Alzheimer’s disease has long been attributed to a buildup of plaque in the brain. As scientists begin to explore alternative theories, new research on astrocytes is getting some attention.
Reactive Astrocytes
Astrocytes are a type of glial cell in the brain and spinal cord that provide structural and metabolic support to neurons. The cells perform a variety of functions, including regulating the chemical environment, promoting synapse formation, and repairing damaged brain tissue.
South Korean scientists recently demonstrated that when astrocytes take up elevated levels of acetates–a type of molecule produced as a byproduct of metabolism–they transform into dangerous reactive astrocytes. Reactive astrocytes have emerged as a suspected culprit in the development and progression of Alzheimer’s disease.
New Imaging Technique
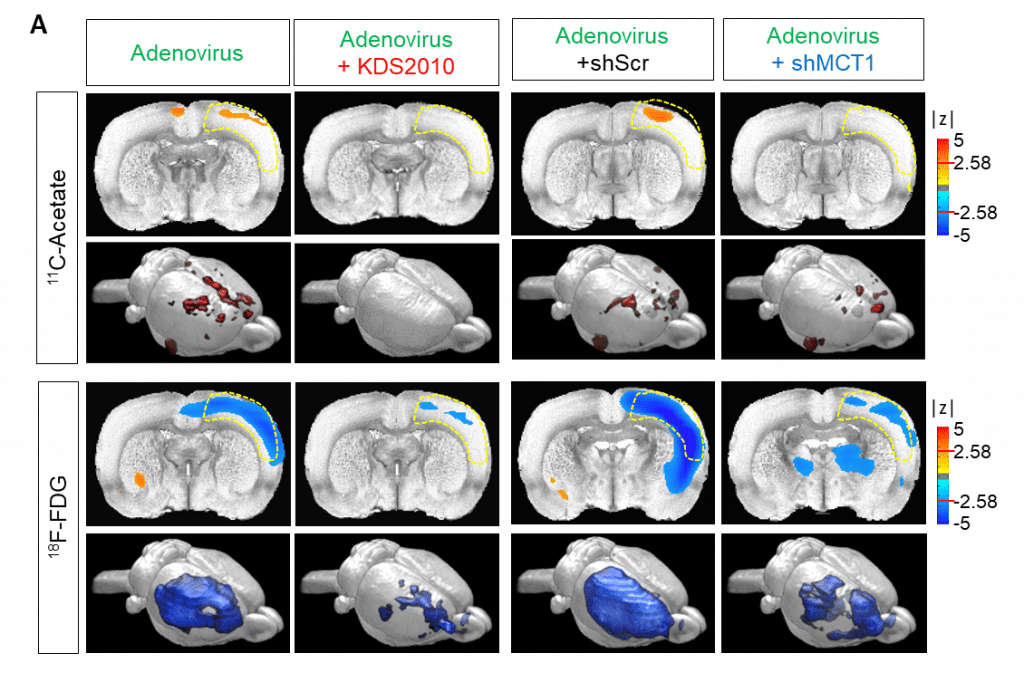
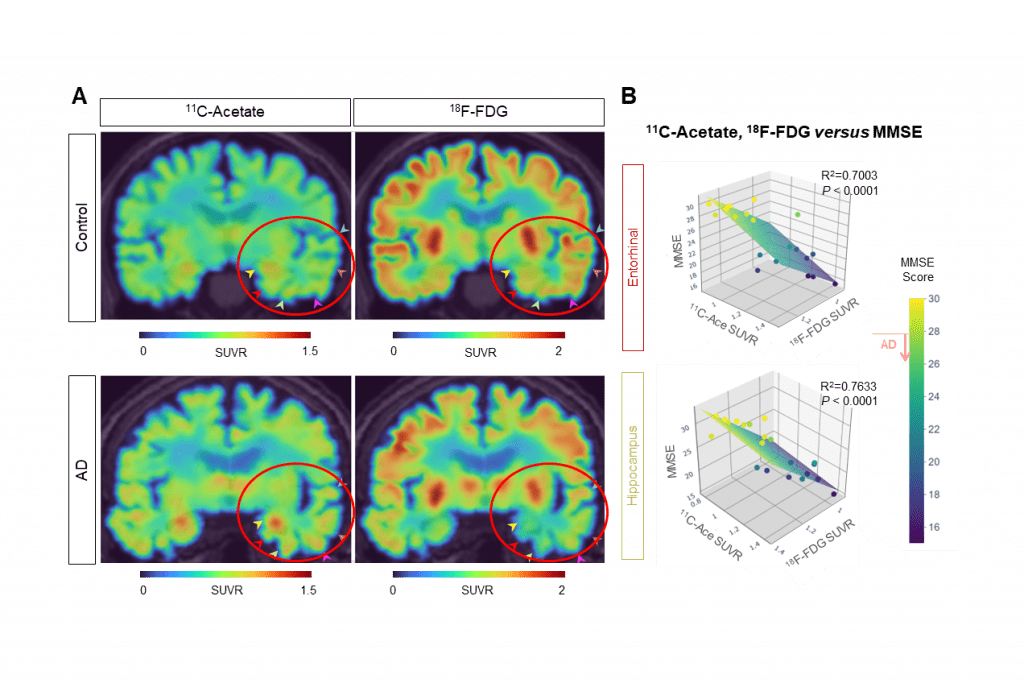
The scientists used their insights to develop a new imaging technique to directly observe the astrocyte-neuron interactions. Using positron emission tomography (PET) imaging with radioactive acetate and glucose probes (11C-acetate and 18F-FDG) they were able to visualize the changes in neuronal metabolism in patients with Alzheimer’s.
Amyloid Load and Depression in Older Adults
Treatment Challenges in Early-Stage AD (Free CME)
Imaging also spotted a strong correlation between cognitive function and the PET signals of both 11C-acetate and 18F-FDG. It found high levels of acetate and low levels of glucose in both mouse and human brains. And, it was able to directly attribute those changes to reactive astrocytes in the brain.
“Reactive astrocytes showed metabolic abnormalities that excessively uptake acetate compared to normal state. We found that the acetate plays an important role in promoting astrocytic inflammatory responses,” YUN Mijin, one of the paper’s authors, commented.
In the mouse model, the researchers took it one step further by reversing the metabolic changes. This inhibited reactive astrogliosis and astrocytic MCT1 expression which, in turn, halted the formation of the harmful reactive astrocytes and prevented them from taking up too much acetate.
GABA Involvement
When astrocytes uptake too much acetate this also boosted the production of another molecule called GABA (gamma-aminobutyric acid), the researchers found. GABA is an inhibitory neurotransmitter that reduces the activity of neurons. By preventing overstimulation, the molecule helps regulate a variety of brain functions, including anxiety, sleep, and muscle control.
Previous studies suggest that an imbalance of GABA is bad for the brain. When levels are too high in certain regions, it can lead to neurological disorders like Alzheimer’s. A similar mechanism has also been implicated in epilepsy, depression, and schizophrenia.
Shifting Theories
More than six million Americans are affected by Alzheimer’s, according to the Alzheimer’s Association. The prevailing hypothesis on this neurodegenerative disease has long been that amyloid beta plaques cause neuroinflammation in the brain. However, targeting these plaques has shown little success in treating or slowing its progression.
The new research provides some early evidence that acetate and the MCT1 transporter could be a new target in Alzheimer’s. The researchers believe that 11C-acetate and 18F-FDG PET imaging might be a tool for early diagnosis of Alzheimer’s dementia. With further development, they added, it could also lead to breakthrough treatments.
__________________________________________________________
The research was funded by the Institute for Basic Science (IBS.) IBS has 4 research institutes and 33 research centers as of January 2023.



