Transdermal delivery is an alternative to oral routes of drug administration and has made considerable contributions to the treatment of various medical diseases. With the advent of new transdermal delivery technologies, higher numbers of medications are being approved for use as transdermal formulations. This route of administration has several innate advantages that have the potential to benefit various patient populations, including those with central nervous system disorders. The current review briefly outlines the history of transdermal medications, discusses the advantages and disadvantages of transdermal formulations, and examines the challenges and opportunities present for the use of transdermal treatments in psychiatry. Patients with psychiatric illnesses have many unmet needs that may be filled through the benefits gained from transdermal treatments, such as reduced dosing frequency, effective control of plasma medication concentrations, improved tolerability, ability to check compliance visually, and avoidance of first-pass hepatic metabolism. Established transdermal treatments for various psychiatric diseases are discussed followed by an introduction to therapies that are being developed as the first patch formulations for the treatment of schizophrenia. Hypothetical schizophrenia patient profiles are also outlined to aid providers in considering how transdermal treatments for this disease may fill unmet needs for specific patients. The evidence demonstrating the potential benefits of transdermal treatments in psychiatry presented in the current review should both encourage health care providers to consider how patients may benefit from transdermal alternatives and promote future innovation in the area of transdermal drug delivery for psychiatric disorders.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

ABSTRACT
Transdermal delivery is an alternative to oral routes of drug administration and has made considerable contributions to the treatment of various medical diseases. With the advent of new transdermal delivery technologies, higher numbers of medications are being approved for use as transdermal formulations. This route of administration has several innate advantages that have the potential to benefit various patient populations, including those with central nervous system disorders. The current review briefly outlines the history of transdermal medications, discusses the advantages and disadvantages of transdermal formulations, and examines the challenges and opportunities present for the use of transdermal treatments in psychiatry. Patients with psychiatric illnesses have many unmet needs that may be filled through the benefits gained from transdermal treatments, such as reduced dosing frequency, effective control of plasma medication concentrations, improved tolerability, ability to check compliance visually, and avoidance of first-pass hepatic metabolism. Established transdermal treatments for various psychiatric diseases are discussed followed by an introduction to therapies that are being developed as the first patch formulations for the treatment of schizophrenia. Hypothetical schizophrenia patient profiles are also outlined to aid providers in considering how transdermal treatments for this disease may fill unmet needs for specific patients. The evidence demonstrating the potential benefits of transdermal treatments in psychiatry presented in the current review should both encourage health care providers to consider how patients may benefit from transdermal alternatives and promote future innovation in the area of transdermal drug delivery for psychiatric disorders.
J Clin Psychiatry 2019;80(4):18nr12554
To cite: Citrome L, Zeni CM, Correll CU. Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry. 2019;80(4):18nr12554.
To share: https://doi.org/10.4088/JCP.18nr12554
© Copyright 2019 Physicians Postgraduate Press, Inc.
aPsychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York
bResearch and Development, Noven Pharmaceuticals, Inc, Jersey City, New Jersey
cDepartment of Psychiatry and Molecular Medicine, Hofstra Northwell School of Medicine, Hempstead, New York
dDepartment of Child and Adolescent Psychiatry, Charité Universitפtsmedizin, Berlin, Germany
*Corresponding author: Leslie Citrome, MD, MPH, 11 Medical Park Drive, Ste 106, Pomona, NY 10970 ([email protected]).
HISTORY OF TRANSDERMAL MEDICATIONS
The oldest existing human medical records include accounts of the administration of drugs through the skin for transdermal management of systemic illnesses.1 Ointments, balms, and patches containing plant and mineral extracts were often used in ancient Egypt and Babylon as early as 3000 bc.1 In the United States, patent medicines from the Civil War era included treatments that were absorbed through the skin (Figure 12,3). Various cases of poisoning due to skin exposure to nitrobenzene, aniline dyes, and phenol in the early- to mid-1900s emphasized both the potential for lethal consequences due to dermal overexposure and the promise of systemic absorption of topical agents.1
The first modern adhesive transdermal delivery devices were developed through experiments in the 1960s by Dale Wurster and Sherman Kramer4 in which they were able to modify the extent of systemic absorption of salicylate esters via a diffusion cell glued to human volunteers’ forearms. Following many years of persistent investigation, the transdermal delivery of medications became common practice in the late 20th century as technology progressed to allow precise, reproducible administration of drugs through the skin with predictable systemic effects.1
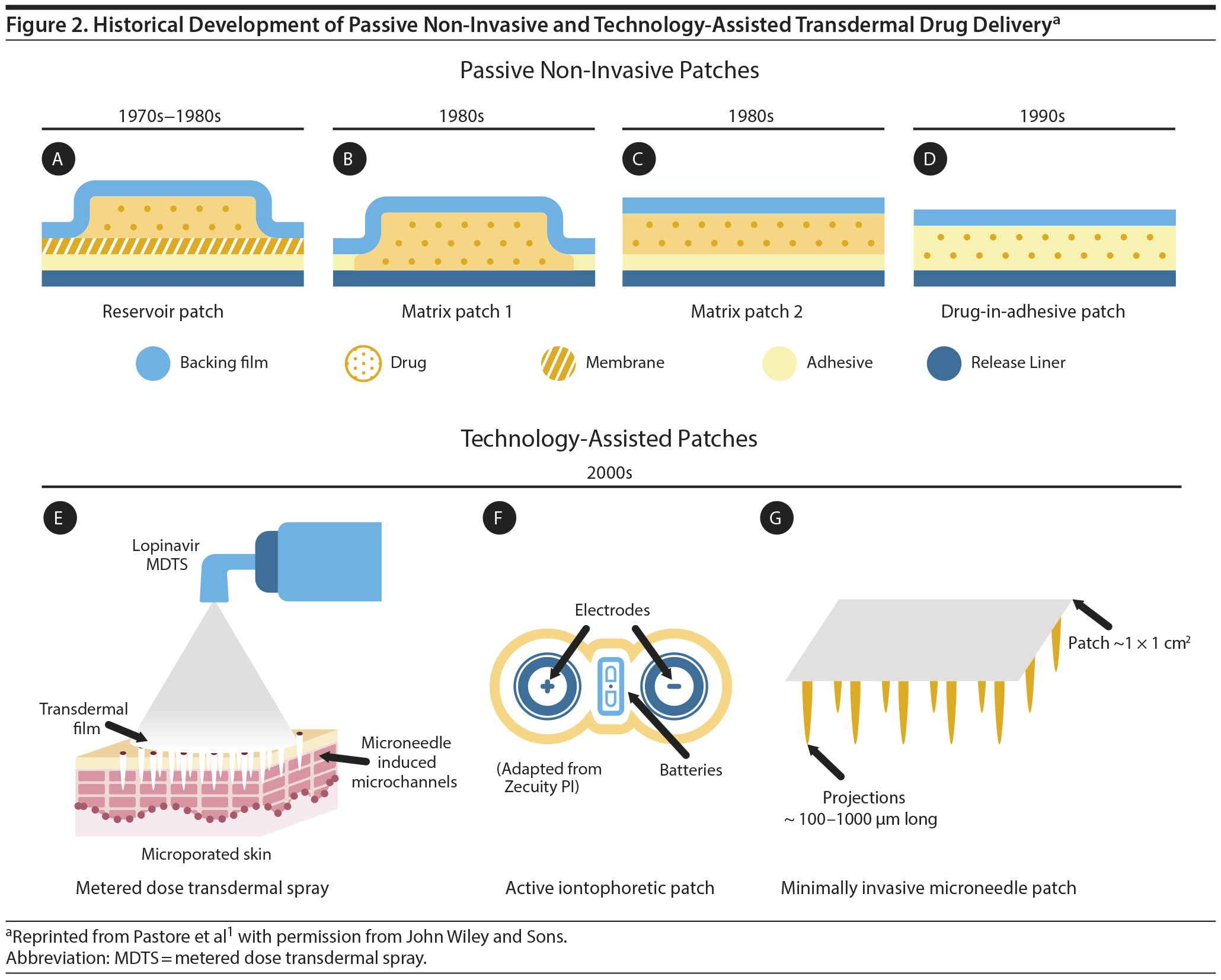
In the 1970s, Alejandro Zaffaroni5-7 patented the first membrane that provided a continual and controlled rate of drug delivery through the skin. Further investigation showed that this method of delivery resulted in steady release and consistent levels of drug in the blood.8 From these discoveries, the first passive, non-invasive patches—reservoir patches—were developed for the treatment of motion-induced nausea (scopolamine), angina pectoris (nitroglycerin), hypertension (clonidine), and post-menopausal hormone replacement (estradiol) (Figure 2).1 The next 20 years brought various additional designs such as matrix patches and drug-in adhesive patches, which have been used to treat numerous medical issues from pain to hypogonadism.
The first “blockbuster” transdermal medication, the nicotine patch, was introduced for smoking cessation in 1984 as both matrix- and reservoir-type delivery systems.1 In addition to the technological progress in the field of passive transdermal drug delivery shown in Figure 2, significant efforts have been made in the area of minimally invasive or active transdermal drug delivery. These efforts focus on the following technology platforms:
- Microneedles—create microchannels in the outermost layer of the epidermis (Supplementary Figure 1)
- Iontophoresis—electrical repulsion at low voltage drives charged molecules through the skin
- Sonophoresis—disrupts the stratum corneum by ultrasound-mediated cavitation
- Electroporation—creates pores in the stratum corneum via short high-voltage electrical pulses
- Radiofrequency ablation—ablates cells and creates microchannels in the stratum corneum via application of electrical current at high frequencies
- Thermal ablation—creates microchannels in the stratum corneum via short heat pulses

- Recent advances in the technology supporting transdermal drug delivery have led to significant contributions toward the treatment of numerous diseases.
- Patients with psychiatric illnesses may benefit from transdermal formulations, as they potentially provide reduced dosing frequency, steady plasma drug concentrations, improved tolerability, and easier compliance monitoring.
- Health care providers should be made aware of the usefulness of transdermal treatments.
Most of the current work in the domain of novel minimally invasive and active transdermal drug delivery has not yet reached the premarket approval stage, with only a few United States Food and Drug Administration (FDA)-approved products to date, such as ultrasound skin enhancement, or sonophoresis, which can be used to administer over-the-counter topical lidocaine.9
CHALLENGES AND OPPORTUNITIES
The skin provides an important barrier function by protecting the body against chemical, microbial, and physical effects of the environment (Supplementary Figure 1). Although the outermost layer of the skin, the stratum corneum, is only approximately 20-µm thick, it is the main contributor to this barrier function and is composed of insoluble keratins and lipids. It is thought that the very high density (1.4 g/cm3 when dry) and low level of hydration (15%-20%) of the stratum corneum contribute to its ability to function as a protective barrier.10 Largely due to the difficulties associated with crossing the stratum corneum, only approximately 1% of the over 2,500 FDA-approved medical entities are available for transdermal delivery via patches in the United States today.11
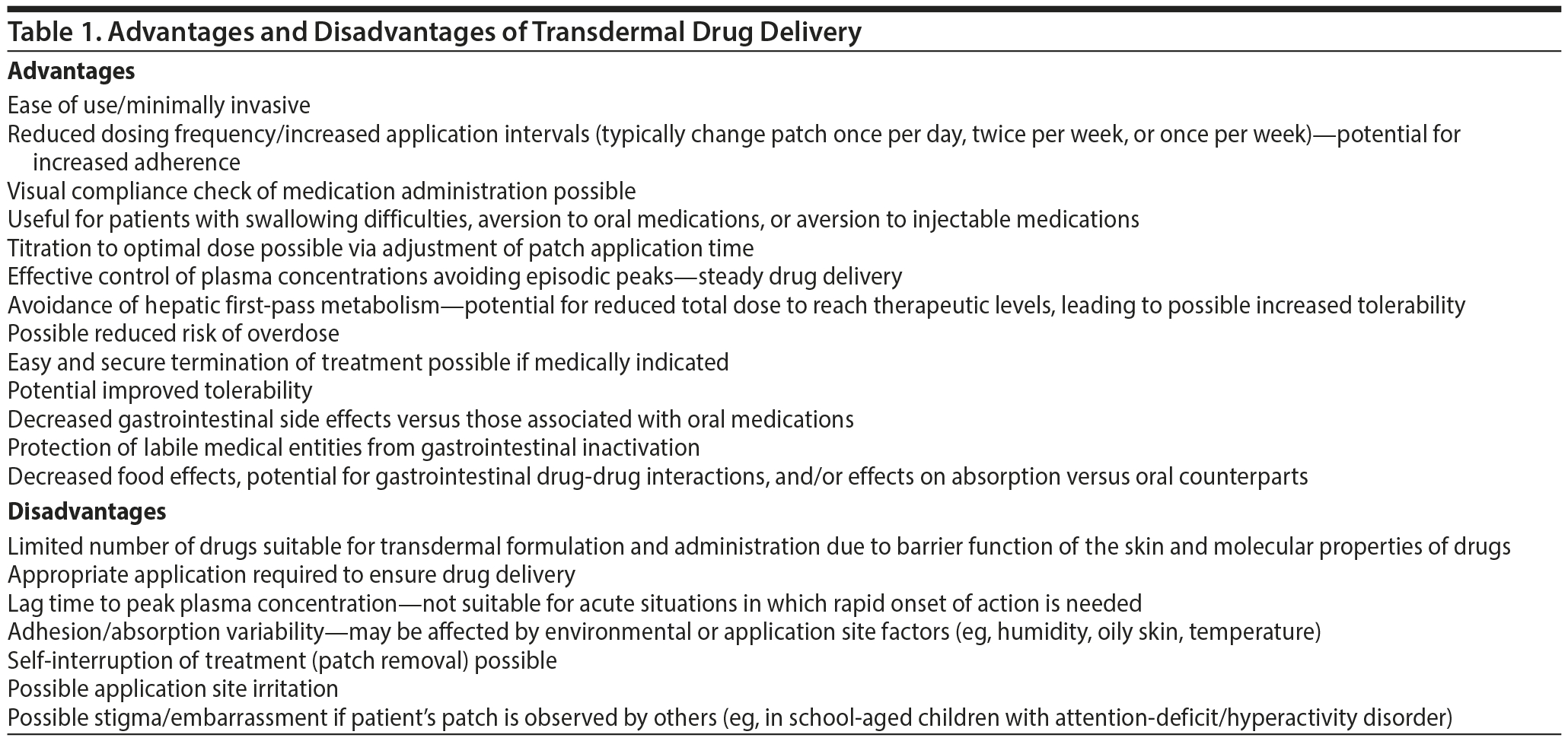
Transdermal treatments have several innate advantages that have the potential to benefit various types of patients (Table 1). Patient and caretaker satisfaction may be markedly increased due to the ease of use and reduced dosing frequency, patients averse to oral/injection formulations may find it less invasive, and patients may experience improved tolerability with transdermal versus other treatments.12,13 Although brief instruction is required, patches are easy to apply and can be administered by either a patient or a caregiver with very little difficulty. Compared to oral medications, adherence to the treatment plan may also increase significantly with transdermal medication delivery due to reduced dosing frequency, because visual confirmation of administration is possible, and because avoidance of the hepatic first-pass metabolism may result in lower therapeutic doses and fewer side effects. There is a potential for reduced risk of overdose or abuse with patches, although the potential for misuse of fentanyl through chewing of patches has been described in a single case study.14 An additional possible advantage of treatment with patches is that removal of the patch stops drug delivery, if necessary, which could make transitioning from other methods of drug delivery to patches less complicated, as exact timing of treatment discontinuation can be identified.
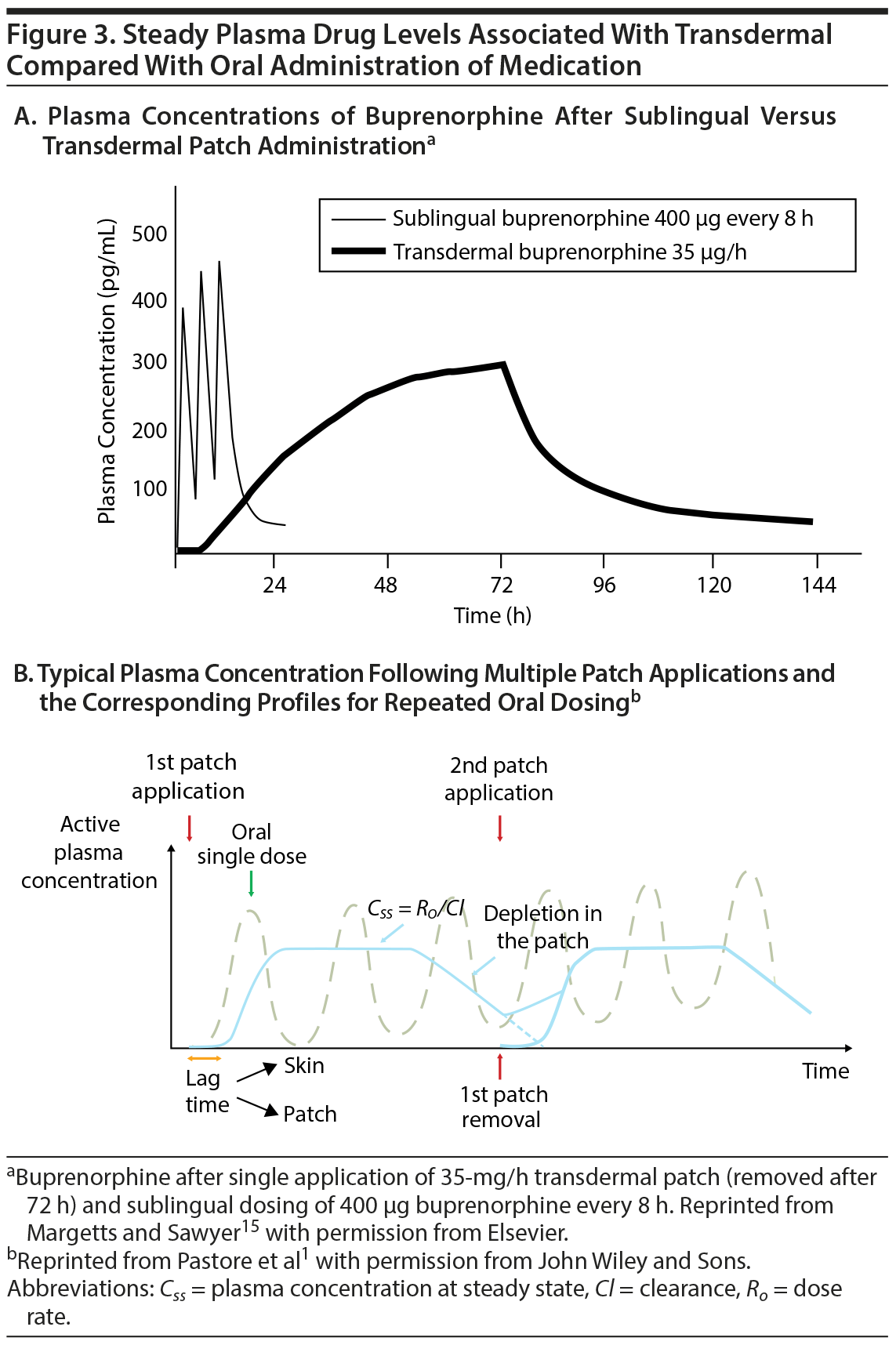
Depending on the specific technology used, transdermal drug delivery is associated with an inherent lag time to reach therapeutically relevant plasma drug concentrations. Once steady-state concentrations of the drug are reached, however, plasma concentrations remain at this level as long as the patch is worn, hence avoiding the episodic peaks and troughs seen with oral formulations of the same drug (Figure 3A).15 This type of steady drug delivery has many advantages, such as reduced side effects and less varied drug response over time.15 In most treatment scenarios in which steady drug delivery is to be maintained, the removal of one patch is followed by immediate application of a subsequent patch. In such an application scenario, the trough concentrations of the pharmacokinetic profile are typically above the minimally effective plasma concentration (Figure 3B)1, which can allow for titration of medication through patch application time while avoiding troughs in drug concentration, something that is not easily achieved with oral administration and may be achieved only through complex dosing schedules. One drawback of delayed time to minimum effective plasma concentration, however, is that passive transdermal systems are not useful in acute situations in which time to maximum plasma drug concentration must be brief to achieve clinical efficacy in a short duration of time. Under these circumstances when urgent care is needed, other types of delivery systems should be pursued (eg, intramuscular or inhaled agents for the management of acute agitation in schizophrenia). Alternate transdermal technologies designed to deliver drugs at a therapeutically relevant level in acute situations, such as microneedle patches, may have future potential in this regard.
A notable disadvantage of medicated patches is that not all drugs are suitable for transdermal delivery because of their physicochemical characteristics. Appropriate candidates for transdermal therapy must have a low molecular weight and high lipid solubility to penetrate the stratum corneum, must be chemically stable during storage and application, and must have dosing levels that equate to an appropriately sized patch.15,16 Additionally, certain medications may directly cause skin irritation or a localized immunologic reaction.17 Although many drugs may, in theory, be more beneficial to patients when applied via transdermal systems, the molecular characteristics of the drug itself along with the practical boundaries of transdermal systems significantly limit development of certain treatments into patch formulations at this time. Additionally, certain therapeutic areas that are limited to treatment with high molecular weight biologics (eg, for psoriasis and diabetes) or molecules are inherently excluded from potential transdermal drug delivery with current technologies.
Adhesives and other components necessary for transdermal applications can also lead to skin reactions, including irritation and sensitization, and should be considered and tested during the development of transdermal drug candidates. Additionally, humidity, heat, and levels of oil in the skin can affect patch adhesion and resultant absorption properties of the active ingredient. Thus, proper patch design considerations, patch storage conditions, and special thought into patient patch application under suitable conditions are necessary for transdermal treatment success.
In situations in which consent and capacity are in question, such as with patients with dementia or cognitive impairment, the use of transdermal systems should be carefully considered. From a caregiver perspective, it might be beneficial to be able to monitor patient adherence to a given regimen by visually assessing whether a patch is being worn by the patient at a certain point in time. However, as patches can be removed by patients at any time, resulting in interruption of treatment, adherence can still be impaired. Long-acting injectables have an inherent advantage in this respect; however, if a patient exhibits intolerability to an injected medication, there is no method to reverse the drug effects after the injection has been administered.
Many of the potential benefits of transdermal administration of medications, such as enhanced adherence, ability to perform a visual compliance check, and potentially improved tolerability, make transdermal formulations a strong option for the treatment of psychiatric and neurologic diseases. Transdermal treatments with central nervous system effects have the potential to address unmet clinical needs for patients by altering duration of treatment, minimizing first-pass metabolism, potentially reducing the number of drug interactions, and decreasing the risk for gastrointestinal adverse effects. As the opportunities for transdermal drug delivery are increasing through application of new technologies, it is important to examine how established transdermal treatments in psychiatry have fared and to look ahead to the possibilities for development of novel transdermal treatments for patients with psychiatric and neuropsychiatric diseases.
HISTORY OF TRANSDERMAL MEDICATIONS IN PSYCHIATRY AND NEUROPSYCHIATRY: ESTABLISHED AND EMERGING TREATMENTS
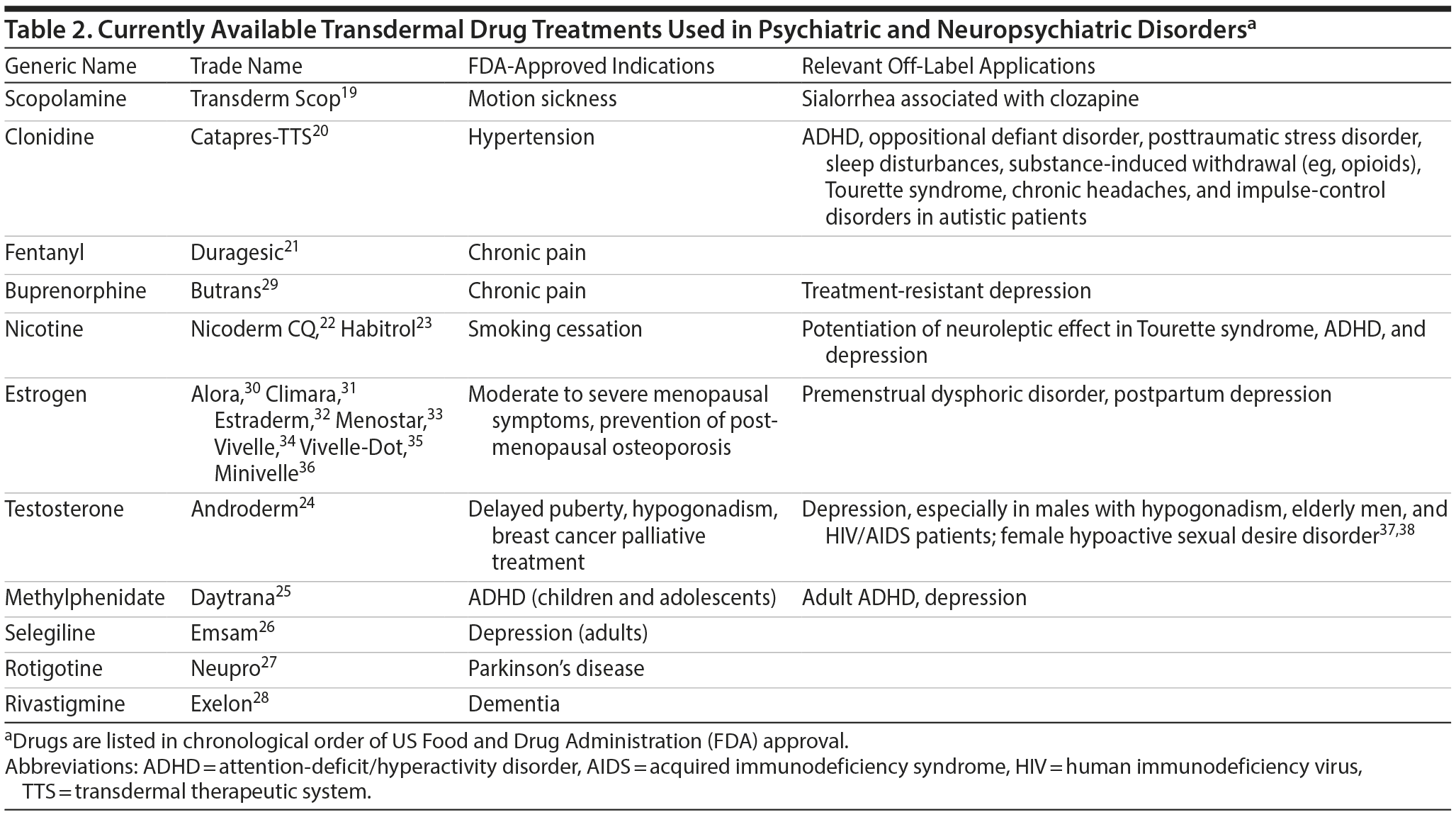
Innovations in transdermal drug delivery have led to the availability of treatment options for various psychiatric and neuropsychiatric conditions. Nicotine for smoking cessation was the first transdermal treatment to achieve substantial commercial success, which significantly raised the profile of transdermal treatments within the general population and promoted the development of other transdermal medications.18 Table 2 outlines transdermal systems used in the management of psychiatric and neuropsychiatric disorders in the order of their development.19-38 As mentioned, a transdermal patch for motion sickness using scopolamine was the first patch to be introduced to the United States market.1 Of note, transdermal scopolamine treatment is also used off label to reduce hypersalivation that occurs following treatment with clozapine.39
Transdermal clonidine was the next patch developed with the intent that it would provide steady-state plasma concentrations and reduce the incidence of most adverse effects seen with oral formulations.18,20 The α2-adrenergic agonist clonidine is indicated for the treatment of hypertension in adults40 but has a wide range of uses in psychiatric patients, such as attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (Table 2). Transdermal clonidine provides extended therapeutic efficacy, as it is applied only once every 7 days. Clinical trials with transdermal clonidine, however, showed that up to 50% of patients had localized skin reactions after 7 days of wearing the patch.20 Further analyses of patients who discontinued treatment due to skin irritation showed that the risk of discontinuation due to contact dermatitis was greatest between treatment weeks 6 and 26, although sensitivity can develop at any point.20
Attention-Deficit/Hyperactivity Disorder
Patients with ADHD can benefit from transdermal treatment formulations, as caregivers can administer and monitor the medication, the patch can be removed and applied in a manner that allows for specific timing of onset and offset of desired effects, and the medication can be steadily delivered for hours without interruption during the time when children may need to be particularly attentive in school or during homework activities. Methylphenidate transdermal system was the first patch to be developed and approved for pediatric patients and remains the only transdermal treatment approved by the FDA for ADHD in children and adolescents (2006).25 Methylphenidate transdermal system is a drug-in adhesive patch for which the dose delivered is dependent on the corresponding size of the patch and wear time.18,25 Steady dosing leads to higher peak plasma concentrations of methylphenidate compared with extended-release oral formulations, indicating that absorption of this drug may be greater through the skin, very likely due to a lack of first-pass metabolism.41 Additionally, several laboratory classroom studies have shown that steady dosing provided by the methylphenidate patch over many hours results in significantly improved scores on ADHD measures.42
Pharmacokinetic studies indicate that peak plasma levels are reached 10 hours after a single application and 8 hours after subsequent applications when worn for 9 hours.25 Recommended application of the patch is 2 hours before the desired effect; thus, onset of action for the methylphenidate transdermal system is slower than that of most oral formulations. In a long-term, open-label, 6-month study25 in 162 adolescents, adverse dermatologic reactions to transdermal methylphenidate were reported in 1.9% of patients.
As with most chronic diseases, adherence to ADHD medication is challenging, and analyses of large claims databases show that patients typically discontinue medication < 1 year after starting treatment.43 Individualizing treatment for ADHD may increase adherence, and transdermal treatments can facilitate personalizing therapy as duration of dosing is easily altered by simply removing the patch. Thus, in addition to reducing the total number of administrations and providing steady plasma levels, transdermal applications also provide the flexibility to modify duration of treatment in a manner that corresponds to the patient’s needs. Both patients and caregivers report improvements in health-related quality of life and satisfaction after switching from an extended-release oral formulation to the methylphenidate patch and for treatment with the methylphenidate patch versus a placebo transdermal system.42
Depression
Monoamine oxidase inhibitors (MAOIs) can be helpful for patients with depressive disorders, but drug-drug interactions and dietary restrictions to avoid hypertensive crisis have limited their use.44 Transdermal formulations may reduce the need for these dietary restrictions and medication warnings, making MAOIs easier to use. A transdermal formulation of the MAOI selegiline received FDA marketing approval for the treatment of major depressive disorder in 2006, with no hypertensive crises reported in any of the controlled clinical trials.18,26 Transdermal selegiline has a better safety and tolerability profile than oral MAOIs, which results in less need for dietary restrictions at lower doses.45 A quantitative review45 of 5 studies that investigated effects of transdermal selegiline on major depressive disorder found the highest significant adverse event versus placebo was application site reaction, with 28.5% for selegiline versus 13.5% for placebo, resulting in a number needed to harm (NNH) of 7 (95% CI, 6-10). Discontinuation due to an adverse effect resulted in an overall NNH value of 32 (95% CI, 19-132) versus placebo. Similar to other transdermal medications, the selegiline patch can provide continuous 24-hour dosing, avoids first-pass metabolism, and may help patients more easily reach and maintain therapeutic doses of drug.
Parkinson’s Disease
Transdermal formulations of Parkinson’s disease medications may improve treatment outcomes compared to other routes of administration, as patches can provide steady drug delivery without episodic peaks and troughs. Additionally, patients with Parkinson’s disease may develop swallowing difficulties, making oral medications challenging; thus transdermal treatments can be highly advantageous under these circumstances. Rotigotine is a dopamine agonist of the non-ergoline class developed in the mid-1980s that is indicated for the treatment of Parkinson’s disease.18,46 The patch formulation of rotigotine received FDA approval in 2007 and is the first transdermal treatment for Parkinson’s disease.27 Despite the benefits gained from dopaminergic treatments, the inability to deliver these drugs in a continuous manner has limited their efficacy.47 It is thought that the motor fluctuations and dyskinesias that persist in Parkinson’s patients, regardless of treatment, may be due to pulsatile stimulation of striatal dopamine receptors caused by discontinuous administration of drug.48 Treatment with transdermal medications, such as rotigotine, could reduce the erratic plasma drug levels seen with oral medications and limit complications due to pulsatile administration.
In clinical trials, treatment with transdermal rotigotine resulted in significantly higher incidences of application-site reactions compared with placebo in a dose-dependent manner in Parkinson’s disease patients (early-stage Parkinson’s : placebo, 19%; rotigotine 4 mg, 19%; 6 mg, 32%; 8 mg, 43%; late-stage Parkinson’s : placebo, 13%; rotigotine 8 mg, 36%; 12 mg, 46%).27,48,49 Nonetheless, rotigotine can be coadministered with l-DOPA, allowing for a reduction in l-DOPA dosing, and once-daily patch application can reduce medication burden for advanced Parkinson’s patients with concomitant diseases.47 In cases in which motor disturbances can interfere with patch self-administration, family members or caregivers may apply the patch.
Alzheimer’s Disease and Dementia
Patients with Alzheimer’s disease and other types of dementia often receive help from a caregiver, and transdermal treatments are easy to administer and to check for compliance. The reduced frequency of dosing necessary with transdermal medications in patients who already have a high medication burden could also increase adherence to treatment. In addition, steady dosing and lack of gastrointestinal exposure via patch treatments could help reduce the side effects and drug-drug interactions often observed with oral formulations of dementia medications. Oral rivastigmine, an acetylcholinesterase inhibitor, was approved by the FDA in 2000 for dementia in Alzheimer’s disease and Parkinson’s disease.50 Noncompliance with oral medications is a common problem in patients with dementia, and patients rarely remain on oral treatments for Alzheimer’s for longer than 1 year.51,52 The rivastigmine transdermal system was developed with compliance in mind and was approved by the FDA in 2007.28 Higher doses of the oral cholinesterase inhibitors and the episodic peaks seen with oral formulations result in unwanted gastrointestinal effects, which may impede the ability of patients to reach optimal therapeutic doses.51 Side effects associated with the oral formulations of rivastigmine were attenuated when daily doses were administered over 3 doses versus 2,53,54 indicating that reduced gastrointestinal exposure may improve tolerability. The rivastigmine 9.5-mg/24-hour patch provides similar exposure to that of a 12-mg/d oral dose with lower fluctuations in plasma concentrations and 30%-54% less intersubject variability versus the oral formulation.28 In addition, studies have demonstrated that this dose of transdermal rivastigmine had similar efficacy to the highest oral dose with 3-fold reductions in reports of nausea and vomiting.55 Contact dermatitis, such as pruritus and rash, has been observed in increased numbers for treatment with transdermal (pruritus, 3.1%; rash, 2.2%) versus oral rivastigmine (pruritis, 0.2%; rash, 0.1%), indicating that application site reactions should be monitored by patients and caregivers when using the patch.28,56,57
Patient autonomy and caregiver responsibilities are a major concern, particularly for patients who are elderly and struggling with dementia. Caregiver preference and satisfaction for rivastigmine patches were investigated in a large double-dummy, double-blind trial58 of Alzheimer’s patients who received both the capsule and patch formulations. As shown in Supplementary Table 1, per the Alzheimer’s Disease Caregiver Preference Questionnaire, caregivers preferred the patch formulation in overall satisfaction, for ease of following a schedule, and for ease of use over the capsule.
In a large, non-interventional, real-world study56 of Alzheimer’s patients who were exposed to both oral and transdermal treatment with rivastigmine, significantly more caregivers and 80% of physicians preferred transdermal monotherapy and significantly more patients were adherent with the transdermal therapy over oral medications. Efficacy, tolerability, and patient/caregiver preference data for the rivastigmine patch indicate that this established transdermal treatment for patients with dementia is as effective as and more tolerable than oral routes with better patient compliance rates.28,56 A transdermal patch with the acetylcholinesterase inhibitor donepezil for patients with mild-to-moderate Alzheimer’s disease is currently in development with a randomized, phase 3, active comparator trial estimated to be completed in 2019 (NCT03197740).
Schizophrenia
Currently, there are no FDA-approved transdermal antipsychotics; however, the innate advantages of transdermal patch formulations may align well with the needs of people with schizophrenia. The use of transdermal systems for antipsychotics has the potential to increase tolerability through smoother and more continuous plasma drug concentrations, fewer gastrointestinal side effects, and lower effective doses due to lack of first-pass metabolism. Antipsychotic patches may also improve adherence through elimination of the need for multiple daily doses of medication, reduction in side effects, and ability to perform visual compliance checks and by providing an option for patients who are averse to taking oral or injectable medications. The optimal antipsychotic treatment is one that provides low-dose efficacy with minimal side effects and addresses the issues surrounding poor compliance among patients with schizophrenia; thus, development of novel transdermal treatments has the potential to fill some of these unmet needs.
Currently, there are 2 antipsychotics in development in the United States as possible transdermal patch treatments: aripiprazole once weekly and asenapine once daily. Aripiprazole is an atypical antipsychotic for which the treatment of schizophrenia is among its indications.59-61 A randomized, double-blind, single-dose, placebo-controlled safety and bioavailability study62 of once-weekly transdermal aripiprazole in healthy volunteers (N = 12) indicated that sustained delivery of therapeutic doses of the drug could be administered over 7 days with no safety issues. A second pharmacokinetic, safety, multidose study63 demonstrated that steady state of the transdermal formulation was reached within 3 weeks, with concentrations similar to those found with the oral medication and no safety signal in healthy males. The FDA recently indicated that a relative bioavailability study comparing steady-state pharmacokinetics between the transdermal and oral formulations of aripiprazole would be an acceptable entrance into an abbreviated development path if bioequivalence is established.64 The FDA also outlined standard studies required for transdermal reservoir patches that would need to be completed.
Asenapine is an atypical antipsychotic that is being developed as a transdermal patch. The only formulation of asenapine currently available is a sublingual tablet, approved in the United States for the treatment of schizophrenia and bipolar I disorder.65 Asenapine tablets are ineffective when swallowed because of high hepato-gastrointestinal first-pass metabolism. Although sublingual asenapine was proven safe and effective in short- and long-term studies,65-67 oral hypoesthesia and dysgeusia can be significant concerns in terms of tolerability and are directly related to its sublingual route of administration. Administration of asenapine via the sublingual route is also associated with variability in plasma concentrations due to inter- and intraindividual differences in absorption.68 Moreover, if the asenapine tablet is inadvertently swallowed rather than allowed to orally disintegrate and be absorbed via the oral mucosa, bioavailability is negligible.68 Patients are therefore instructed not to swallow the tablet and are advised to refrain from eating and drinking 10 minutes after administration. The need for twice-daily dosing poses yet another challenge associated with the sublingual formulation. Because of these limitations, both patients and caregivers may find adherence to sublingual asenapine cumbersome.
A recently completed phase 3, randomized, double-blind, 6-week trial (NCT02876900) examined the efficacy and safety of high- and low-dose transdermal asenapine patches (equivalent to sublingual asenapine 10 mg and 5 mg twice daily, respectively) versus placebo in 616 adults with schizophrenia experiencing an acute exacerbation of their illness.69 Data from this study demonstrated that treatment with transdermal asenapine resulted in significantly greater improvements in change from baseline in Positive and Negative Syndrome Scale total score versus placebo from week 2 to week 6 of treatment with low-dose asenapine and from week 3 to week 6 with high-dose asenapine (P ≤ .05 for all; primary endpoint of week 6, P < .01 for both doses). Changes from baseline in the Clinical Global Impressions-Severity of Illness scale were also significantly improved in patients on asenapine treatment compared with placebo starting at week 2 through week 6 for both doses. No deaths or serious adverse events occurred throughout the study, and the systemic safety profile of the asenapine transdermal system was found to be consistent with that known for asenapine. Treatment-emergent adverse events at the patch application site were higher in the transdermal asenapine groups compared with placebo; however, most of these events were mild or moderate in severity.69
Transdermal Schizophrenia Patient Profile
Because transdermal antipsychotic medications have not yet been introduced in the United States for the treatment of schizophrenia, defining patients that may benefit from the 2 atypical antipsychotic transdermal medications in development may facilitate forethought regarding unmet patient needs that may be addressed with these treatment regimens.
In addition to patients who may be looking to try a novel antipsychotic to better manage their symptoms, patients who currently have or have previously had success with symptom management on asenapine or aripiprazole treatment but who have experienced issues with taking them as prescribed for various reasons may be good candidates for transdermal therapy. For example, a patient who has previously had general treatment success with sublingual asenapine but reported troubling dysgeusia would very likely tolerate the asenapine patch formulation well and may transition easily from the sublingual to the transdermal formulation without difficulty. Similarly, a patient who has experienced symptom management with aripiprazole but also suffered persistent medication-induced nausea that resulted in decreased adherence would be a candidate for transdermal aripiprazole. The ideal treatment outcome with either transdermal medication for this patient would be continued symptom management, resolved nausea, and increased adherence to medication due to the potential resolution of associated adverse events, possible reduced dosing frequency, and reduced restrictions around dosing due to potential food or drug effects.
An additional setting in which transdermal treatment with either of these atypical antipsychotics would most likely be beneficial would be in an inpatient setting where visual compliance checks are useful and administering more invasive treatment may be difficult. As previously indicated, currently available patches are not ideal for acute situations for which rapid drug onset of action is needed; however, once optimal plasma levels are reached, they remain steady as long as the patch is applied.
Finally, patients with schizophrenia who have had success with sublingual asenapine or oral aripiprazole and are taking multiple oral medications, have cognitive deficits that may impede their ability to recall whether they have taken their medication, or are unable to adhere to a treatment plan may also be good candidates for transdermal treatment with either of these atypical antipsychotics. If a patient is taking multiple oral medications, it is possible that switching to a transdermal route for even one of them may reduce the burden of polypharmacy significantly and should be considered. Cognitive deficits are common in schizophrenia70; thus, if patients are continually not able to recall if they have taken their medication, switching to a transdermal route may be a pragmatic solution. A visual check for themselves or caretakers can be highly beneficial and, in some instances, may reduce the chances of overdose. Nonadherence is a significant issue with chronic disorders such as schizophrenia, and, as discussed, reduced dosing and decreased side effects may result in increased compliance. If patients have had success with asenapine or aripiprazole but have had difficulties with compliance, they may be good candidates for transdermal treatment.
CONCLUSIONS AND FUTURE DIRECTIONS
The many advantages of transdermal treatments include their ease of use, reduced dosing frequency, steady delivery of drug, potential for similar efficacy with lower dosage, increased tolerability, reduced drug-drug interactions, and avoidance of first-pass metabolism. Due to these advantages, several drugs have been developed as transdermal medications, some of which now include interventions that treat psychiatric and neuropsychiatric disorders. Established transdermal treatments for ADHD, depression, Parkinson’s disease, Alzheimer’s disease, and other forms of dementia have shown that treatments of this type can fill unmet needs in patients and offer evidence that establishing various formulations for medications is a useful endeavor.
Transdermal therapies are currently in development for the treatment of schizophrenia, a disorder for which no transdermal formulation has previously been available in the United States. Persons with schizophrenia constitute a population that has the propensity to benefit from the potential advantages of transdermal formulations of atypical antipsychotics, including asenapine and aripiprazole. Having additional treatment choices in terms of drug delivery and formulations will help address individual patient needs, values, and preferences.
Submitted: August 31, 2018; accepted March 20, 2019.
Published online: July 16, 2019.
Potential conflicts of interest: In the past 48 months, Dr Citrome has engaged in collaborative research with, or received consulting or speaking fees, from Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Intra-Cellular Therapeutics, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven Pharmaceuticals, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda; is a stock shareholder (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (all purchased more than 10 years ago); and has received royalties from Wiley (Editor in Chief, International Journal of Clinical Practice), UpToDate (reviewer), and Springer Healthcare (book). Dr Zeni is an employee of Noven Pharmaceuticals. In the last 36 months, Dr Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Angelini, Gerson Lehrman Group, IntraCellular Therapies, Janssen/Johnson & Johnson, LB Pharma, Lundbeck, Medavante, Medscape, Merck, Neurocrine, Noven Pharmaceuticals, Otsuka, Pfizer, ROVI, Servier, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, ROVI, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a shareholder of LB Pharma.
Funding/support: This report was funded by Noven Pharmaceuticals, Inc, Jersey City, New Jersey, United States, a wholly-owned subsidiary of Hisamitsu Pharmaceutical Co.
Role of the sponsor: Noven Pharmaceuticals, Inc, provided support for writing and editorial assistance.
Acknowledgments: The authors thank Debika Chatterjea, PhD, of MedVal Scientific Information Services, LLC for medical writing and editorial assistance, which was sponsored by Noven Pharmaceuticals, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines.” Dr Chatterjea has no conflicts of interest to declare.
Supplementary material: See accompanying pages.
REFERENCES
1.Pastore MN, Kalia YN, Horstmann M, et al. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172(9):2179-2209. PubMed CrossRef
2.Gitner H, Miller R. The appeal of this Civil War-era proprietary stamp will never die. 2017. Linn’s Stamp News website. https://www.linns.com/news/us-stamps-postal-history/2017/december/appeal-civil-war-era-proprietary-stamp-never-die.html. Accessed February 14, 2018.
3.Brush Hatcher J. Flashback: panaceas of the sixties had their own revenue stamps. 2009. Collectors Weekly website. https://www.collectorsweekly.com/articles/panaceas-of-the-sixties-had-their-own-revenue-stamps/. Accessed February 15, 2018.
4.Wurster DE, Kramer SF. Investigation of some factors influencing percutaneous absorption. J Pharm Sci. 1961;50(4):288-293. PubMed CrossRef
5.Zaffaroni A. Bandage for administering drugs. US patent 3,598,122. Alza Corporation;1971.
6.Zaffaroni A. Bandage for controlled release of vasodilatators. US patent 3,742,951. Alza Corporation;1973.
7.Zaffaroni A. Bandage for the administration of drug by controlled metering through microporous materials. US patent 3,797,494. Alza Corporation;1974.
8.Riegelman S. Pharmacokinetics: pharmacokinetic factors affecting epidermal penetration and percutaneous adsorption. Clin Pharmacol Ther. 1974;16(5 part 2):873-883. PubMed CrossRef
9.US Food and Drug Administration. 510(k) summary of safety and effectiveness: Sonoprep ultrasonic skin permeation system and procedure tray. 2004. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf4/K040525.pdf. Accessed March 26, 2018.
10.Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438-470. PubMed CrossRef
11.US Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. 2018. FDA website. https://www.accessdata.fda.gov/scripts/cder/ob/. Accessed March 22, 2018.
12.Rabin CR, Siegel SJ. Delivery systems and dosing for antipsychotics. Handb Exp Pharmacol. 2012;212(212):267-298. PubMed CrossRef
13.Aggarwal G, Dhawan S. Psychotropic drugs and transdermal delivery: an overview. Int J Pharma Bio Sci. 2010;1(2):1-12.
14.Dale E, Ashby F, Seelam K. Report of a patient chewing fentanyl patches who was titrated onto methadone. BMJ Case Rep. 2009;2009:bcr0120091454. PubMed CrossRef
15.Margetts L, Sawyer R. Transdermal drug delivery: principles and opioid therapy. Contin Educ Anaesth Crit Care Pain. 2007;7(5):171-176. CrossRef
16.Lombardo AJ, Kuzniecky R, Powers RE, et al. Altered brain sodium channel transcript levels in human epilepsy. Brain Res Mol Brain Res. 1996;35(1-2):84-90. PubMed CrossRef
17.Ale I, Lachapelle JM, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: an overview. Adv Ther. 2009;26(10):920-935. PubMed CrossRef
18.Stevens JR, Justin Coffey M, Fojtik M, et al. The use of transdermal therapeutic systems in psychiatric care: a primer on patches. Psychosomatics. 2015;56(5):423-444. PubMed CrossRef
19.Transderm ScÅp (scopolamine) transdermal system patch [package insert]. Princeton, NJ: Sandoz Inc; October 2017.
20.Catapres-TTS (clonidine) [package insert]. Ridgefield, CT: Boehringer Ingelheim International GmbH; November 2009.
21.Duragesic (fentanyl transdermal system) [package insert]. Titusville, NJ: Janssen Pharmaceutica Products, LP; 2003.
22.Nicoderm CQ: safety data sheet. Research Triangle Park, NC: GlaxoSmithKline US; September 2014.
23.Habitrol patch: safety data sheet. Hyderabad, India: Dr. Reddy’s Laboratories Ltd; March 2015.
24.Androderm (testosterone transdermal system), for topical use CIII [package insert]. Irvine, CA: Allergan USA, Inc; October 2016.
25.Daytrana (methylphenidate transdermal system) [package insert]. Wayne, PA: Shire Pharmaceuticals Ireland Limited; June 2010.
26.Emsam (selegiline transdermal system) [package insert]. Morgantown, WV: Mylan Specialty L.P.; September 2014.
27.Neupro (rotigotine transdermal system) [package insert]. Smyrna, GA: UCB, Inc.; September 2016.
28.Exelon PATCH (rivastigmine transdermal system) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; November 2016.
29.Butrans (buprenorphine) Transdermal System for transdermal administration [package insert]. Stamford, CT: Purdue Pharma L.P.; June 2011.
30.Alora (estradiol transdermal system, USP) [package insert]. Irvine, CA: Allergan; February 2018.
31.Climara (estradiol transdermal system) [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc.; 2007.
32.Estraderm (estradiol transdermal system) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2012.
33.Menostar (estradiol transdermal system) [package insert]. St. Paul, MN: 3M Pharmaceuticals; December 2005.
34.Vivelle (estradiol transdermal system) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; February 2000.
35.Vivelle-Dot (estradiol transdermal system) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; March 2003.
36.Minivelle (estradiol transdermal system) [package insert]. Miami, FL: Noven Pharmaceuticals Inc; October 2012.
37.Achilli C, Pundir J, Ramanathan P, et al. Efficacy and safety of transdermal testosterone in postmenopausal women with hypoactive sexual desire disorder: a systematic review and meta-analysis. Fertil Steril. 2017;107(2):475-482.e15. PubMed CrossRef
38.Ganesan K, Habboush Y, Sultan S. Transdermal testosterone in female hypoactive sexual desire disorder: a rapid qualitative systematic review using grading of recommendations assessment, development and evaluation. Cureus. 2018;10(3):e2401. PubMed
39.Mathews M, Mathews M, Mathews J. How to remedy excessive salivation in patients taking clozapine. Curr Psychiatr. 2003;2(12):80.
40.Catapres (clonidine hydrochloride, USP) [package insert]. Ridgefield, CT: Boehringer Ingelheim International GmbH; October 2009.
41.Pierce D, Dixon CM, Wigal SB, et al. Pharmacokinetics of methylphenidate transdermal system (MTS): results from a laboratory classroom study. J Child Adolesc Psychopharmacol. 2008;18(4):355-364. PubMed CrossRef
42.Findling RL, Dinh S. Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS). CNS Drugs. 2014;28(3):217-228. PubMed CrossRef
43.Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. PubMed CrossRef
44.Asnis GM, Henderson MA. EMSAM (deprenyl patch): how a promising antidepressant was underutilized. Neuropsychiatr Dis Treat. 2014;10:1911-1923. PubMed CrossRef
45.Citrome L, Goldberg JF, Portland KB. Placing transdermal selegiline for major depressive disorder into clinical context: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2013;151(2):409-417. PubMed CrossRef
46.Horn AS, Tepper P, Van der Weide J, et al. Synthesis and radioreceptor binding activity of N-0437, a new, extremely potent and selective D2 dopamine receptor agonist. Pharm Weekbl Sci. 1985;7(5):208-211. PubMed
47.Boroojerdi B, Wolff HM, Braun M, et al. Rotigotine transdermal patch for the treatment of Parkinson’s disease and restless legs syndrome. Drugs Today (Barc). 2010;46(7):483-505. PubMed CrossRef
48.Zhou CQ, Li SS, Chen ZM, et al. Rotigotine transdermal patch in Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2013;8(7):e69738. PubMed CrossRef
49.Chen F, Jin L, Nie Z. Safety and efficacy of rotigotine for treating Parkinson’s disease: a meta-analysis of randomised controlled trials. J Pharm Pharm Sci. 2017;20(0):285-294. PubMed CrossRef
50.Exelon (rivastigmine tartrate) capsules, for oral use. EXELON (rivastigmine tartrate) oral solution [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; November 2016.
51.Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer’s disease. Expert Opin Drug Deliv. 2008;5(12):1377-1386. PubMed CrossRef
52.Small G, Dubois B. A review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23(11):2705-2713. PubMed CrossRef
53.Farlow MR, Somogyi M. Transdermal patches for the treatment of neurologic conditions in elderly patients: a review. Prim Care Companion CNS Disord. 2011;13(6):doi:10.4088/PCC.11r01149. PubMed CrossRef
54.Feldman HH, Lane R; Study 304 Group. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2007;78(10):1056-1063. PubMed CrossRef
55.Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease: rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007;22(5):456-467. PubMed CrossRef
56.Pai MC, Aref H, Bassil N, et al. Real-world evaluation of compliance and preference in Alzheimer’s disease treatment. Clin Interv Aging. 2015;10:1779-1787. PubMed CrossRef
57.Alva G, Cummings JL, Galvin JE, et al. Skin reactions at the application site of rivastigmine patch (4.6 mg/24 h, 9.5 mg/24 h or 13.3 mg/24 h): a qualitative analysis of clinical studies in patients with Alzheimer’s disease. Int J Clin Pract. 2015;69(5):518-530. PubMed CrossRef
58.Winblad B, Kawata AK, Beusterien KM, et al. Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(5):485-491. PubMed CrossRef
59.Abilify (aripiprazole) tablets. Abilify Discmelt (aripiprazole) orally disintegrating tablets. Abilify (aripiprazole) oral solution. Abilify (aripiprazole) injection for intramuscular use only [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co, Ltd; August 2016.
60.Abilify Maintena (aripiprazole) for extended-release injectable suspension, for intramuscular use [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co, Ltd; March 2018.
61.Aristada (aripiprazole lauroxil) extended-release injectable suspension, for intramuscular use [package insert]. Waltham, MA: Alkermes, Inc; January 2018.
62.Aequus Pharmaceuticals. Aequus announces positive results from single-dose bioavailability study of aripiprazole transdermal patch [press release]. 2016. Aequus Pharmaceuticals website. https://www.aequuspharma.ca/news-releases/aequus-announces-positive-results-from-single-dose-bioavailability-study-of-aripiprazole-transdermal-patch. Accessed June 7, 2018.
63.Aequus Pharmaceuticals. Aequus announces positive results from second proof of concept study for long-acting aripiprazole transdermal patch [press release]. 2017. Aequus Pharmaceuticals website. https://www.aequuspharma.ca/news-releases/aequus-announces-positive-results-from-second-proof-of-concept-study-for-long-acting-aripiprazole-transdermal-patch. Accessed June 7, 2018.
64.Aequus Pharmaceuticals. Aequus announces abbreviated development path for transdermal aripiprazole [press release]. 2017. Aequus Pharmaceuticals website. https://www.aequuspharma.ca/news-releases/aequus-announces-abbreviated-development-path-for-transdermal-aripiprazole. Accessed February 28, 2018.
65.Saphris (asenapine) sublingual tablets [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; March 2015.
66.Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903. PubMed CrossRef
67.Citrome L. Asenapine review, part II: clinical efficacy, safety and tolerability. Expert Opin Drug Saf. 2014;13(6):803-830. PubMed
68.US Food and Drug Administration. Briefing book. July 30, 2009 PDAC. Saphris (asenapine) sublingual tablets. 2009. Alliance for Human Research Protection website. http://ahrp.org/wp-content/uploads/2009/08/www.fda.gov_downloads_AdvisoryCommittees_CommitteesMeetingMaterials_Drugs_PsychopharmacologicDrugsAdvisoryCommittee_UCM173877.pdf. Accessed January 24, 2019.
69.Citrome L, Walling D, Zeni C, et al. Efficacy and safety of an asenapine transdermal patch (asenapine transdermal system, HP-3070) in the treatment of adults with schizophrenia: a phase 3 randomized, double- blind, placebo-controlled, 6-week, inpatient study [abstract]. Neuropsychopharmacology. 2018;43(suppl 1):S116-S117.
70.Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141-168. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top