery criteria during the open-label phase. None of the 11 subjects who entered the open-label phase in remission experienced a relapse or recurrence during the 12 months.
Subjects Entering 12-Month Open-Label Phase With Response but Not Remission
Four subjects entered the 12-month open-label phase showing a response without full remission at the end of the double-blind phase.
The mean HDRS-17 at 12 months for completers (n = 3) was 9.3 ± 9.3, and at the last visit for all subjects (n = 4) was 13.0 ± 10.6. Sustained response occurred at all visits through the end of 12 months in 3 of the 4 subjects; 1 subject who sustained a response at 3 months and met remission criteria at 6 months lost the response at 9 months and met relapse criteria at 12 months.
Three subjects (75%) met remission criteria during the open-label phase. Remission occurred at 3 months in 1 subject (25%) and at 6 months in 2 subjects (50%). Two subjects met full recovery criteria.
Subjects Entering 12-Month Open-Label Phase With No Response
Fifty-three subjects who failed to show a response at the end of the double-blind phase entered the 12-month open-label phase. All 53 subjects had at least 1 follow-up visit after receiving open-label treatment. Thirty-four nonresponders (64%) completed 12 months of open-label therapy, and 47 subjects (89%) completed at least 6 months of therapy. The time of onset for response and remission rates during the 12-months of open-label therapy with 15-mg l-methylfolate for the subjects (n = 53) who failed to respond during the acute, double-blind phase is shown in Figure 2A.
Thirty-seven subjects (70%) who entered the open-label phase as nonresponders achieved a response at some point during the 12-month open-label phase. Of the 37 subjects who achieved a response during the open-label phase, 92% reported a response within the first 3-6 months of the 12-month phase. Twenty-four subjects (45%) achieved response by 3 months, 10 subjects (19%) by 6 months, 1 subject (2%) by 9 months, and 2 subjects (4%) by 12-months (Figure 2A).
Thirty-two subjects (60%) who failed to show a response at the end of the acute therapy phase achieved remission during the 12-month open-label phase. For the 34 subjects who completed 12 months of therapy, 17 (50%) were in remission at 12 months. Of the 32 subjects who achieved remission during the open-label phase, 91% reported remission within the first 3-6 months of the 12-month phase. Eighteen subjects (34%) achieved remission by 3 months, 11 subjects (21%) by 6 months, and 3 subjects (6%) by 12 months. Among the 37 subjects who achieved a response, 32 subjects (86%) demonstrated full remission, suggesting that, for subjects responding to 15-mg l-methylfolate, the majority demonstrated a complete remission.
Fourteen subjects (26%) who entered the open-label phase as nonresponders, met recovery criteria during the 12-month open-label phase. There were no reports of recurrence in the 10 subjects who met criteria for recovery at 6 months, and no relapse occurred for the 2 subjects who recovered at 9 months. One additional subject met remission criteria at 3 and 9 months but had an HDRS-17 score of 8 at 6 months and a score of 11 at 12 months and, therefore, did not meet relapse criteria.
Outcome in Nonresponders From Double-Blind Phase Based on Treatment Prior to Open-Label Treatment
A subanalysis of response and remission during the 12 months of open-label therapy in the subjects (n = 53) who failed to respond to l-methylfolate 7.5 or 15 mg or placebo prior to receiving open-label l-methylfolate is shown in Figure 2B. Open-label l-methylfolate resulted in response in 16 subjects (64%) and remission in 12 (48%) of the placebo nonresponders (n = 25), response and remission in 6 subjects (86%) who were 7.5-mg l-methylfolate nonresponders (n = 7), and response in 15 (71%) and remission in 14 (67%) of 15-mg l-methylfolate nonresponders (n = 21). The majority of subjects in all 3 groups responded or remitted within the first 3-6 months of open-label therapy. Among the 10 subjects who failed to respond to the initial acute 60 days of 15 mg, 8 subjects (80%) achieved remission within 3-6 months of open-label therapy, and nearly half (n = 4) of the subjects achieved recovery, suggesting that a subgroup of subjects may require longer therapy to achieve and sustain recovery beyond the initial 8 weeks of 15-mg l-methylfolate.
Safety, Tolerability, and Retention
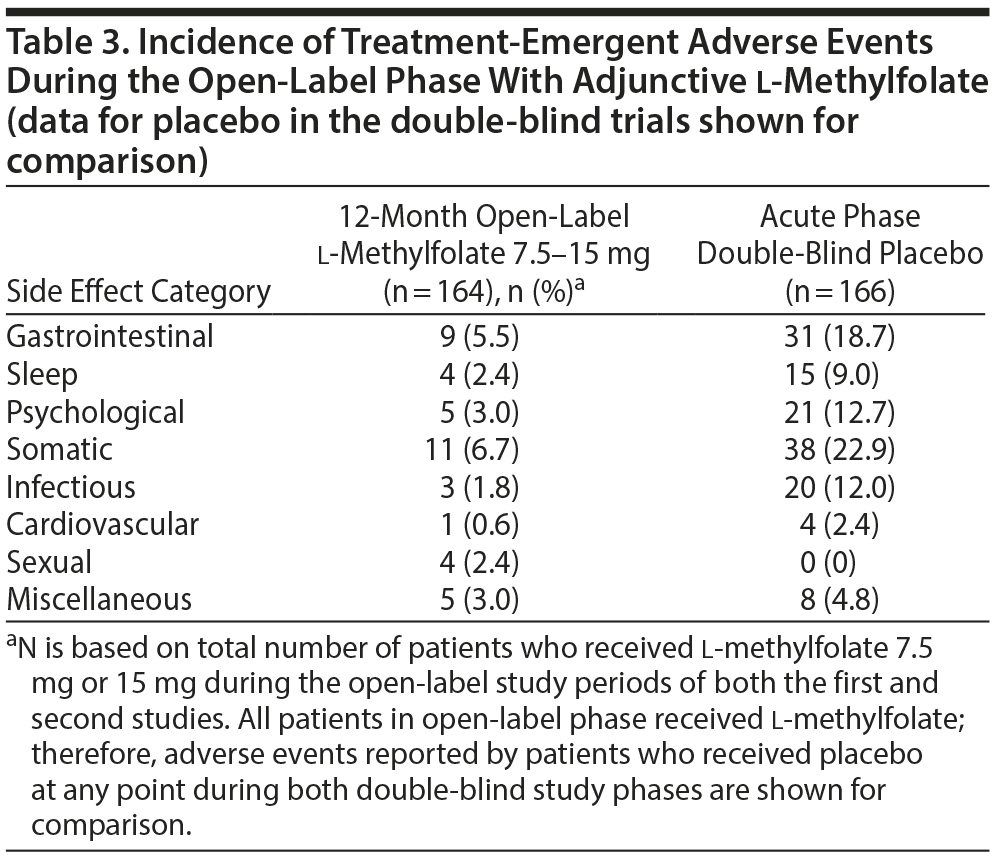
Safety, tolerability, and retention of all subjects (n = 165) who entered the open-label phase from both acute double-blind studies were analyzed. One subject withdrew before receiving open-label treatment. One hundred fifty (91%) subjects received at least 1 dose of 15-mg l-methylfolate. One hundred thirteen (69%) of 164 subjects who entered the open-label phase and completed 12 months of therapy were analyzed for safety and tolerability. Reasons for discontinuation among 164 subjects who received at least 1 dose of adjunctive l-methylfolate included lost to follow-up, 16% (n = 27); noncompliance, 4% (n = 7); withdrew consent, 4% (n = 6); lack of efficacy, 4% (n = 6); adverse event, 2% (n = 3); and other/unspecified, 1% (n = 2).
Among the 164 subjects exposed to l-methylfolate during the 12-month phase, 42 subjects reported adverse events (Table 3). The incidence of adverse events to l-methylfolate was comparable to placebo during the acute, double-blind, placebo-controlled phase. One subject had a serious adverse event after 2 weeks of open-label therapy that was not related to study medication. For the 68 subjects included in the efficacy analyses, there were no serious adverse events, and no subject experienced an adverse event that resulted in early termination during the 12-month open-label phase.
DISCUSSION
Despite the open-label design, several findings were noted that may address unmet needs in current strategies for long-term treatment of MDD. These results support previous findings of the acute efficacy of adjunctive l-methylfolate 15 mg and provide further support as a long-term, adjunctive therapy to achieve and sustain remission (recovery) and prevent recurrence of new depressive episodes in a population with an inadequate response to monotherapy SSRI. The data also indicate that, when used as adjunctive therapy with an SSRI, l-methylfolate 15 mg has a safety and tolerability profile comparable to placebo during 12-months of treatment. None of the 68 subjects who continued in the 12 month open-label phase discontinued for adverse events.
For the 11 subjects who entered the open-label phase in remission, 91% completed 12 months, and 10 of 11 subjects were taking 15 mg l-methylfolate at the time they entered the 12-month open-label phase, supporting 15 mg as an effective dose. For subjects who showed a response without remission at the end of the double-blind phase, 75% completed the 12-month open-label phase, and 2 of 4 subjects who showed a response without remission on 7.5 mg l-methylfolate remitted with a sustained remission after the dose was increased to 15 mg during the 12-month open-label phase. The majority (64%) of nonresponders from the acute double-blind phase completed the 12-month open-label phase, and the majority converted to a response (70%) or full remission (60%) with 15-mg l-methylfolate. Recovery occurred in 26%, with no subject experiencing a recurrence and 1 subject meeting relapse criteria. This subgroup of subjects could represent those who require a period of treatment longer than 30 days to achieve a remission.
The majority of subjects who entered the open-label phase without remission (nonresponders and responders or nonremitters) achieved remission during the open-label phase and converted in the first 3 or 6 months of therapy. This finding may be expected in subjects who failed the double-blind, acute therapy with adjunctive placebo or a subtherapeutic dose of l-methylfolate (7.5 mg); however, it was also observed in subjects who received 15 mg during the 8 weeks of acute treatment, demonstrating 80% remission and recovery rates within the first 6 months of continuing 15 mg l-methylfolate. The reported remission and recovery rates in this subgroup of nonresponders suggest that some subjects who fail to respond or remit to the initial 4 to 8 weeks of adjunctive 15-mg l-methylfolate may benefit from an extended trial.
This study underscores the importance of achieving remission to ultimately attain recovery and prevent recurrence. The data from these acute and open-label studies suggest that the majority of subjects treated with l-methylfolate 15 mg who demonstrated a clinical response met full remission criteria. Among the subjects who entered the open-label phase in remission and were already on 15-mg treatment, none experienced a recurrence. Of the 57 subjects entering the open-label phase as responders/nonremitters or nonresponders, 56% achieved remission and 26.3% achieved full recovery within the first 6 months of open-label treatment. Only 10 of these 57 were on l-methylfolate 15 mg for 8 weeks prior to the open-label phase; 23 were on l-methylfolate 15 mg for the 30 days prior to the open-label phase. This suggests a robust and sustained response within 3-6 months of receiving adjunctive l-methylfolate.
Among the most notable findings in this 12-month study were the high rates of sustained remission and no reported recurrence in those who recovered. This supports the durability of the remission and recovery with 15-mg adjunctive l-methylfolate combined with an SSRI. This is similar to the data for subjects entering the open-label phase already in remission, as well as for data on those entering open label with a response without full remission. The high retention rate may be explained by several factors, including the favorable tolerability, previous treatment with an SSRI, and a blinded adjunctive therapy (l-methylfolate or placebo) for at least 30 days prior to entering the long-term therapy, and by the high rates of sustained remission (recovery) and lack of recurrence during the long-term phase.
The results from this study are limited by the open-label design; however, all subjects were enrolled from double-blind, placebo-controlled trials, and this long-term study was an extension study using the same sites and scientific rigor utilized for the acute, controlled studies. Additionally, the subjects who completed the acute double-blind studies were given the option to enter the open-label phase, which may have resulted in an enriched population who were already improving during the acute phase; however, the majority of the subjects in the long-term analysis included nonresponders during the double-blind phase. Another limitation was the long duration (12 weeks) between study visits, and subjects may have experienced outcome events at an earlier time than the 3-month assessments. A long-term maintenance study for the prevention of relapse and recurrence requires subjects in remission to be followed over time.
CONCLUSION
This study is among the first to suggest the potential benefits of l-methylfolate 15 mg with an SSRI as an adjunctive therapy for MDD. This study demonstrates the safety, tolerability, and high retention rates of adjunctive l-methylfolate 15 mg over 12 months. The potential benefits of adjunctive l-methylfolate 15 mg over 12 months are supported by the reported high rates of response, remission, and recovery. The absence of recurrence in all subjects who achieved recovery and the high rate of sustained remission suggest the potential durable efficacy over 12 months with adjunctive l-methylfolate 15 mg and an SSRI. The majority of the patients may respond to adjunctive l-methylfolate 15 mg in the first 3-6 months of therapy, although there may be a subgroup of patients who require extended therapy to achieve remission and recovery.
Other pharmacologic agents as well as nonpharmacologic therapies explored as adjunctive therapy or augmentation in patients not responding to antidepressants have demonstrated efficacy, but their use may be limited by adverse events, and evidence is limited with many approaches.24,25 Based on these results, adjunctive l-methylfolate can be considered an early option in patients who fail to adequately respond to monotherapy SSRI, with preliminary evidence demonstrating sustained remission and recovery. Further controlled studies are warranted to clarify the acute and long-term benefits of using l-methylfolate in patients with MDD and to ascertain subpopulations who may benefit from early intervention with adjunctive l-methylfolate 15 mg for inadequate response to monotherapy.
Submitted: June 16, 2015; accepted January 27, 2016.
Online first: March 15, 2016.
Drug names: fluoxetine (Prozac and others), l-methylfolate calcium (Deplin).
Potential conflicts of interest: Dr Zajecka has received grant/research support from Actavis, Alkermes, Allergan, Assurex Health, AstraZeneca, Cyberonics, ElMindA, Forest Laboratories, Cheryl T. Herman Foundation, Hoffman-LaRoche, Janssen, Naurex, National Institutes of Health, Shire, and Takeda; has served on the consultant/advisory board for Avanir (Data Safety Monitoring Board), Eli Lilly, Forest Laboratories, Lundbeck, Nestlé Health-Pamlab, Shire, and Takeda; and has received other financial support: Trustee, Cheryl T. Herman Foundation; expert witness for psychiatric testimony; and partner, Psychiatric Medicine Associates. Dr Fava has received research support from Abbot, Alkermes, American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clintara, Covance, Covidien, Eli Lilly, EnVivo, Euthymics Bioscience, Forest, Ganeden Biotech, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen R&D, Jed Foundation, Johnson & Johnson Pharmaceutical Research & Development, Lichtwer GmbH, Lorex, Lundbeck, MedAvante, Methylation Sciences, National Alliance for Research on Schizophrenia and Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Neuralstem, Novartis AG, Organon, PamLab, Pfizer, Pharmacia-Upjohn, Pharmaceutical Research Associates, Pharmavite, PharmoRx Therapeutics, Photothera, Reckitt Benckiser, Roche, RCT Logic (formerly Clinical Trials Solutions), Sanofi-Aventis, Shire, Solvay, Stanley Medical Research Institute, Synthelabo, and Wyeth-Ayerst Laboratories; has been an advisor/consultant for Abbott, Affectis AG, Alkermes, Amarin Pharma, Aspect Medical Systems, AstraZeneca, Auspex, Avanir, AXSOME Therapeutics, Bayer AG, Best Practice Project Management, BioMarin, Biovail, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, CNS Response, Compellis, Cypress, DiagnoSearch Life Sciences (P), Dainippon Sumitomo, Dov, Edgemont, Eisai, Eli Lilly, EnVivo, ePharmaSolutions, EPIX, Euthymics Bioscience, Fabre-Kramer, Forest, Forum, GenOmind, GlaxoSmithKline, Grunenthal GmbH, i3 Innovus/Ingenis, Janssen, Jazz, Johnson & Johnson Pharmaceutical Research & Development, Knoll, Labopharm, Lorex, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, Naurex, Nestle Health Sciences, Neuralstem, Neuronetics, NextWave, Novartis AG, Nutrition 21, Orexigen Therapeutics, Organon, Otsuka, Pamlab, Pfizer, PharmaStar, Pharmavite, PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa, Pharmaceutical Product Development, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic (formerly Clinical Trials Solutions), Rexahn, Ridge Diagnostics, Roche, Sanofi-Aventis, Sepracor, Servier Laboratories, Schering-Plough, Solvay, Somaxon, Somerset, Sunovion, Supernus, Synthelabo, Takeda, Tal Medical, Tetragenex, TransForm, Transcept, and Vanda; has received speaking/publishing support from Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis AG, Organon, Pfizer, PharmaStar, United BioSource, Wyeth-Ayerst; has equity holdings in Compellis and PsyBrain; holds a patent for Sequential Parallel Comparison Design, licensed by MGH to Pharmaceutical Product Development; holds a patent application for a combination of ketamine plus scopolamine in major depressive disorder licensed by MGH to Biohaven; and receives copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire, Sexual Functioning Inventory, Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs & Symptoms, Symptoms of Depression Questionnaire, and SAFER and from Lippincott Williams & Wilkins, Wolkers Kluwer, and World Scientific Publishing. Dr Shelton was a consultant for Bristol-Myers Squibb, Cerecor, Clintara, Cyberonics, Forest, Janssen, Medtronic, MSI Methylation Sciences, Naurex, Nestlé Health-Pamlab, Pfizer, Ridge Diagnostics, Shire Pic, and Takeda; and has received grant/research support from Alkermes, Assurex Health, Avanir, Cerecor, Elan, Forest, Janssen, Naurex, Novartis, Otsuka, Nestlé Health-Pamlab, and Takeda. Dr Papakostas has served as a consultant for Abbott Laboratories, AstraZeneca, Avanir, Brainsway Ltd, Bristol-Myers Squibb, Cephalon, Dey Pharma, Eli Lilly, Genentech, GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc, Janssen Global Services, Jazz, Johnson & Johnson, Novartis AG, One Carbon Therapeutics, Otsuka, PAMLAB, Pfizer, Pierre-Fabre, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire, Sunovion, Takeda, Theracos, and Wyeth; has received honoraria from Abbott, AstraZeneca, Avanir, Bristol-Myers Squibb, Brainsway, Cephalon, Dey, Eli Lilly, Evotec AG, GlaxoSmithKline, Inflabloc, Jazz, H. Lundbeck A/S, Novartis AG, Otsuka, PAMLAB, Pfizer, Pierre-Fabre, Ridge Diagnostics, Shire, Sunovion, Takeda, Theracos, Titan, and Wyeth; has received research support from AstraZeneca, Bristol-Myers Squibb, Forest, the National Institute of Mental Health, PAMLAB, Pfizer, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion, and Theracos; and has served (not currently) on the speaker’s bureau for Bristol-Myers Squibb and Pfizer. Mss Barrentine and Young are employees of Nestlé Health-Pamlab.
Funding/support: This work was supported by grants from Nestlé Health Science-Pamlab, Inc, Covington, Louisiana.
Role of the sponsor: The sponsor participated in the review of the manuscript, but final approval and the decision to submit the manuscript was the sole decision of the lead author.
Acknowledgment: The authors acknowledge the editorial assistance of Richard S. Perry, PharmD (medical writer/independent contractor), with this article, which was supported by Nestlé Health Science-Pamlab Inc, Covington, Louisiana.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Keller MB. Remission versus response: the new gold standard of antidepressant care. J Clin Psychiatry. 2004;65(suppl 4):53-59. PubMed
2. Thase ME. Introduction: defining remission in patients treated with antidepressants. J Clin Psychiatry. 1999;60(suppl 22):3-6. PubMed
3. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340. PubMed doi:10.1001/jama.1997.03540280071036
4. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180. PubMed doi:10.1017/S0033291700033146
5. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. PubMed doi:10.1176/appi.ajp.163.1.28
6. Trivedi MH, Daly EJ. Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin Neurosci. 2008;10(4):377-384. PubMed
7. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed doi:10.1176/ajp.2006.163.11.1905
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530, quiz 1665. PubMed doi:10.1176/ajp.2006.163.9.1519
9. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252. PubMed doi:10.1056/NEJMoa052964
10. Zajecka J, Goldstein C, Barowski J. Combining medications to achieve remission. In: Schwartz TL, Petersen T, eds. Depression: Treatment Strategies and Management. 2nd ed. New York, NY: Informa Healthcare: 2009: 54-100.
11. Rush AJ, Kraemer HC, Sackeim HA, et al; ACNP Task Force. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841-1853. PubMed doi:10.1038/sj.npp.1301131
12. Solomon DA, Leon AC, Mueller TI, et al. Tachyphylaxis in unipolar major depressive disorder. J Clin Psychiatry. 2005;66(3):283-290. PubMed doi:10.4088/JCP.v66n0302
13. Posternak MA, Zimmerman M. Dual reuptake inhibitors incur lower rates of tachyphylaxis than selective serotonin reuptake inhibitors: a retrospective study. J Clin Psychiatry. 2005;66(6):705-707. PubMed doi:10.4088/JCP.v66n0605
14. Zimmerman M, Thongy T. How often do SSRIs and other new-generation antidepressants lose their effect during continuation treatment? evidence suggesting the rate of true tachyphylaxis during continuation treatment is low. J Clin Psychiatry. 2007;68(8):1271-1276. PubMed doi:10.4088/JCP.v68n0814
15. Zajecka JM. Clinical issues in long-term treatment with antidepressants. J Clin Psychiatry. 2000;61(suppl 2):20-25. PubMed
16. Ereshefsky L, Saragoussi D, Despiégel N, et al. The 6-month persistence on SSRIs and associated economic burden. J Med Econ. 2010;13(3):527-536. PubMed doi:10.3111/13696998.2010.511050
17. Nelson JC. The evolving story of folate in depression and the therapeutic potential of l-methylfolate. Am J Psychiatry. 2012;169(12):1223-1225. PubMed doi:10.1176/appi.ajp.2012.12091207
18. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274. PubMed doi:10.1176/appi.ajp.2012.11071114
19. Papakostas GI, Shelton RC, Zajecka JM, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863. PubMed doi:10.4088/JCP.13m08947
20. Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov Clin Neurosci. 2011;8(1):19-28. PubMed
21. Fava M, Evins AE, Dorer DJ, et al. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72(3):115-127. PubMed doi:10.1159/000069738
22. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
23. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
24. Epstein I, Szpindel I, Katzman MA. Pharmacological approaches to manage persistent symptoms of major depressive disorder: rationale and therapeutic strategies. Psychiatry Res. 2014;220(suppl 1):S15-S33. PubMed doi:10.1016/S0165-1781(14)70003-4
25. Wright BM, Eiland EH 3rd, Lorenz R. Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy. 2013;33(3):344-359. PubMed doi:10.1002/phar.1204
This PDF is free for all visitors!
Save
Cite