Objective: Little is known about the overall prevalence of major depressive disorder (MDD) in persons with dementia (ie, “depression in dementia”: DpD). The aim of this systematic review and meta-analysis was to determine the prevalence and factors associated with DpD among older adults (age range 58.7-87.8 years). The protocol was registered in the PROSPERO registry (2015:CRD42015020681).
Data Sources: We searched the following electronic databases: MEDLINE (1946-February 2017), Embase (1980-2017 week 5), and PsycINFO (1967-February 2017) using medical subject headings and free-text search terms for studies in the English language.
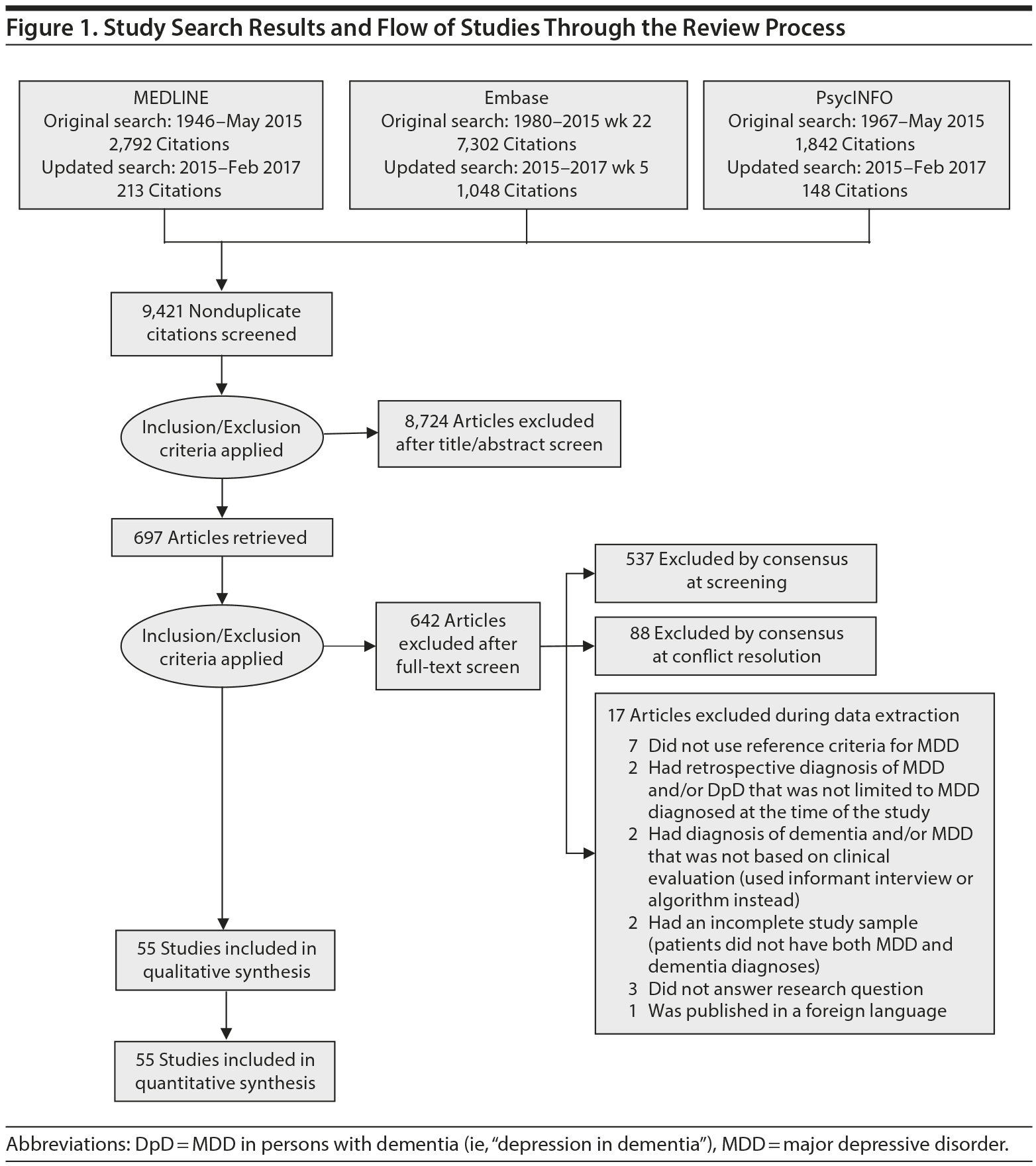
Study Selection: We screened 9,421 studies, and 55 met the inclusion criteria (ie, used validated criteria for both MDD and dementia).
Data Extraction: Two independent reviewers extracted data from included studies. Meta-analysis was used to determine the pooled estimates and 95% confidence intervals for the prevalence of DpD. Prevalence across dementia subtypes, study setting, diagnostic criteria, and dementia severity was compared in subgroup analyses.
Results: The prevalence of MDD in all-cause dementia was 15.9% (95% CI, 12.6%-20.1%). The prevalence of MDD was higher among individuals with vascular dementia (24.7%) compared to Alzheimer’s disease (14.8%). Studies using the provisional diagnostic criteria for DpD reported a higher prevalence (35.6%) compared to studies using either the DSM-III-R (13.2%) or DSM-IV (17.3%) criteria.
Conclusions: Depression is common among individuals with dementia, and the type of dementia and diagnostic criteria affect prevalence estimates of DpD. Further studies are required to understand factors that lead to the development of DpD and strategies to prevent and treat DpD.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Meta-Analysis of the Prevalence of Major Depressive Disorder Among Older Adults With Dementia

ABSTRACT
Objective: Little is known about the overall prevalence of major depressive disorder (MDD) in persons with dementia (ie, “depression in dementia”: DpD). The aim of this systematic review and meta-analysis was to determine the prevalence and factors associated with DpD among older adults (age range 58.7-87.8 years). The protocol was registered in the PROSPERO registry (2015:CRD42015020681).
Data Sources: We searched the following electronic databases: MEDLINE (1946-February 2017), Embase (1980-2017 week 5), and PsycINFO (1967-February 2017) using medical subject headings and free-text search terms for studies in the English language.
Study Selection: We screened 9,421 studies, and 55 met the inclusion criteria (ie, used validated criteria for both MDD and dementia).
Data Extraction: Two independent reviewers extracted data from included studies. Meta-analysis was used to determine the pooled estimates and 95% confidence intervals for the prevalence of DpD. Prevalence across dementia subtypes, study setting, diagnostic criteria, and dementia severity was compared in subgroup analyses.
Results: The prevalence of MDD in all-cause dementia was 15.9% (95% CI, 12.6%-20.1%). The prevalence of MDD was higher among individuals with vascular dementia (24.7%) compared to Alzheimer’s disease (14.8%). Studies using the provisional diagnostic criteria for DpD reported a higher prevalence (35.6%) compared to studies using either the DSM-III-R (13.2%) or DSM-IV (17.3%) criteria.
Conclusions: Depression is common among individuals with dementia, and the type of dementia and diagnostic criteria affect prevalence estimates of DpD. Further studies are required to understand factors that lead to the development of DpD and strategies to prevent and treat DpD.
J Clin Psychiatry 2018;79(5):17r11772
To cite: Asmer MS, Kirkham J, Newton H, et al. Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J Clin Psychiatry. 2018;79(5):17r11772.
To share: https://doi.org/10.4088/JCP.17r11772
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Queen’s University, Kingston, Ontario, Canada
bDepartments of Psychiatry and Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta, Canada
cDepartment of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada
*Corresponding author: Dallas P. Seitz, MD, PhD, FRCPC, Providence Care Hospital, 752 King St West, Kingston, Ontario, Canada K7L 4X3 ([email protected]).
With population aging, there will be increasing numbers of older adults with Alzheimer’s disease and dementia.1 Dementia currently affects 24 million individuals worldwide, and its prevalence is expected to quadruple by 2050.2 While dementia is associated with cognitive changes, behavioral changes such as depression also frequently occur3 with up to 20% of individuals reporting some degree of clinically significant depressive symptoms.4 Depressive symptoms are even common in mild cognitive impairment (MCI)-a recent meta-analysis found that the prevalence of depression in MCI was an estimated 32%.5 Depressive symptoms can have adverse consequences for patients and their care givers,6 and thus a clear understanding of the prevalence of depression in dementia is warranted.
Individuals with dementia are twice as likely as age-matched controls to be diagnosed with depression7; conversely, the presence of chronic depression increases the risk of dementia later in life.8 As operationalized in the construct mild behavioral impairment,9 the emergence of new depressive symptoms in older adults is associated with increased risk of cognitive decline and dementia, suggesting that depression may be a prodrome of dementia in addition to a factor associated with poorer outcomes when comorbid with dementia.10,11 It is hypothesized that depression and dementia may be linked by common risk factors such as vascular disease, hypothalamic-pituitary-axis dysfunction, and increased expression of inflammatory cytokines.8,12,13 The occurrence of major depressive disorder (MDD) in dementia (ie, “depression in dementia”: DpD) may accelerate cognitive and functional decline, lead to poor medical outcomes, hasten admission to long-term care, and increase mortality.14-21 DpD is also associated with increased burden and depression among caregivers of people with dementia.14,22
Although there are known associations between MDD and dementia, the overall prevalence of DpD has not been well described. An estimated 60% of dementia patients with depressive symptoms meet the criteria for MDD, and 1 study reported a prevalence of MDD in dementia of 12.7%.23 Other studies12,14,24,25 have observed results that range from 8.0% to 40.0%, with many estimates near the lower end of the range. Possible reasons for the wide range may be due to different diagnostic criteria used to define MDD, methodological variation, and differences in the underlying study populations.
The goal of our study was to conduct a systematic review and meta-analysis to determine the prevalence of DpD among older adults (age range 58.7-87.8 years) in studies that used validated criteria for the diagnosis of both depression and dementia. We also investigated factors associated with DpD including subtype and severity of dementia, clinical setting of studies, and clinical criteria for diagnosing dementia and depression. This information may help us better understand the overall burden of DpD and inform screening, clinical evaluation, and management decisions.
METHODS
Literature Search and Inclusion Criteria
Our review was approved by Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. Prior to beginning the literature review, our protocol was registered in the PROSPERO registry (2015:CRD42015020681).26 We searched MEDLINE (1946 to February 2017), Embase (1980 to 2017 week 5) and PsycINFO (1967 to February 2017) databases for relevant articles. We used medical subject headings and free-text search terms to identify studies measuring the prevalence of depression in patients with dementia. See Supplementary Figure 1 for search terms used in the MEDLINE database; these terms were modified slightly for the other databases.
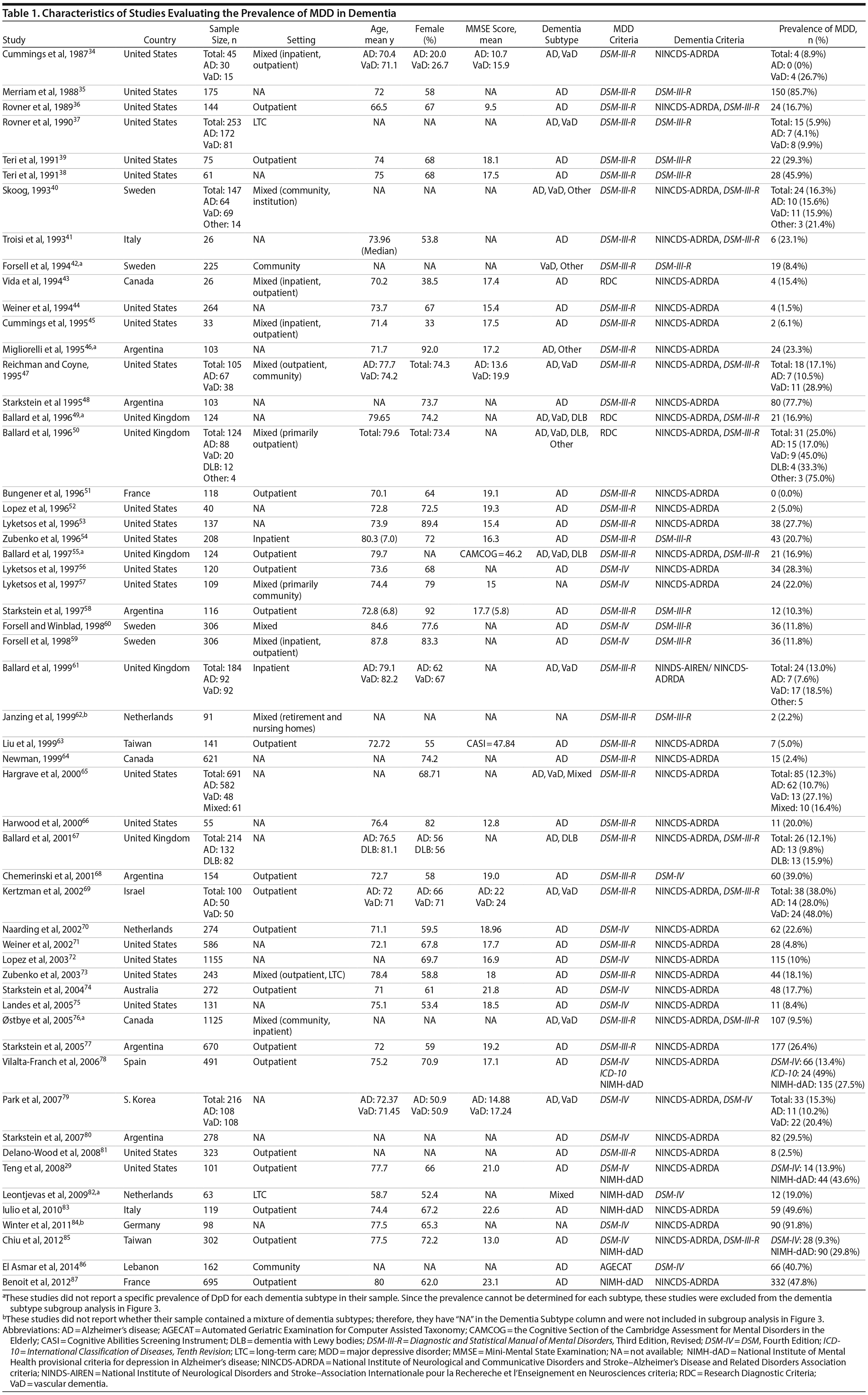
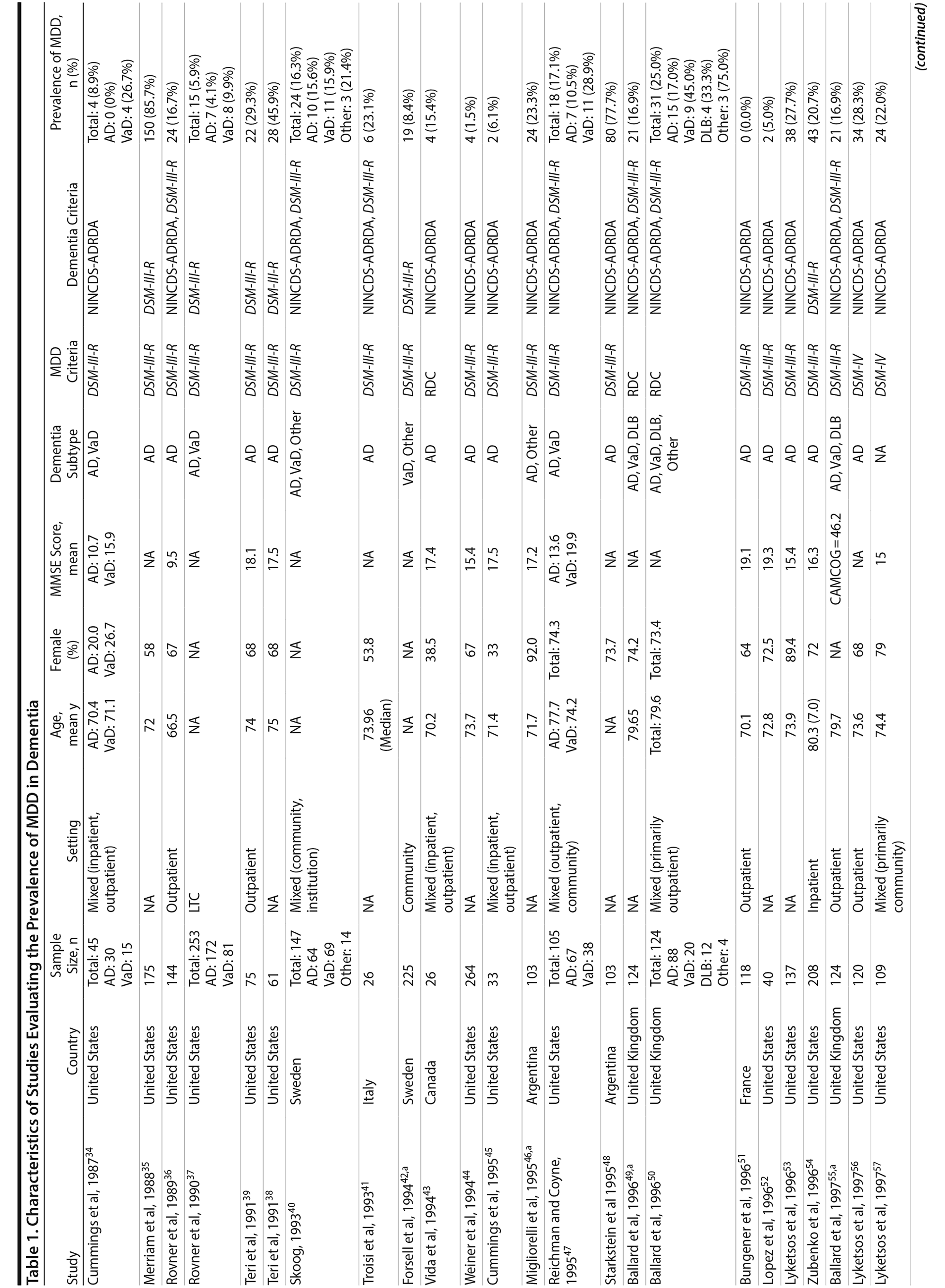
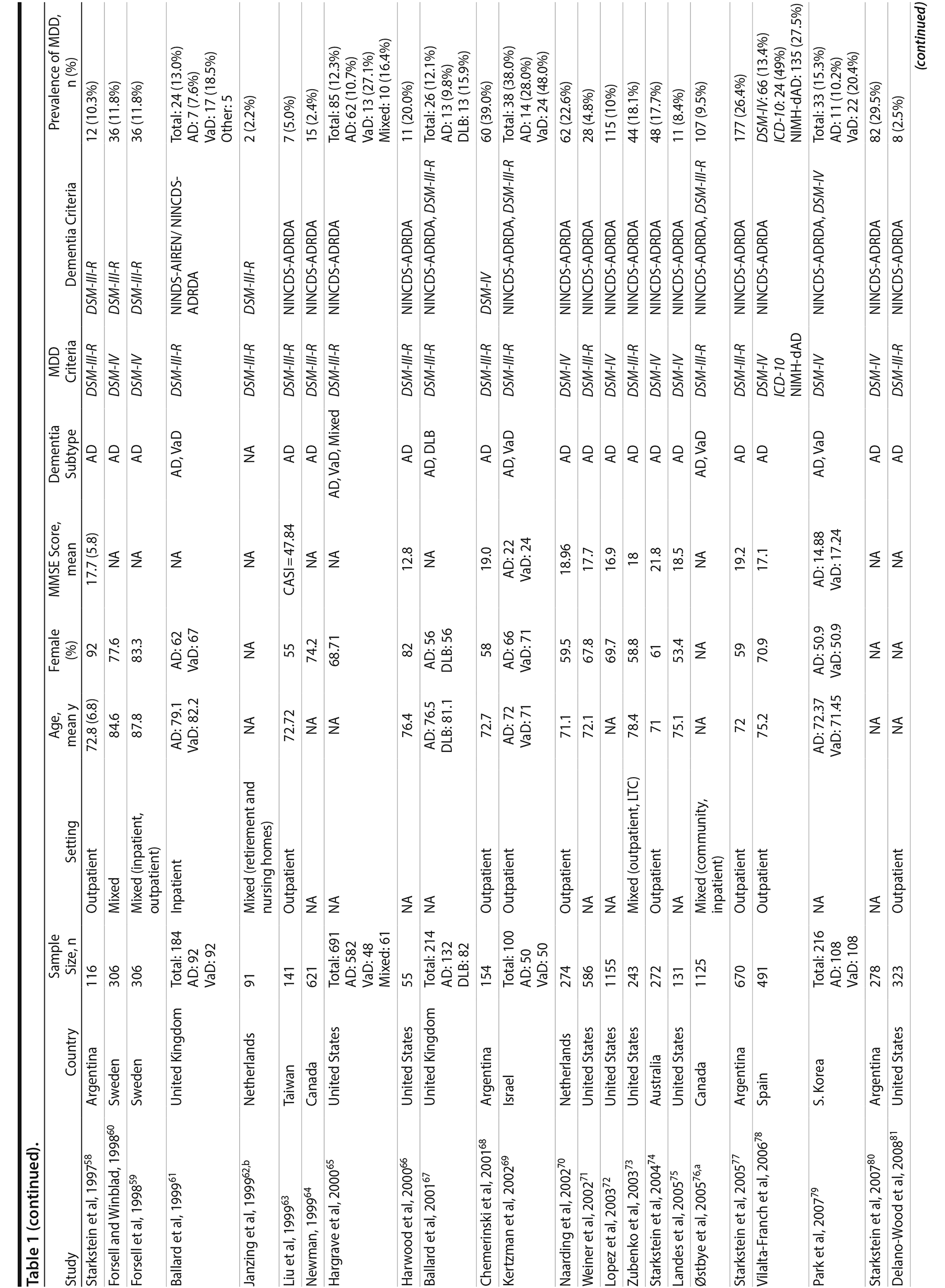
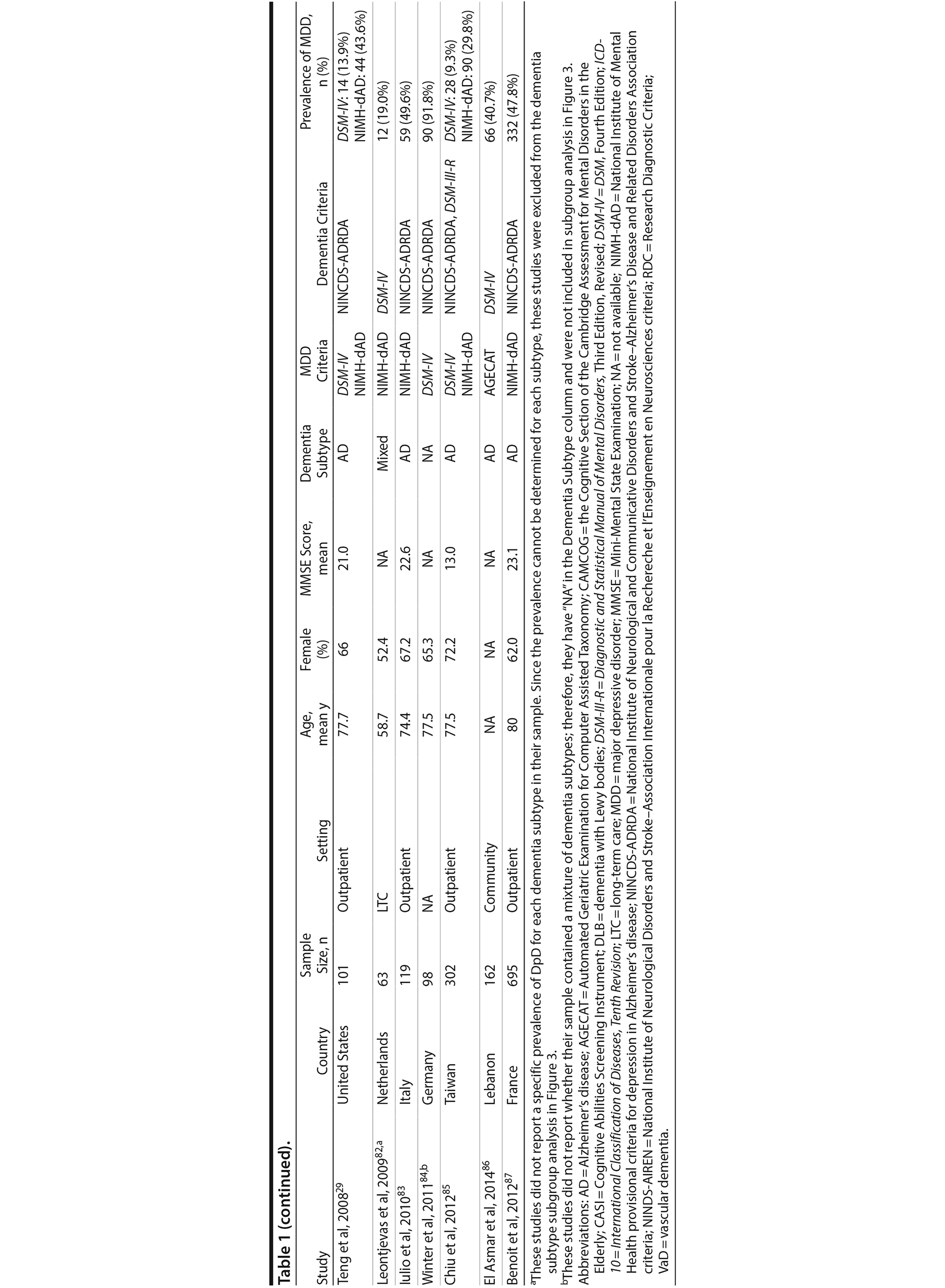
We included prospective and retrospective observational studies published in the English language. Depression was defined as MDD diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R) or more recent versions27 or International Classification of Diseases, Ninth Edition (ICD-9) or more recent versions.28 In addition to the generic criteria for MDD, we also included studies that used National Institute of Mental Health provisional criteria for depression in Alzheimer’s disease (NIMH-dAD). These criteria were derived from the DSM-IV criteria for MDD with some modifications to the duration and frequency of depressive symptoms to reflect unique features of DpD.29,30 Dementia was defined as all-cause dementia as diagnosed using DSM-III-R (or more recent) and ICD-9 (or more recent) and as specific types of dementia using National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for Alzheimer’s disease31 and National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’ Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia.32

- Depressive symptoms commonly occur in people with dementia, although the prevalence of major depressive disorder and factors associated with depression in dementia are not well understood.
- For all patients with dementia, particularly those with vascular dementia, clinicians should be alert to the high prevalence of major depressive disorder.
We excluded studies that only measured depressive symptoms without the criteria for MDD, studies of only Parkinson’s disease and frontotemporal dementia, and studies that defined dementia solely using cognitive screening tests. We also excluded studies where the depression or dementia diagnosis was made retrospectively using chart reviews rather than clinical evaluations.
Titles and abstracts of citations that were retrieved from the electronic databases were screened by 2 authors, and relevant studies were retrieved for full-text review. In cases where there was conflict between reviewers, a third author reviewed the citation.
Data Extraction
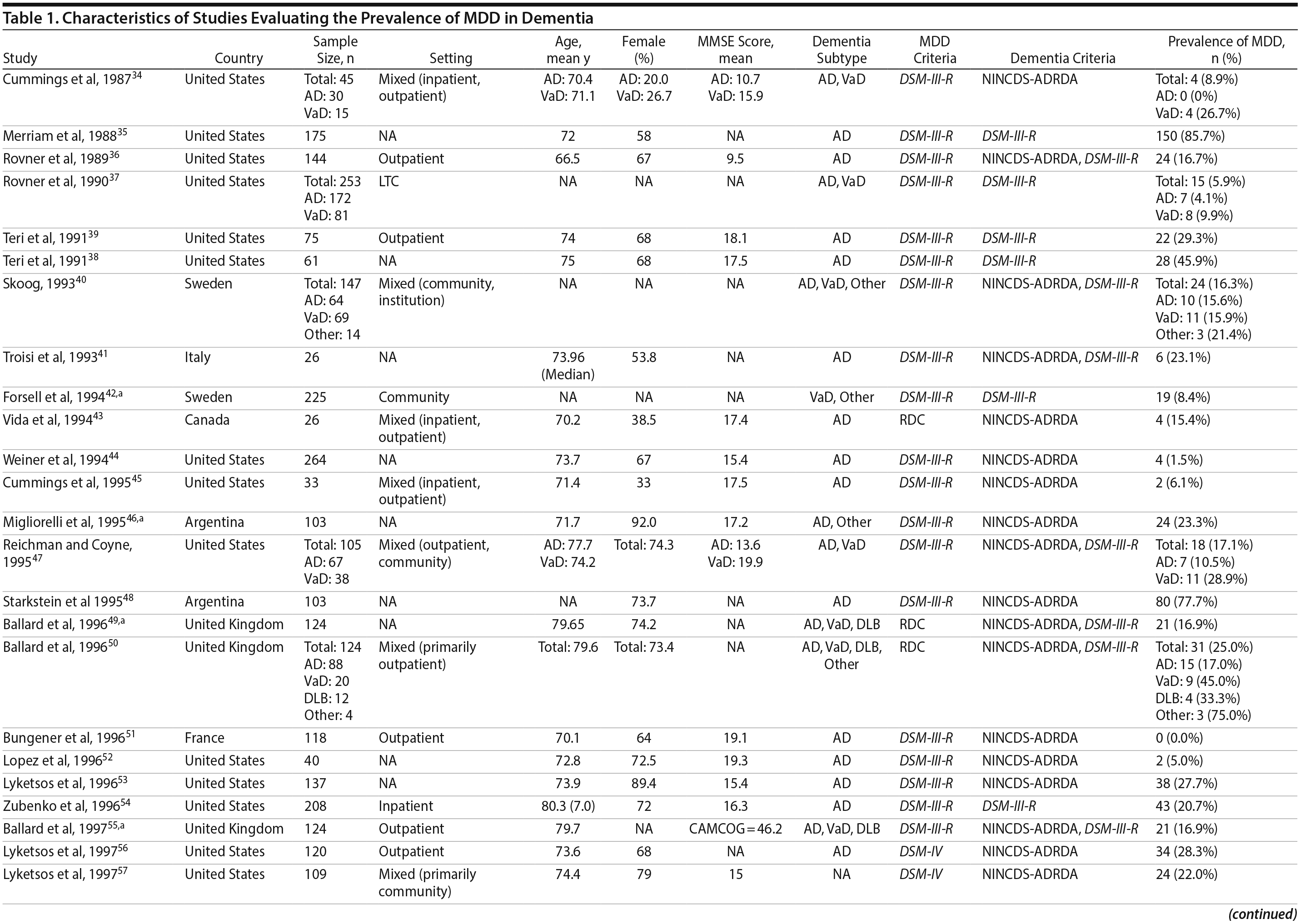
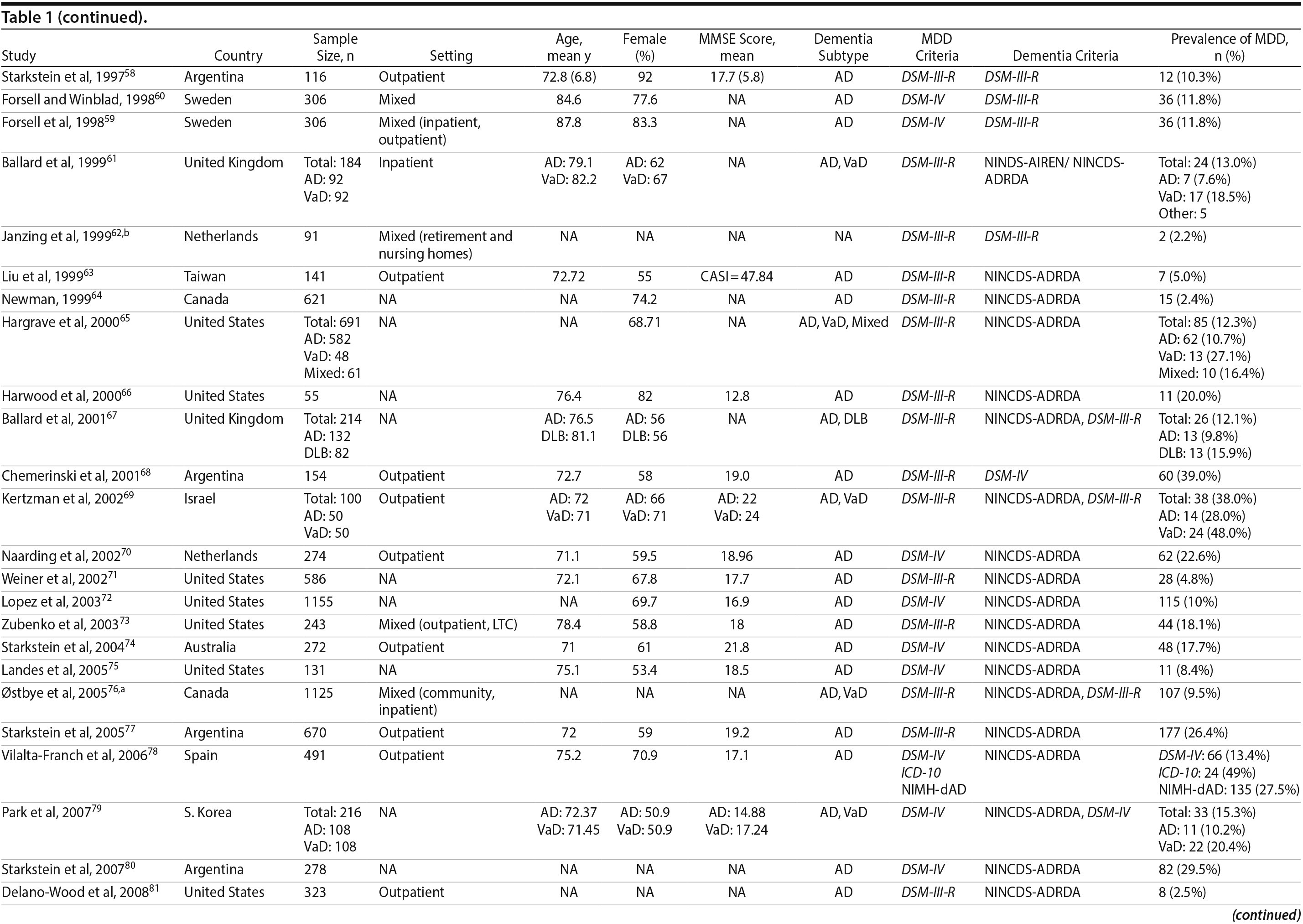
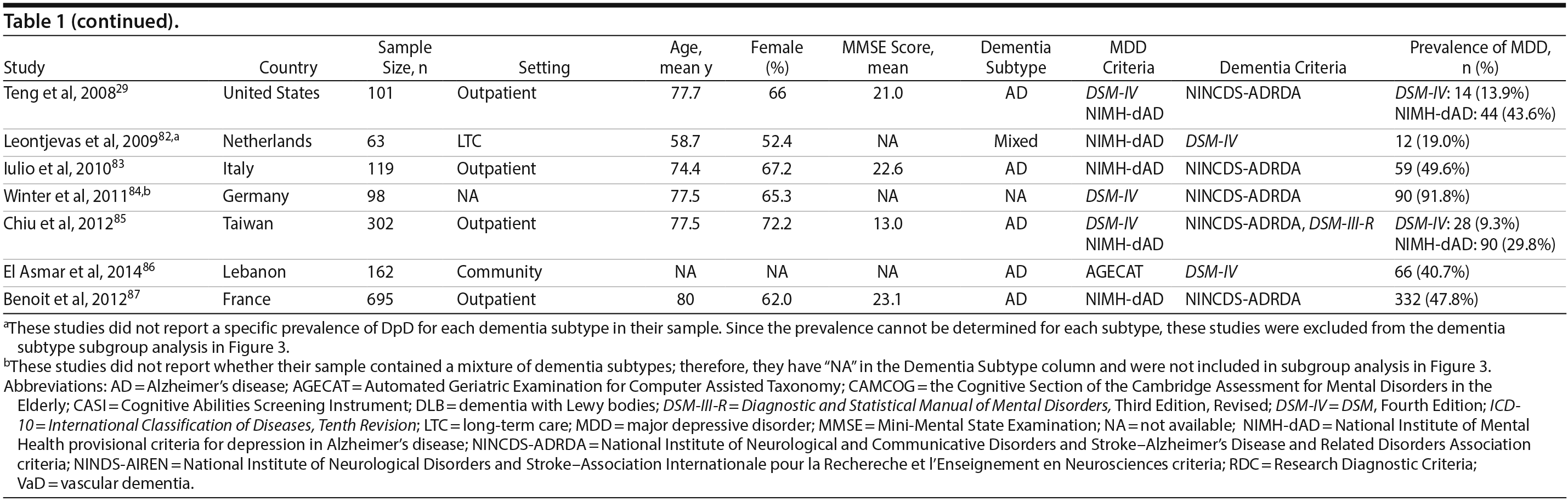
Two authors used a standardized form to independently extract information from each study regarding the total sample size and proportion of individuals with dementia who met criteria for MDD. Demographic information such as mean age, sex, study setting (eg, community, outpatient, long-term care, inpatient), and study country, when reported, was extracted. Clinical data regarding dementia subtype (eg, Alzheimer’s disease, vascular dementia [VaD], mixed dementia types [ie, VaD and Alzheimer’s disease], or dementia with Lewy bodies [DLB]), diagnostic criteria for the diagnosis of MDD and dementia, mean scores on cognitive testing (eg, Mini-Mental State Examination [MMSE] or other tests), and dementia severity scales (eg, Clinical Dementia Rating), when reported, were also extracted.
Assessment of Study Quality
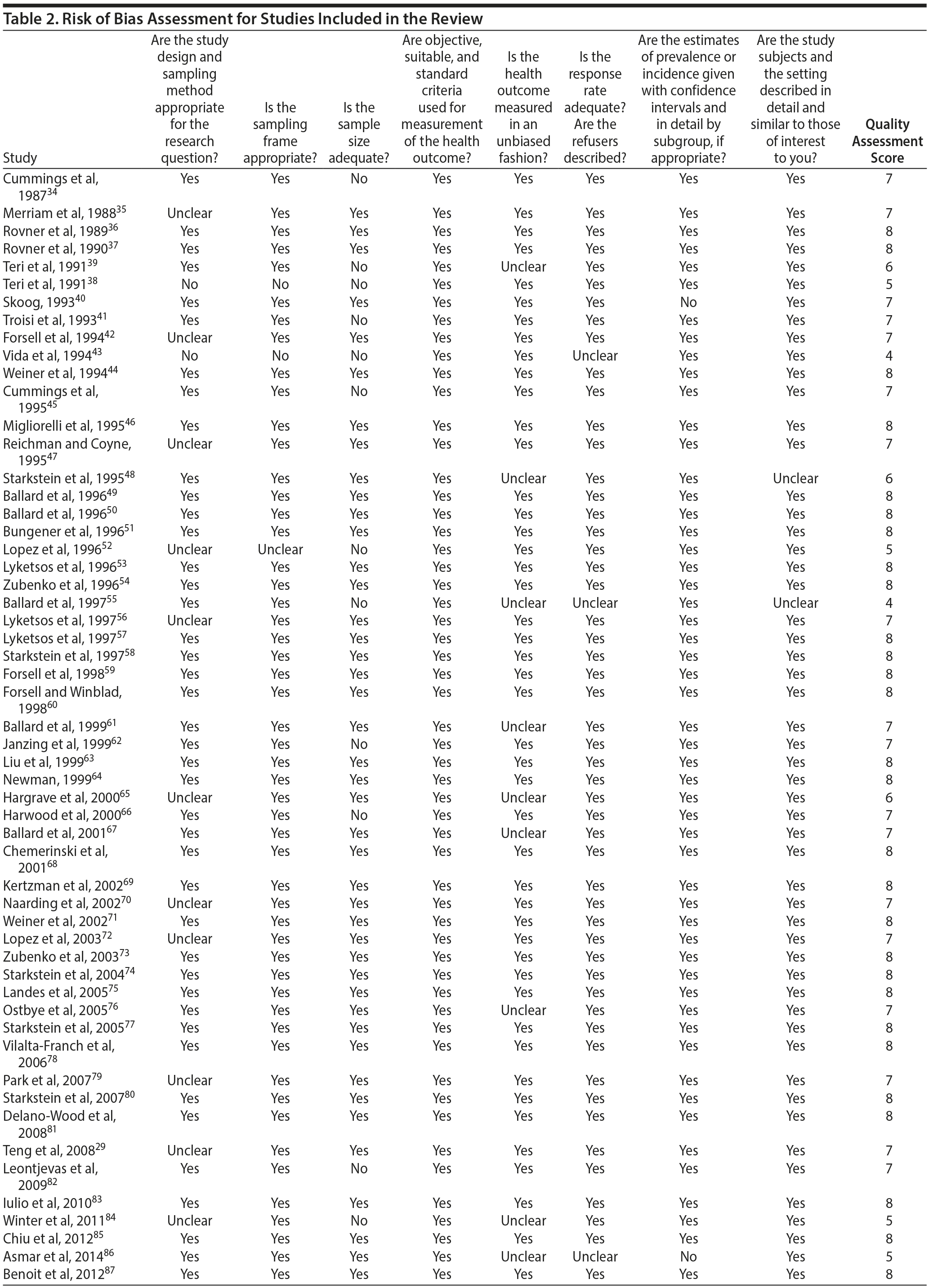
Study quality was assessed using the Loney criteria.33 These criteria assess study bias through 8 characteristics: representativeness of the study population, sampling method, sample size, use of standardized criteria, and unbiased assessment of diagnoses and prevalence. We also considered the presence and size of confidence intervals in the estimates of prevalence. Each characteristic was rated “yes,” “no,” or “unclear” (see Table 2); “yes” answers indicated a lower risk of bias in the respective characteristics.
Data Synthesis and Meta-Analysis
Studies were assessed qualitatively to determine appropriateness to combine in meta-analysis. Estimates from clinically homogeneous study populations in individual studies were then combined using the logit transformation in a random-effects meta-analysis to arrive at the pooled estimates and 95% confidence intervals for the prevalence of DpD. We assessed the statistical heterogeneity of studies within the meta-analysis using the Q and I2 statistics, with evidence of statistical heterogeneity defined as Q (χ2) statistic P values of < .1 or I2 values > 50%. In all analyses, 2-sided P values < .05 were used as the threshold for statistical significance. Publication bias was visually assessed using funnel plots. The software package R version 3.2.3 was used for all statistical analyses (R Development Core Team and R Foundation for Statistical Computing, Vienna, Austria).
Subgroup Analyses
A comparison of the prevalence of depression by dementia subtype (ie, Alzheimer’s disease, VaD, mixed dementia types, or DLB), the criteria used to diagnose MDD and dementia, study continent, study setting (eg, community, outpatient, long-term care, inpatient), and severity of cognitive impairment was examined in subgroup analyses. Community setting was defined as participants from epidemiologic samples assessed in a general community setting or at home. Outpatient setting was defined as outpatients in a hospital or another health-care outpatient setting. Long-term care was defined as participants living in a long-term care or nursing-home facility. Inpatient setting included participants being admitted to a psychiatric inpatient unit at the time of their assessment. The severity of dementia was also examined in subgroup analyses and categorized as “mild” if the mean MMSE score was between 18-24, “moderate” if the mean MMSE score was between 10-17, and “severe” if the mean MMSE score was < 10. We also reported subgroup analyses on the basis of Clinical Dementia Rating scale scores of 0.5, 1, 2, and 3.
Sensitivity Analysis
To determine if study quality had an impact on the estimates of DpD and heterogeneity, we conducted a sensitivity analysis where the primary analysis of DpD was completed after excluding studies with a quality score of < 7 of 8.
RESULTS
Study Selection and Description of Included Studies
A total of 9,421 nonduplicate citations were found, and 8,724 were excluded after review of title and abstract. We included for full-text review 697 studies of which 642 were excluded. The flow diagram and reasons for exclusion are summarized in Figure 1. A total of 55 studies29,34-87 met inclusion criteria (Table 1). Together, these studies included a total of 13,172 subjects. Thirty studies were conducted in unspecified or mixed outpatient and inpatient settings, 2 studies were conducted in a long-term care setting, 18 were conducted in outpatient settings, 3 were conducted in community settings, and 2 were conducted in inpatient settings.
Assessment of Study Quality
Most studies were found to be of high methodological quality. Of the 55 included studies, 46 studies had a score of 7 or 8 on the quality assessment score. The remaining 9 studies received scores ranging from 4 to 6 (Table 2).
Meta-Analysis of the Prevalence of MDD in Older Adults With Dementia
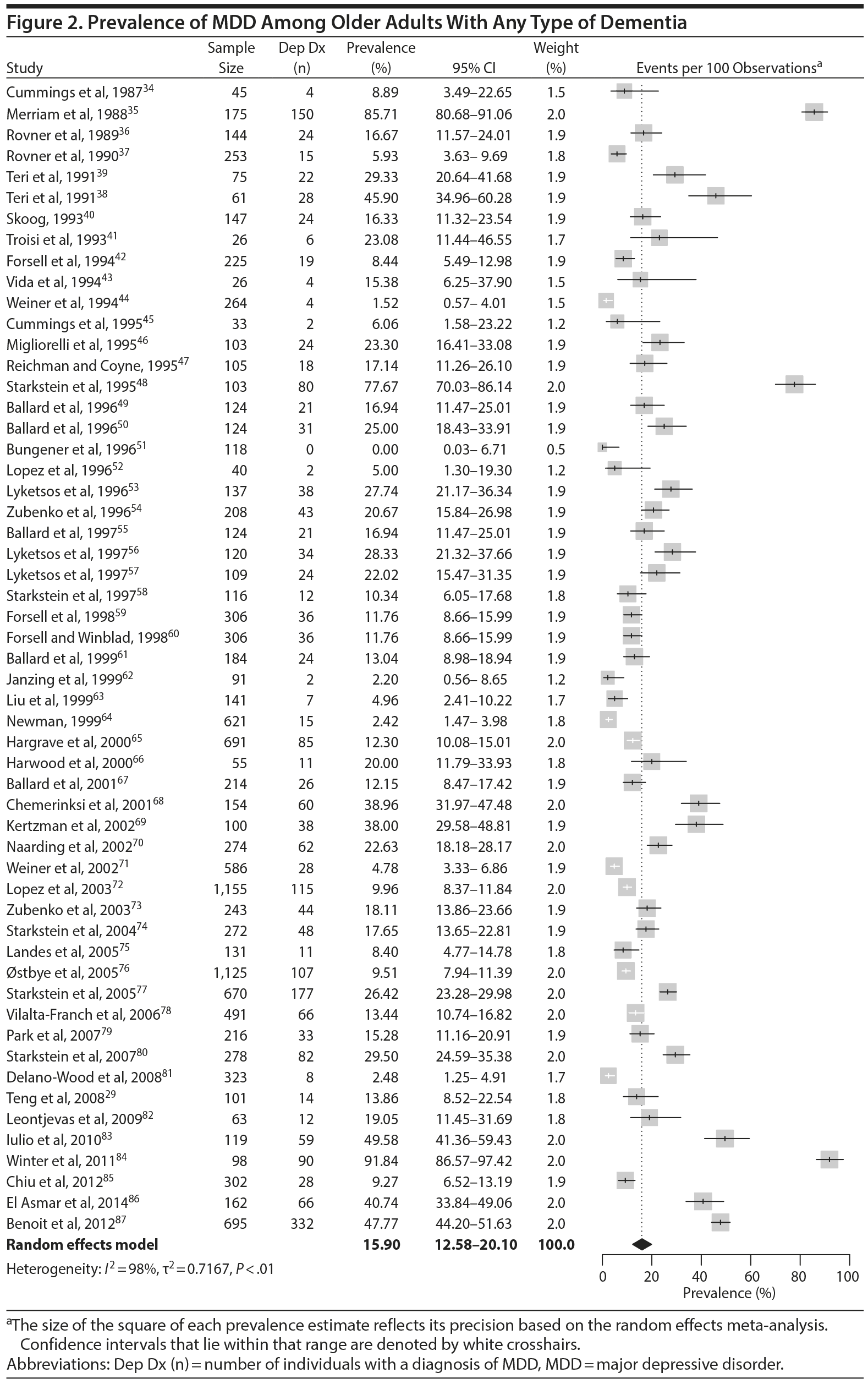
The prevalence of DpD across all dementia subtypes varied from 0.0% to 91.8% in the individual studies (Figure 2). In meta-analysis, the overall prevalence of DpD for any definition of MDD and all-cause dementia was 15.9% (95% CI, 12.6%-20.1%; Figure 2). There was a high degree of heterogeneity noted in the overall meta-analysis (I2 = 98%, P < .01). There was no evidence of publication bias in this analysis, according to a visual inspection of the funnel plot (Supplementary Figure 2).
Subgroup Analyses
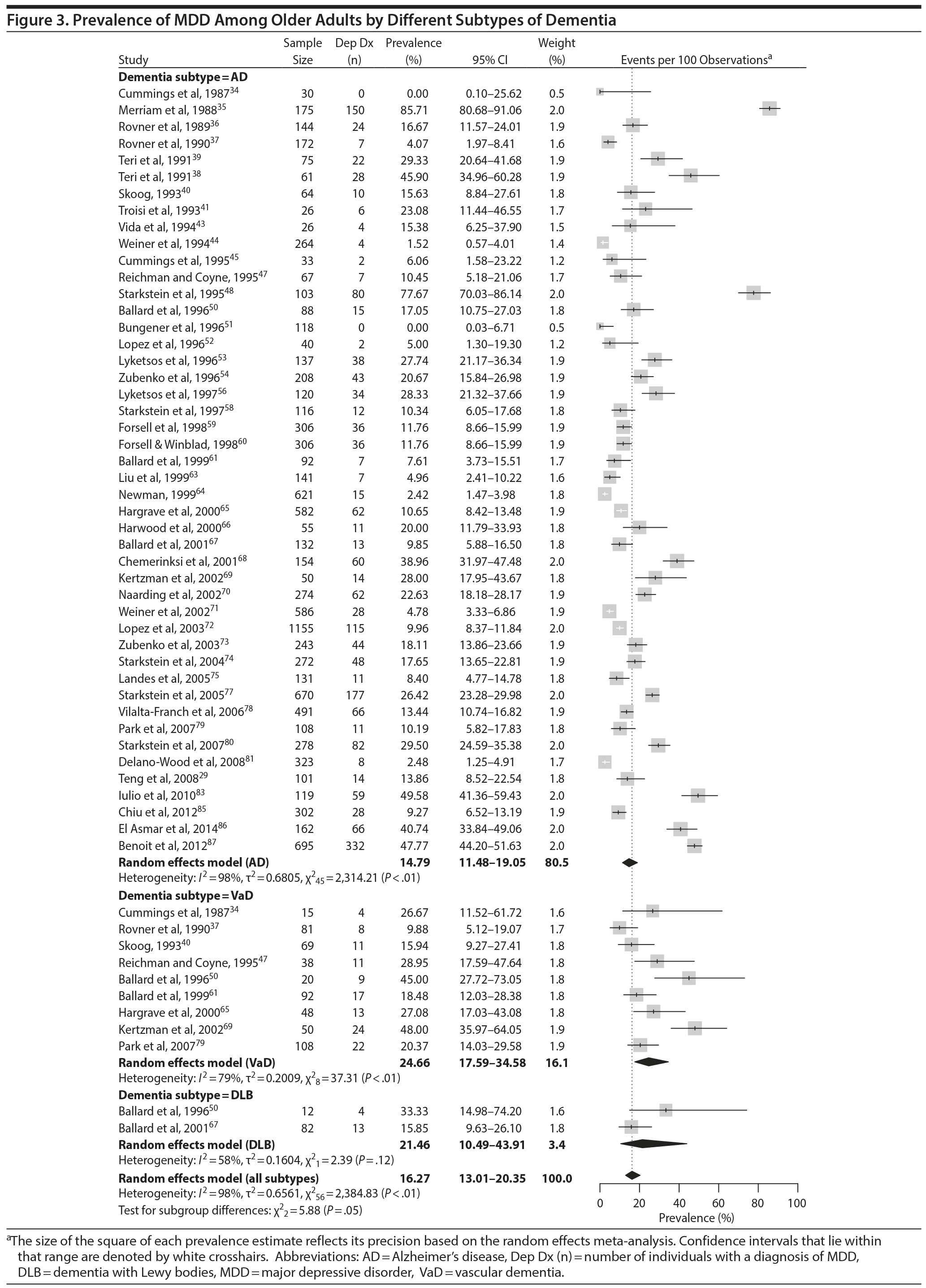
Prevalence of DpD by dementia subtypes. Of the 55 studies, 46 reported on the prevalence of DpD among individuals with Alzheimer’s disease and 9 reported similarly among individuals with VaD. Two studies also reported on individuals with DLB. The pooled prevalence of DpD among older adults with AD was 14.8% (95% CI, 11.5-19.1), 24.7% (95% CI, 17.6-34.6) among those with VaD, and 21.5% (95% CI, 10.5-43.9) among those with DLB (Figure 3). There was a statistically significant difference between the 3 groups (Q = 5.9, P = .05), and pairwise comparisons of each subtype showed a statistical difference between Alzheimer’s disease and VaD (Q = 5.63, P = .02) but none between the other comparisons (Alzheimer’s disease vs DLB: Q = 0.93, P = .34; VaD vs DLB: Q = 0.12, P = .73). There was evidence of statistical heterogeneity within the Alzheimer’s disease (Q = 2,314.2, P < .01, I2 = 98%) and the VaD subgroups (Q = 37.3, P < .01, I2 = 79%).
Prevalence of DpD by diagnostic criteria for MDD. All 55 studies reported at least one diagnostic criterion used to diagnose MDD. In reporting the prevalence of DpD, 34 studies used the DSM-III-R, 11 used the DSM-IV, 3 used the Research Diagnostic Criteria, 3 used the NIMH-dAD, 3 used multiple diagnostic criteria, and 1 used Automated Geriatric Examination for Computer Assisted Taxonomy to define MDD. Within the DSM-III-R and DSM-IV subgroups, the pooled prevalence of DpD was 13.2% (95% CI, 9.4%-18.6%) and 17.3% (95% CI, 9.6%-31.4%), respectively. The prevalence of DpD using NIMH-dAD was 35.6% (95% CI, 27.6%-46.0%). The difference between the 3 subgroups was statistically significant (Q = 22.1, P < .01), and pairwise comparisons of each subgroup showed a statistical difference between DSM-III-R and NIMH-dAD (Q = 20.78, P < .01) and DSM-IV and NIMH-dAD (Q = 4.79, P = .03) but none between DSM-III-R and DSM-IV (Q = 0.61, P = .43) (Supplementary Figure 3).
Prevalence of DpD by severity of cognitive impairment. A total of 10 studies reported on the prevalence of DpD among individuals with mild dementia (MMSE, 18-24) and 9 studies reported on individuals with moderate dementia (MMSE, 10-17). The pooled prevalence of DpD among those with mild dementia was 22.1% (95% CI, 15.7%-30.9%). The pooled prevalence of DpD among those with moderate dementia was 11.6% (95% CI, 6.9%-19.7%). The difference between the 2 subgroups did not meet the threshold for statistical significance (Q = 4.03, P = .04) (Supplementary Figure 4). There were also no differences in prevalence of DpD according to the Clinical Dementia Rating scale scores in the 4 studies reporting this information (Supplementary Figure 5).
Prevalence of DpD by study setting. A total of 18 studies reported on the prevalence of DpD in outpatient settings, 2 reported on those examined in long-term care, 3 reported on those in the community, and 2 reported on those sampled from inpatient settings. Studies in outpatient clinical settings reported a pooled prevalence estimate of DpD of 18.4% (95% CI, 13.9%-24.4%). The pooled prevalence of DpD among individuals in long-term care was 10.6% (95% CI, 3.4%-33.3%), in the community was 20.0% (95% CI, 8.3%-48.0%), and in inpatient samples was 16.7% (95% CI, 10.7%-26.3%). There was no significant difference between subgroups (Q = 1.0, P = .81) (Supplementary Figure 6.
Prevalence of DpD by continent of study sample. We pooled the studies by continent for subgroup analysis: 5 were from Asia, 1 from Australia, 19 from Europe, 25 from North America, and 5 from South America. The pooled prevalence of DpD from studies in Asia was 17.0% (95% CI, 8.8%-32.7%); in Australian studies, 17.7% (95% CI, 13.7%-22.8%); in European studies, 18.1% (95% CI, 12.4%-26.5%); in North American studies, 12.2% (95% CI, 7.5%-19.8%); and in South American studies, 29.5% (95% CI, 16.1%-54.1%). The differences between subgroups were not statistically significant (Q = 5.1, P = .28) (Supplementary Figure 7).
Sensitivity Analysis
After excluding studies with a quality score of less than 7, the prevalence of DpD from the remaining 46 studies was 14.1% (95% CI, 10.9%-18.3%), and heterogeneity remained high (Q = 2,364.5, P < .01, I2 = 98%).
DISCUSSION
Our review found that MDD is common among older adults with dementia with a prevalence of 15.9%. There was a high degree of heterogeneity in the estimates of the prevalence of DpD. Some of the factors that were associated with the prevalence of DpD included type of dementia as well as the diagnostic criteria used for dementia. We did not observe that study setting or dementia severity had a significant impact on the prevalence of DpD. The findings from our review are consistent with a previous meta-analysis23 of DpD, which reported a prevalence of 12.7%, although this study included only 25 studies as compared to the 55 studies that were included in our review. Overall, our review provides evidence supporting a high prevalence of DpD, highlighting its clinical importance in this population.
Our review indicates that individuals with dementia have an increased prevalence of MDD when compared to individuals without dementia, where depression prevalence is approximately 1.8%.88 The reasons for this difference in prevalence are most likely multifactorial. Neurodegenerative changes associated with dementia may lead to alterations in neurotransmitters that contribute to the increased prevalence of depression.79,89 Depression or depressive symptoms, common among individuals with MCI,5 may also be a prodrome of dementia in many individuals,10,12 and the presence of symptoms may be associated with an increased risk of subsequent conversion to dementia.90 Chronic depression has also been identified as a risk factor for the development of dementia91,92 and may contribute to the high prevalence of DpD. Individuals with dementia also frequently experience stressful life events and limited social supports that further contribute to the increased risk of depression.93 There are quite likely multiple pathways that lead to the development of DpD, and further studies are required to understand how these different factors contribute.
One challenge with accurately diagnosing DpD is that depression itself is associated with cognitive impairment.94 Individuals with major depression can display clinically significant cognitive deficits, particularly in measures of executive functioning and attention, both during depressive episodes and in the euthymic state.94 The presence of cognitive deficits in depression can also be a marker of poor response to antidepressant treatment.95 Our review focused on the diagnosis of MDD and dementia using validated diagnostic criteria, and as such, misclassification of individuals as having dementia when these cognitive deficits may have been caused by depression should have been minimal. However, additional studies are required to identify both neuropsychological profiles94,96 and imaging biomarkers,97 which may be useful in distinguishing between cognitive deficits caused by depression versus those of early dementia. In clinical practice, actively treating depression and other comorbidities that may be contributing to cognitive impairment would be recommended for individuals presenting with clinically significant depressive symptoms and cognitive impairment prior to establishing a diagnosis of dementia.
In our review, the prevalence of MDD in older adults with VaD (24.7%) was significantly higher than in those with Alzheimer’s disease (14.8%). While there may be multiple reasons for this difference, it is most likely due to the direct relationship between cerebrovascular disease pathology and the risk of developing depression. Cerebrovascular disease is known to be an independent risk factor for depression among individuals without dementia; therefore, the higher rate of DpD in VaD is consistent with the increased risk of depression.98 VaD is also a heterogeneous disease, and specific patterns of cerebrovascular disease in VaD (eg, multiinfarct dementia vs large cortical infarct) may be associated with different rates of MDD prevalence.99 Our study suggests that individuals with VaD may be at particularly high risk of depression, and clinicians should carefully monitor mood symptoms in this population.
We found that the NIMH-dAD provisional diagnostic criteria for DpD were associated with higher prevalence estimates for DpD when compared to MDD criteria that are not specific to dementia. The NIMH-dAD criteria were derived from DSM-IV criteria with modifications that include reducing the number of core depressive symptoms from 5 to 3 symptoms, omitting the concentration criteria, and allowing symptoms to be present at any point within a 2-week period, which is in contrast to the DSM-IV requirement that all the symptoms be present for most of the time in a 2-week period.30 Our review found that the NIMH-dAD criteria are quite likely more sensitive to detecting depression in older adults with dementia than the DSM-IV criteria, although the increased sensitivity may be associated with a loss of specificity.29 Whether the NIMH-dAD is susceptible to false positives or the DSM-III-R or DSM-IV is susceptible to false negatives is unknown, and whether 1 set of criteria is preferred or more accurate than another requires further study. Clinicians should be aware that the diagnostic criteria used to identify DpD may impact the case definition and prevalence estimates, and the criteria most relevant for clinical application may depend on the specific situation and intended clinical purpose such as screening or confirmation of diagnosis.
The estimated prevalence of DpD according to dementia severity was numerically higher among those with milder dementia than moderate dementia, although this was not statistically significant. While a recent meta-analysis100 showed no association between dementia severity and frequency of depressive symptoms, another review23 of DpD found a higher prevalence of MDD among individuals with milder severity of dementia. It is likely that depression criteria are more accurate among individuals with mild dementia where awareness of symptoms is preserved to a greater extent than among individuals with more advanced dementia.101 Whether the accuracy of depression criteria differs across severities of dementia requires further study. Although not statistically significant, the prevalence of depression among individuals in long-term care was lower than in other settings. This may in part be related to the severity of dementia, which tends to be higher among long-term care residents, although other factors may also play a potential role in these differences as well.
The high prevalence of DpD has important clinical implications14-21 and supports recommendations that clinicians should be vigilant for MDD in dementia.102 Given the relatively high baseline probability of depression in dementia and the availability of screening tools with good diagnostic test properties,103 screening for depression in dementia may be clinically feasible. Once depression is identified, guidelines recommend nonpharmacologic and supportive measures for all individuals with dementia,104,105 and there is increasing evidence for specific psychotherapies for treatment of DpD.106 Guidelines also recommend a trial of antidepressants when there is an inadequate response to nonpharmacologic management of MDD,104,105 although the efficacy of antidepressants in treating DpD is controversial.107
Our study used rigorous review methods and included only studies that used standardized criteria for both MDD and dementia. Most of the included studies were of high methodological quality. However, significant statistical heterogeneity was observed in our meta-analyses. While we could identify some potential contributors to this heterogeneity, additional differences in underlying methodologies and study populations very likely contributed to this heterogeneity. There are some limitations to our review. Although our review was focused on MDD among individuals with dementia, subsyndromal symptoms of depression are also common in dementia and associated with poor outcomes.4 The prevalence of subsyndromal depression and factors associated with these symptoms have been evaluated in mild cognitive impairment,5 and examination of depressive symptoms more broadly among individuals with dementia should also be considered as a separate topic for meta-analysis. Information on the use of antidepressants was not reported in the majority of studies included in our review, although antidepressant use has been reported to be common among individuals with dementia.108 The effectiveness of antidepressants for treating DpD is currently questionable,18 and whether antidepressant treatment impacts the prevalence estimates is unknown.
CONCLUSION
MDD is common among individuals with dementia, and the prevalence of DpD varies with the type of dementia and depression criteria. Persons with dementia, caregivers, and health care providers should be aware of the high prevalence of MDD in dementia. Further research is needed to clarify the relationships between dementia and depression as well as to develop optimal strategies to identify and manage depression in individuals with dementia once it develops.
Submitted: June 28, 2017; accepted December 7, 2017.
Published online: July 31, 2018.
Potential conflicts of interest: Dr Seitz has been involved as a site investigator for clinical research trials sponsored by Roche. Drs Asmer, Kirkham, Ismail, and Mss Newton, Elbayoumi, and Leung have no conflicts to disclose.
Funding/support: This work was supported by the Canadian Institutes of Health Research Knowledge Synthesis Grant KRS-141000 and the Canadian Consortium on Neurodegeneration in Aging CNA-137794.
Role of the sponsor: The supporters had no role in the design, analysis, interpretation, or publication of this study.
Previous presentation: Poster presented at the 2017 annual meeting of the American Association for Geriatric Psychiatry; March 24-27; Dallas, Texas and the Canadian Academy of Geriatric Psychiatry; November 4-5; Toronto, Ontario.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res. 2012;43(8):600-608. PubMed CrossRef
2. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640-651. PubMed CrossRef
3. van der Linde RM, Dening T, Matthews FE, et al. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. 2014;29(6):562-568. PubMed CrossRef
4. Savva GM, Zaccai J, Matthews FE, et al. Prevalence, correlates and course of behavioral and psychological symptoms of dementia in the population. Br J Psychiatry. 2009;194(3):212-219. PubMed CrossRef
5. Ismail Z, Elbayoumi H, Smith EE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58-67. PubMed CrossRef
6. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475-1483. PubMed CrossRef
7. Andreasen P, Lonnroos E, von Euler-Chelpin MC. Prevalence of depression among older adults with dementia living in low- and middle-income countries: a cross-sectional study. Eur J Public Health. 2014;24(1):40-44. PubMed CrossRef
8. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538. PubMed CrossRef
9. Ismail Z, Aguera-Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929-938. PubMed CrossRef
10. Ismail Z, Malick A, Smith EE, et al. Depression versus dementia: is this construct still relevant? Neurodegener Dis Manag. 2014;4(2):119-126. PubMed CrossRef
11. Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202. PubMed CrossRef
12. Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24(6):461-472. PubMed CrossRef
13. Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35(6):776-781. PubMed CrossRef
14. Curran EM, Loi S. Depression and dementia. Med J Aust. 2013;199(6 suppl):S40-S44. PubMed
15. Feng L, Scherer SC, Tan BY, et al. Comorbid cognitive impairment and depression is a significant predictor of poor outcomes in hip fracture rehabilitation. Int Psychogeriatr. 2010;22(2):246-253. PubMed CrossRef
16. Lavretsky H, Zheng L, Weiner MW, et al. Association of depressed mood and mortality in older adults with and without cognitive impairment in a prospective naturalistic study. Am J Psychiatry. 2010;167(5):589-597. PubMed CrossRef
17. Mehta KM, Yaffe K, Langa KM, et al. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. J Gerontol A Biol Sci Med Sci. 2003;58(5):M461-M467. PubMed CrossRef
18. Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011;59(4):577-585. PubMed CrossRef
19. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117. PubMed CrossRef
20. Prado-Jean A, Couratier P, Druet-Cabanac M, et al. Specific psychological and behavioral symptoms of depression in patients with dementia. Int J Geriatr Psychiatry. 2010;25(10):1065-1072. PubMed CrossRef
21. Rapp MA, Schnaider-Beeri M, Wysocki M, et al. Cognitive decline in patients with dementia as a function of depression. Am J Geriatr Psychiatry. 2011;19(4):357-363. PubMed CrossRef
22. Watson LC, Lewis CL, Moore CG, et al. Perceptions of depression among dementia caregivers: findings from the CATIE-AD trial. Int J Geriatr Psychiatry. 2011;26(4):397-402. PubMed CrossRef
23. Chi S, Wang C, Jiang T, et al. The prevalence of depression in Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res. 2015;12(2):189-198. PubMed CrossRef
24. Huang CQ, Wang ZR, Li YH, et al. Cognitive function and risk for depression in old age: a meta-analysis of published literature. Int Psychogeriatr. 2011;23(4):516-525. PubMed CrossRef
25. Lyketsos CG. The interface between depression and dementia: where are we with this important frontier? Am J Geriatr Psychiatry. 2010;18(2):95-97. PubMed CrossRef
26. Konetzka RT, Brauner DJ, Shega J, et al. The effects of public reporting on physical restraints and antipsychotic use in nursing home residents with severe cognitive impairment. J Am Geriatr Soc. 2014;62(3):454-461. PubMed CrossRef
27. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders, Third Edition, Revised. Washington, DC: American Psychiatric Association; 1987.
28. Practice Management Information Corp. ICD-9-CM: International Classification of Diseases (9th Rev.) and Clinical Modification. 6th ed. Los Angeles, CA: PMIC; 2004.
29. Teng E, Ringman JM, Ross LK, et al. Diagnosing depression in Alzheimer disease with the national institute of mental health provisional criteria. Am J Geriatr Psychiatry. 2008;16(6):469-477. PubMed CrossRef
30. Olin JT, Schneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(2):125-128. PubMed CrossRef
31. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed CrossRef
32. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250-260. PubMed CrossRef
33. Loney PL, Chambers LW, Bennett KJ, et al. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19(4):170-176. PubMed
34. Cummings JL, Miller B, Hill MA, et al. Neuropsychiatric aspects of multi-infarct dementia and dementia of the Alzheimer type. Arch Neurol. 1987;44(4):389-393. PubMed CrossRef
35. Merriam AE, Aronson MK, Gaston P, et al. The psychiatric symptoms of Alzheimer’s disease. J Am Geriatr Soc. 1988;36(1):7-12. PubMed CrossRef
36. Rovner BW, Broadhead J, Spencer M, et al. Depression and Alzheimer’s disease. Am J Psychiatry. 1989;146(3):350-353. PubMed CrossRef
37. Rovner BW, German PS, Broadhead J, et al. The prevalence and management of dementia and other psychiatric disorders in nursing homes. Int Psychogeriatr. 1990;2(1):13-24. PubMed CrossRef
38. Teri L, Baer LC, Reifler BV. Depression in Alzheimer’s patients: investigation of symptom patterns and frequency. Clin Gerontol. 1991;11(1):47-57. CrossRef
39. Teri L, Wagner AW. Assessment of depression in patients with Alzheimer’s disease: concordance among informants. Psychol Aging. 1991;6(2):280-285. PubMed CrossRef
40. Skoog I. The prevalence of psychotic, depressive and anxiety syndromes in demented and non-demented 85-year-olds. Int J Geriatr Psychiatry. 1993;8(3):247-253. CrossRef
41. Troisi A, Pasini A, Gori G, et al. Assessment of depression in Alzheimer’s disease: symptoms, syndrome, and computed tomography findings. Dementia. 1993;4(2):87-93. PubMed
42. Forsell Y, Jorm AF, Winblad B. Outcome of depression in demented and non-demented elderly: observations from a three-year follow-up in a community-based study. Int J Geriatr Psychiatry. 1994;9(1):5-10. CrossRef
43. Vida S, Des Rosiers P, Carrier L, et al. Prevalence of depression in Alzheimer’s disease and validity of Research Diagnostic Criteria. J Geriatr Psychiatry Neurol. 1994;7(4):238-244. PubMed CrossRef
44. Weiner MF, Edland SD, Luszczynska H. Prevalence and incidence of major depression in Alzheimer’s disease. Am J Psychiatry. 1994;151(7):1006-1009. PubMed CrossRef
45. Cummings JL, Ross W, Absher J, et al. Depressive symptoms in Alzheimer disease: assessment and determinants. Alzheimer Dis Assoc Disord. 1995;9(2):87-93. PubMed CrossRef
46. Migliorelli R, Teson A, Sabe L, et al. Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry. 1995;152(1):37-44. PubMed CrossRef
47. Reichman WE, Coyne AC. Depressive symptoms in Alzheimer’s disease and multi-infarct dementia. J Geriatr Psychiatry Neurol. 1995;8(2):96-99. PubMed CrossRef
48. Starkstein SE, Migliorelli R, Teson A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60. PubMed CrossRef
49. Ballard CG, Patel A, Solis M, et al. A one-year follow-up study of depression in dementia sufferers. Br J Psychiatry. 1996;168(3):287-291. PubMed CrossRef
50. Ballard C, Bannister C, Solis M, et al. The prevalence, associations and symptoms of depression amongst dementia sufferers. J Affect Disord. 1996;36(3-4):135-144. PubMed CrossRef
51. Bungener C, Jouvent R, Derouesne C. Affective disturbances in Alzheimer’s disease. J Am Geriatr Soc. 1996;44(9):1066-1071. PubMed CrossRef
52. Lopez OL, Gonzalez MP, Becker JT, et al. Symptoms of depression and psychosis in Alzheimer’s disease and frontotemporal dementia: exploration of underlying mechanisms. Cogn Behav Neurol. 1996;9(3):154-161.
53. Lyketsos CG, Tune LE, Pearlson G, et al. Major depression in Alzheimer’s disease: an interaction between gender and family history. Psychosomatics. 1996;37(4):380-384. PubMed CrossRef
54. Zubenko GS, Mulsant BH, Sweet RA, et al. Premorbid history of major depression and the depressive syndrome of Alzheimer’s disease. Am J Geriatr Psychiatry. 1996;4(1):85-90. PubMed
55. Ballard C, Coope B, Qyebode F, et al. Depression in dementia sufferers—comparison of diagnostic criteria. J Am Geriatr Soc. 1997;45(1):123-124. PubMed CrossRef
56. Lyketsos CG, Baker L, Warren A, et al. Depression, delusions, and hallucinations in Alzheimer’s disease: no relationship to apolipoprotein E genotype. J Neuropsychiatry Clin Neurosci. 1997;9(1):64-67. PubMed CrossRef
57. Lyketsos CG, Steele C, Baker L, et al. Major and minor depression in Alzheimer’s disease: prevalence and impact. J Neuropsychiatry Clin Neurosci. 1997;9(4):556-561. PubMed CrossRef
58. Starkstein SE, Chemerinski E, Sabe L, et al. Prospective longitudinal study of depression and anosognosia in Alzheimer’s disease. Br J Psychiatry. 1997;171(1):47-52. PubMed CrossRef
59. Forsell Y, Jorm F, Winblad B. The outcome of depression and dysthymia in a very elderly population: results from a three-year follow-up study. Aging Ment Health. 1998;2(2):100-104. CrossRef
60. Forsell Y, Winblad B. Major depression in a population of demented and nondemented older people: prevalence and correlates. J Am Geriatr Soc. 1998;46(1):27-30. PubMed CrossRef
61. Ballard C, Neill D, O’ Brien J, et al. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000;59(2):97-106. PubMed CrossRef
62. Janzing J, Teunisse R, Bouwens P, et al. Mood and motivation disturbance in elderly subjects with and without dementia: a replication study. J Nerv Ment Dis. 1999;187(2):117-119. PubMed CrossRef
63. Liu CY, Fuh JL, Teng EL, et al. Depressive disorders in Chinese patients with Alzheimer’s disease. Acta Psychiatr Scand. 1999;100(6):451-455. PubMed CrossRef
64. Newman SC. The prevalence of depression in Alzheimer’s disease and vascular dementia in a population sample. J Affect Disord. 1999;52(1-3):169-176. PubMed CrossRef
65. Hargrave R, Reed B, Mungas D. Depressive syndromes and functional disability in dementia. J Geriatr Psychiatry Neurol. 2000;13(2):72-77. PubMed CrossRef
66. Harwood DG, Barker WW, Ownby RL, et al. Clinical characteristics of community-dwelling black Alzheimer’s disease patients. J Natl Med Assoc. 2000;92(9):424-429. PubMed
67. Ballard CG, O’ Brien JT, Swann AG, et al. The natural history of psychosis and depression in dementia with Lewy bodies and Alzheimer’s disease: persistence and new cases over 1 year of follow-up. J Clin Psychiatry. 2001;62(1):46-49. PubMed CrossRef
68. Chemerinski E, Petracca G, Sabe L, et al. The specificity of depressive symptoms in patients with Alzheimer’s disease. Am J Psychiatry. 2001;158(1):68-72. PubMed CrossRef
69. Kertzman SG, Treves IA, Treves TA, et al. Hamilton Depression Scale in dementia. Int J Psychiatry Clin Pract. 2002;6(2):91-94. PubMed CrossRef
70. Naarding P, Leentjens AF, van Kooten F, et al. Disease-specific properties of the Rating Scale for Depression in patients with stroke, Alzheimer’s dementia, and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(3):329-334. PubMed CrossRef
71. Weiner MF, Doody RS, Sairam R, et al. Prevalence and incidence of major depressive disorder in Alzheimer’s disease: findings from two databases. Dement Geriatr Cogn Disord. 2002;13(1):8-12. PubMed CrossRef
72. Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15(3):346-353. PubMed CrossRef
73. Zubenko GS, Zubenko WN, McPherson S, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry. 2003;160(5):857-866. PubMed CrossRef
74. Starkstein SE, Garau ML, Cao A. Prevalence and clinical correlates of disinhibition in dementia. Cogn Behav Neurol. 2004;17(3):139-147. PubMed CrossRef
75. Landes AM, Sperry SD, Strauss ME. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2005;17(3):342-349. PubMed CrossRef
76. טstbye T, Kristjansson B, Hill G, et al. Prevalence and predictors of depression in elderly Canadians: the Canadian Study of Health and Aging. Chronic Dis Can. 2005;26(4):93-99. PubMed
77. Starkstein SE, Jorge R, Mizrahi R, et al. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162(11):2086-2093. PubMed CrossRef
78. Vilalta-Franch J, Garre-Olmo J, Lopez-Pousa S, et al. Comparison of different clinical diagnostic criteria for depression in Alzheimer disease. Am J Geriatr Psychiatry. 2006;14(7):589-597. PubMed CrossRef
79. Park JH, Lee SB, Lee TJ, et al. Depression in vascular dementia is quantitatively and qualitatively different from depression in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(2):67-73. PubMed CrossRef
80. Starkstein SE, Jorge R, Mizrahi R, et al. Insight and danger in Alzheimer’s disease. Eur J Neurol. 2007;14(4):455-460. PubMed CrossRef
81. Delano-Wood L, Houston WS, Emond JA, et al. APOE genotype predicts depression in women with Alzheimer’s disease: a retrospective study. Int J Geriatr Psychiatry. 2008;23(6):632-636. PubMed CrossRef
82. Leontjevas R, van Hooren S, Mulders A. The Montgomery-Asberg Depression Rating Scale and the Cornell Scale for Depression in Dementia: a validation study with patients exhibiting early-onset dementia. Am J Geriatr Psychiatry. 2009;17(1):56-64. PubMed CrossRef
83. Di Iulio F, Palmer K, Blundo C, et al. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer’s disease and mild cognitive impairment subtypes. Int Psychogeriatr. 2010;22(4):629-640. PubMed CrossRef
84. Winter Y, Korchounov A, Zhukova TV, et al. Depression in elderly patients with Alzheimer dementia or vascular dementia and its influence on their quality of life. J Neurosci Rural Pract. 2011;2(1):27-32. PubMed CrossRef
85. Chiu PY, Steffens D, Chen PK, et al. Depression in Taiwanese patients with Alzheimer’s disease determined by the National Institute of Mental Health Provisional Criteria. Int Psychogeriatr. 2012;24(8):1299-1305. PubMed CrossRef
86. El Asmar K, Chaaya M, Phung KT, et al. Prevalence of depression and anxiety among older adults in Lebanon: preliminary data from Beirut and Mount Lebanon. Alzheimers Dement. 2014;10(4):P595. CrossRef
87. Benoit M, Berrut G, Doussaint J, et al. Apathy and depression in mild Alzheimer’s disease: a cross-sectional study using diagnostic criteria.J Alzheimers Dis. 2012;31(2):325-34. doi: 10.3233/JAD-2012-112003.
88. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174(4):307-311. PubMed CrossRef
89. Kandimalla R, Reddy P. Therapeutics of neurotransmitters in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1049-1069. PubMed CrossRef
90. Brendel M, Pogarell O, Xiong G, et al. Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur J Nucl Med Mol Imaging. 2015;42(5):716-724. PubMed CrossRef
91. Sacuiu S, Insel PS, Mueller S, et al. Chronic depressive symptomatology in mild cognitive impairment is associated with frontal atrophy rate which hastens conversion to Alzheimer dementia. Am J Geriatr Psychiatry. 2016;24(2):126-135. PubMed CrossRef
92. Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329-335. PubMed CrossRef
93. Waite A, Bebbington P, Skelton-Robinson M, et al. Life events, depression and social support in dementia. Br J Clin Psychol. 2004;43(pt 3):313-324. PubMed CrossRef
94. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-2040. PubMed CrossRef
95. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psychiatry. 2016;24(1):31-41. PubMed CrossRef
96. Beck IR, Schmid NS, Berres M, et al. Establishing robust cognitive dimensions for characterization and differentiation of patients with Alzheimer’s disease, mild cognitive impairment, frontotemporal dementia and depression. Int J Geriatr Psychiatry. 2014;29(6):624-634. PubMed CrossRef
97. Amen DG, Krishnamani P, Meysami S, et al. Classification of depression, cognitive disorders, and co-morbid depression and cognitive disorders with perfusion SPECT neuroimaging. J Alzheimers Dis. 2017;57(1):253-266. PubMed CrossRef
98. Ayerbe L, Ayis S, Wolfe CD, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14-21. PubMed CrossRef
99. Erkinjuntti T. Types of multi-infarct dementia. Acta Neurol Scand. 1987;75(6):391-399. PubMed CrossRef
100. Verkaik R, Nuyen J, Schellevis F, et al. The relationship between severity of Alzheimer’s disease and prevalence of comorbid depressive symptoms and depression: a systematic review. Int J Geriatr Psychiatry. 2007;22(11):1063-1086. PubMed CrossRef
101. van Vliet D, de Vugt ME, Köhler S, et al. Awareness and its association with affective symptoms in young-onset and late-onset Alzheimer disease: a prospective study. Alzheimer Dis Assoc Disord. 2013;27(3):265-271. PubMed CrossRef
102. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143-1153. PubMed CrossRef
103. Goodarzi ZS, Mele BS, Roberts DJ, et al. Depression case finding in individuals with dementia: a systematic review and meta-analysis. J Am Geriatr Soc. 2017;65(5):937-948. PubMed CrossRef
104. Moore A, Patterson C, Lee L, et al. Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia: recommendations for family physicians. Can Fam Physician. 2014;60(5):433-438. PubMed
105. Rabins P, Robner B, Rummans T, et al. Guideline Watch (October 2014): Practice Guideline for the Treatment of Patients with Alzheimer’s Disease and Other Dementias. Psychiatryonline website. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/alzheimerwatch.pdf. 2014. Accessed July 31, 2016.
106. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298. PubMed CrossRef
107. Leong C. Antidepressants for depression in patients with dementia: a review of the literature. Consult Pharm. 2014;29(4):254-263. PubMed CrossRef
108. Laitinen M-L, Lönnroos E, Bell JS, et al. Use of antidepressants among community-dwelling persons with Alzheimer’s disease: a nationwide register-based study. Int Psychogeriatr. 2015;27(4):669-672. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!
Save
Cite