LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2023;25(1):22f03257
To cite: Burke H, Jiang S, Stern TA. Assessment and management of delirium in pediatric patients. Prim Care Companion CNS Disord. 2023;25(1):22f03257.
To share: https://doi.org/10.4088/PCC.22f03257
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Human Behavior, Warren Alpert Medical School of Brown University, Providence, Rhode Island
bDepartment of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, California
cDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
dHarvard Medical School, Boston, Massachusetts
*Corresponding author: Heather Burke, MD, Department of Psychiatry and Human Behavior, Warren Alpert Medical School of Brown University, 222 Richmond St, Providence, RI 02903 ([email protected]).
Have you ever been uncertain about how to assess an altered mental status in children and adolescents? Have you been unsure about how delirium can present in pediatric patients? Have you worried about missing a life-threatening cause of an altered mental status in younger patients? Have you wondered about whether and how you can prevent and treat delirium in children and adolescents? If you have, then the following case vignette and discussion should prove useful.
CASE VIGNETTE
An 8-year-old boy with a history of asthma was brought to his pediatrician’s office by his parents with an asthma exacerbation that had started after he had contracted a viral upper respiratory infection. On physical examination, he was wheezing upon both inhalation and expiration, and he was using his accessory muscles to breathe. The pediatrician sent him to the emergency department (ED), where he was started on a nebulizer (albuterol 0.15 mg/kg every 20 minutes and dexamethasone 0.6 mg/kg IV), but his breathing continued to worsen, and he became cyanotic. His oxygen saturation continued to decline to 83%, and he was intubated and admitted to the hospital with respiratory failure.
Two days later, he was extubated. However, his parents noted that he was now anxious and was not acting like he usually did. When he awakened, he needed to be reminded that he was in the hospital. The nurses noted that he had been sleeping during the day, was awake most of the night, and was having nightmares. His medication regimen in the hospital included Ativan 2 mg every 8 hours for anxiety.
DISCUSSION
How Can the Mental Status Examination of Children and Adolescents Be Assessed?
Mental status and neurologic examinations vary according to the age and developmental milestones of children and adolescents. Their interpretation also depends on the child’s baseline functioning and presence or absence of a developmental delay. Given this variability, the neurologic/mental status examination should be informed by an interview with the child’s caregivers to determine the child’s baseline as well as to gather the medical and family history of neurologic diseases or developmental delay.1–3
In neonates, periods of wakefulness typically alternate with active and quiet sleep. However, presence of lethargy or irritability is considered abnormal. Since older infants should be able to fixate on objects or people, watching how they respond to visual, verbal, and physical stimuli is critical in assessing mentation, as is watching how they eat.1–3
In toddlers and children up to 6 years old, most of the mental status examination can be completed by observing day-to-day behaviors. Watching how a child plays with a toy can provide evidence of the level of alertness, as well as motor function and coordination.2
Children aged 6 years and older (who are without a developmental delay) should be able to participate in conversations and describe their symptoms; this can provide evidence of their level of alertness, speech and language, motor behavior, and orientation. When evaluating cognition, screening questions may include counting to 20, reciting the alphabet, or pointing to various body parts (such as the chin, nose, or knees), but this does not substitute for a more formal cognitive evaluation.3
The assessment of the mental status of adolescents is like that of adults, with testing of language, reading, writing, math, fund of knowledge, abstraction, judgment, insight, and memory. However, their educational level or the presence of a neurodevelopmental delay may affect the assessment.1,2
What Can Cause an Altered Mental Status in Children and Adolescents?
Common causes of an altered mental status in children and adolescents include seizures, trauma, shock, exposure to toxic agents, electrolyte abnormalities (including hyperglycemia or hypoglycemia), and encephalitis. In infants and toddlers, additional age-specific etiologies including brief resolved unexplained events, breath-holding spells, inborn errors of metabolism, nonaccidental trauma, sepsis, and intussusception should be considered. In children and adolescents, a brain mass, syncope, migraine headaches, or infectious etiologies (like shigellosis) should be ruled out. Additional age-specific etiologies in adolescents include a variety of psychiatric conditions and posterior reversible encephalopathy syndrome. A prolonged alteration in mentation after an episode of loss of consciousness with accompanying tongue biting can suggest a seizure as opposed to syncope.4 Delirium has also been reported in adolescents with coronavirus disease 2019 (COVID-19), but further study is needed to determine prevalence.5
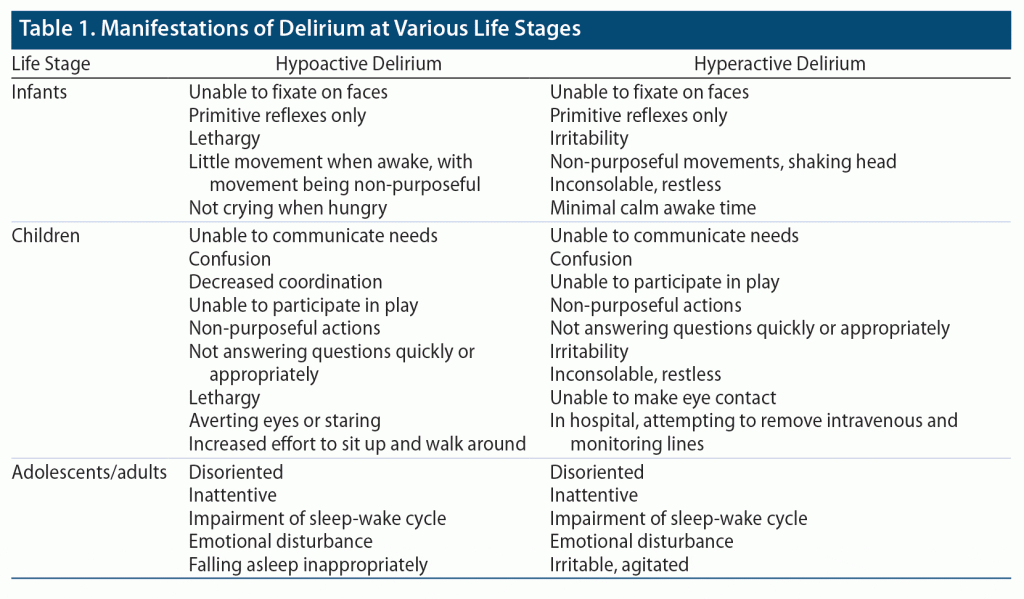
Delirium develops acutely and can be described as a fluctuation in cognition due to an underlying medical condition (such as the etiologies listed previously) (Table 1). Risk factors for delirium include infectious or inflammatory diagnoses, age < 2 years, receiving mechanical ventilation, and using benzodiazepines, narcotics, corticosteroids, or anticholinergic medications. Use of physical restraints and exposure to vasopressors or antiepileptics are also associated with delirium. Children are at higher risk for developing delirium if they have a developmental delay or if they have been in a coma or deeply sedated, and longitudinal studies6–9 have shown that a diagnosis of delirium is most frequently associated with respiratory failure and postsurgical status.
How Can Delirium in Children and Adolescents Be Diagnosed?
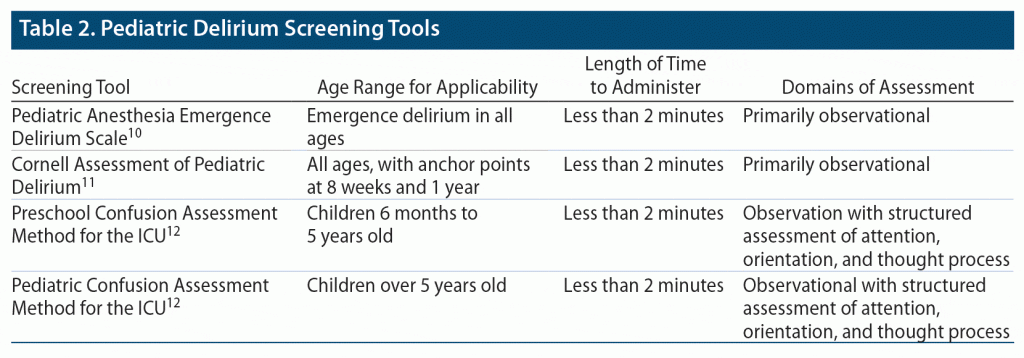
Delirium can be detected and subsequently monitored by using validated assessment tools and scales. Multiple scales, including the Pediatric Anesthesia Emergence Delirium Scale (PAED),10 the Cornell Assessment of Pediatric Delirium (CAP-D),11 and the Confusion Assessment Method for the Intensive Care Unit for both pediatric and preschool populations12 (PCAM-ICU and PSCAM-ICU, respectively) have been shown to have a high sensitivity and specificity for delirium (Table 2). These screenings are intended for use at bedside in the pediatric intensive care unit (PICU). The CAP-D and PAED are primarily observational screenings for delirium. The CAP-D can be performed on children of all ages, whereas the PAED specifically screens for the emergence of delirium or delirium that occurs after being under anesthesia. The PCAM-ICU (for children aged > 5 years) and the PSCAM-ICU (for children aged 6 months to 5 years) rely on both observations of the child and interviews to assess attention, orientation, and thought processes.13
The PSCAM-ICU has even been shown to be a valid screening tool in infants aged 6 months and younger.12 The CAP-D has demonstrated a high interrater reliability, with utilization of anchor points for normal development at 8 weeks and 1 year. Limitations in screening specificity may be seen in patients with significant developmental delay; however, incorporation of the Richmond Agitation Sedation Scale14 has been shown to improve this specificity.11,15 Compared to the other tools, the PAED possesses a lower sensitivity for the diagnosis of hypoactive delirium.13
How Common Is Pediatric Delirium and Why Is It Often Overlooked?
Delirium develops in about 25% of children who are admitted to a PICU, with a 38% incidence seen in children who have been admitted for 6 days or more.7 Hypoactive delirium is more common than hyperactive and mixed delirium, with delirium occurring in up to 34% of those admitted to ICUs. However, hypoactive delirium does not attract the same attention as hyperactive delirium and mixed (hyperactive and hypoactive) delirium with aggression and irritability, which often results in a longer time to diagnosis. Frequent shift changes of nursing staff throughout the day and brief examinations by treatment teams contribute to low rates of detection of delirium. As a result, routine screening is recommended in the ICU.16,17 Delirium in the non-ICU setting has been reported in adults, but little is known about the incidence of pediatric delirium in non-ICU admissions, further complicating its detection and treatment.
What Are the Consequences of Misdiagnosis and Mistreatment of Delirium in Children and Adolescents?
Delirium in children and adolescents has been linked with a higher mortality rate (odds ratio = 4.4).9 Delirium is also associated with a longer length of stay in the PICU (up to twice as long compared to that of patients without delirium) and more time spent receiving mechanical ventilation.9 A diagnosis of delirium has also been associated with costlier hospitalizations (up to an 85% increase in cost).18 For these reasons, early diagnosis and treatment of delirium is crucial.
How Can Delirium in Pediatric Patients Be Managed?
The approach to delirium in pediatric patients involves identifying the underlying illness and minimizing environmental and iatrogenic contributors. Timely evaluation is essential, as myriad medical conditions (involving deoxygenation, hypoperfusion of the central nervous system, drug withdrawal, infections, seizures, toxidromes, metabolic and electrolyte abnormalities, immunologic disorders, and neurologic diseases) can become lethal if left undiagnosed and untreated.9
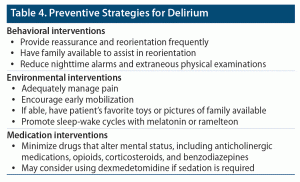
Management of delirium is best accomplished by treating the precipitating problem as specifically as possible. In addition, environmental interventions can reduce delirium’s behavioral accompaniments. A normal sleep-wake cycle can be promoted by implementation of daytime and nighttime routines and by use of melatonin or ramelteon at bedtime. In addition, lightening exogenous sedation, increasing mobility, reorienting frequently, and maintaining a low-stimulus environment can be soothing. Reducing or eliminating iatrogenic triggers is highly recommended (eg, discontinuing anticholinergic medications, opioids, corticosteroids, and benzodiazepines).19,20
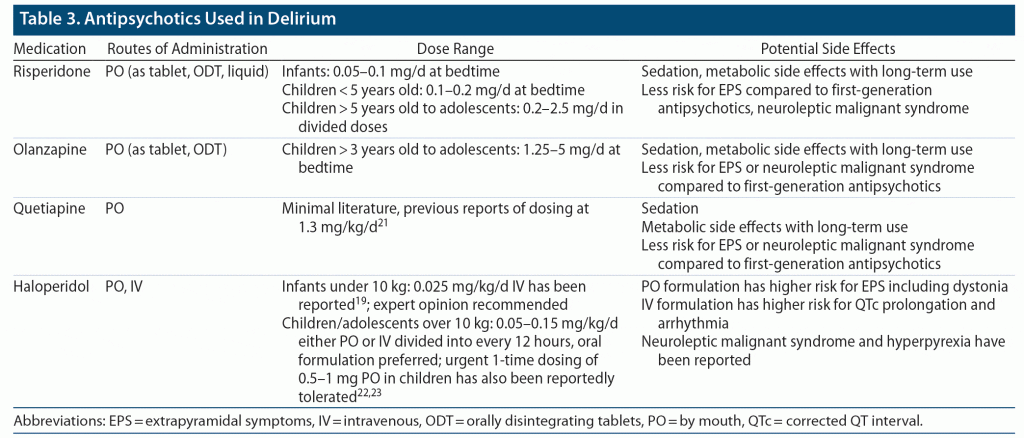
To address the agitation, irritability, and restlessness seen with hyperactive delirium, use of atypical antipsychotics (including risperidone, olanzapine, and quetiapine) or first-generation antipsychotics (eg, haloperidol) has led to positive outcomes (Table 3).19,21–23 Choosing an antipsychotic often depends on the formulation available; for example, risperidone may be prescribed in liquid form or as an oral disintegrating tablet (making it ideal for children who cannot swallow pills). Dexmedetomidine has also been used in the PICU, as it provides sedation and anxiety reduction without increasing the risk for delirium. Dexmedetomidine can be transitioned to other α-agonists (eg, oral clonidine) for continued anxiolysis.20–22
Other medications, such as α2 agonists (eg, guanfacine), can reduce the norepinephrine surge that accompanies hyperactive delirious states. Guanfacine, an α agonist that is typically used for the treatment of attention-deficit/hyperactivity disorder, has shown promise in adults with delirium and in adolescents with delirium secondary to COVID-19 infection.5,24 Specifically for delirium that arises shortly after surgery, dexmedetomidine can be used for purposes of sedation in the PICU and then be transitioned to clonidine to decrease the incidence of delirium.20 Use of ketamine for management of hyperactive delirium in the ED has also shown positive results.25
How Can Delirium in Pediatric Patients Be Prevented?
Prevention of delirium often focuses on manipulating behavioral components as well as on screening and reducing biomedical conditions (eg, hypotension, hypoxemia) (Table 4). Psychosocial and behavioral interventions include having the child be accompanied at the hospital by family members and placing images of family members, favorite objects or toys, and clocks in the room to keep the child oriented. Providing reassurance and reorientation when the child wakes up from anesthesia can also help to reduce anxiety, as well as maintaining proper sleep-wake cycles and minimizing things (including alarms and additional examinations) that could wake the child during the night.20 Adequately managing pain, minimizing iatrogenic contributors, and encouraging early mobilization have decreased the rate of delirium.16
Currently, there are no widely accepted medications for delirium prevention. There is also minimal agreement on which antipsychotics or dosing strategies should be used for the prevention of delirium. In adults, use of antipsychotics to prevent delirium is not routinely recommended.16,26
What Are the Long-Term Consequences of Pediatric Delirium?
In a study,27 delirium was associated with a decreased quality of life, as measured by the Infant-Toddler Quality of Life Questionnaire at 1 month and 3 months after hospital discharge. Psychological consequences of delirium include an increased risk for delusional memories, particularly if children were given benzodiazepines or opioids during their hospitalization.28 Moreover, 5%–28% of children with delirium were later diagnosed with posttraumatic stress disorder (PTSD), and 35%–62% of children with delirium had symptoms of PTSD.29
Delirium, which has been associated with longer length of stays in the neonatal ICU (NICU) and PICU, may also adversely affect long-term cognition. Prolonged stays in the NICU have been associated with a bottom 10% scoring on the Bayley Scales of Infant and Toddler Development on the 9-month and 24-month mental and motor assessments.30 In the PICU, mechanical ventilation and longer ICU length of stays were associated with higher rates of acquired global functional and cognitive disabilities as evidenced by lower scores on the Pediatric Overall Performance Category Scale and the Pediatric Cerebral Performance Category Scale.31 Children who were admitted to the PICU for at least 48 hours with a diagnosis of delirium were found to have a significant decrease in Pediatric Cerebral Performance Category Scale scores (from their preadmission testing to their hospital discharge), with more than a 3-point decline.32 Further research is needed to determine whether delirium independently adversely influences long-term cognition after discharge, as knowing so could provide insight into whether children can benefit from routine cognitive screening or mental status examinations after being discharged from the hospital
What Happened to Our Patient?
Our patient was able to think more clearly after his as-needed benzodiazepines were discontinued, and his pain was adequately treated. He was started on a twice-daily dose of guanfacine for agitation and anxiety and nightly melatonin to improve his sleep-wake cycle. His parents were instructed to reorient him frequently and to keep him engaged throughout the day. With these interventions, he quickly returned to his baseline cognitive state and no longer complained of any nighttime disturbances or anxiety. He was discharged to his home shortly thereafter. Upon follow-up with his primary care doctor, he was screened for PTSD symptoms, during which he reported worsening nightmares since returning home from the hospital. His primary care doctor recommended that if these nightmares persisted or were accompanied by other symptoms of anxiety or PTSD he may benefit from trauma-focused therapy.
CONCLUSION
Delirium is a common and frequently overlooked complication in children who are admitted to the hospital. Contributing factors include worsening underlying medical problems, disruption of sleep-wake cycles, and use of medications that are deliriogenic. Management of delirium should focus on addressing underlying medical problems and managing pain. Management of delirium often includes use of medications, including antipsychotics; however, further study on this issue is needed. Continued monitoring after discharge of both cognitive function and development is indicated, since there may be long-term effects from prolonged NICU and PICU stays. Screening for the psychological impact of hospitalization may also be appropriate to facilitate outpatient follow-up.
Submitted: February 3, 2022; accepted May 6, 2022.
Published online: February 16, 2023.
Relevant financial relationships: None.
Funding/support: None.
CLINICAL POINTS
- Delirium, particularly hypoactive delirium, is frequently underdiagnosed; therefore, routine screening is recommended in pediatric patients.
- When delirium is detected in pediatric patients, management includes identifying the etiology as well as removing any contributing factors that can include environmental and iatrogenic sources.
- There are both short-term and long-term effects from delirium in children and adolescents, including reduced quality of life after discharge from the hospital and impaired cognition.
References (32)

- Ashby AT, Beier AD. Review of pediatric neurologic history and age-appropriate neurologic examination in the office. Pediatr Clin North Am. 2021;68(4):707–714. PubMed CrossRef
- Marcdante KJ, Kliegman R, Nelson WE. Nelson Essentials of Pediatrics. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2019.
- Nurcombe B, Ebert MH. The Psychiatric Interview. In: Ebert MH, Leckman JF, Petrakis IL. eds. Current Diagnosis & Treatment: Psychiatry. 3rd ed. McGraw Hill.
- MacNeill EC, Vashist S. Approach to syncope and altered mental status. Pediatr Clin North Am. 2013;60(5):1083–1106. PubMed CrossRef
- Bauer SC, Moral F, Preloger E, et al. Pediatric COVID-19 delirium: case report of 2 adolescents. WMJ. 2021;120(2):131–136. PubMed
- Patel AK, Bell MJ, Traube C. Delirium in pediatric critical care. Pediatr Clin North Am. 2017;64(5):1117–1132. PubMed CrossRef
- Traube C, Silver G, Reeder RW, et al. Delirium in critically ill children: an international point prevalence study. Crit Care Med. 2017;45(4):584–590. PubMed CrossRef
- Gupta N, Woolley A, Talathi S, et al. Opioid use is associated with ICU delirium in mechanically ventilated children. J Crit Care Med (Targu Mures). 2020;6(3):167–174. PubMed CrossRef
- Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891–898. PubMed CrossRef
- Blankespoor RJ, Janssen NJJF, Wolters AMH, et al. Post-hoc revision of the Pediatric Anesthesia Emergence Delirium Rating Scale: clinical improvement of a bedside-tool? Minerva Anestesiol. 2012;78(8):896–900. PubMed
- Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. 2014;42(3):656–663. PubMed CrossRef
- Canter MO, Tanguturi YC, Ellen Wilson J, et al. Prospective validation of the Preschool Confusion Assessment Method for the ICU to screen for delirium in infants less than 6 months old. Crit Care Med. 2021;49(10):e902–e909. PubMed CrossRef
- Daoud A, Duff JP, Joffe AR; Alberta Sepsis Network. Diagnostic accuracy of delirium diagnosis in pediatric intensive care: a systematic review. Crit Care. 2014;18(5):489. PubMed CrossRef
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. PubMed CrossRef
- Kaur S, Silver G, Samuels S, et al. Delirium and developmental disability: improving specificity of a pediatric delirium screen. Pediatr Crit Care Med. 2020;21(5):409–414. PubMed CrossRef
- Marra A, Ely EW, Pandharipande PP, et al. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33(2):225–243. PubMed CrossRef
- Semple D, Howlett MM, Strawbridge JD, et al. A systematic review and pooled prevalence of delirium in critically ill children. Crit Care Med. 2021;29(suppl 1):i31–i32. PubMed CrossRef
- Traube C, Mauer EA, Gerber LM, et al. Cost associated with pediatric delirium in the ICU. Crit Care Med. 2016;44(12):e1175–e1179. PubMed CrossRef
- Silver GH, Kearney JA, Bora S, et al; Pathways for Clinical Care Workgroup. A clinical pathway to standardize care of children with delirium in pediatric inpatient settings. Hosp Pediatr. 2019;9(11):909–916. PubMed CrossRef
- Turkel SB. Pediatric delirium: recognition, management, and outcome. Curr Psychiatry Rep. 2017;19(12):101. PubMed CrossRef
- Joyce C, Witcher R, Herrup E, et al. Evaluation of the safety of quetiapine in treating delirium in critically ill children: a retrospective review. J Child Adolesc Psychopharmacol. 2015;25(9):666–670. PubMed CrossRef
- Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982–1983. PubMed CrossRef
- Kishk OA, Simone S, Lardieri AB, et al. Antipsychotic treatment of delirium in critically ill children: a retrospective matched cohort study. J Pediatr Pharmacol Ther. 2019;24(3):204–213. PubMed CrossRef
- Jiang S, Czuma R, Cohen-Oram A, et al. Guanfacine for hyperactive delirium: a case series. J Acad Consult Liaison Psychiatry. 2021;62(1):83–88. Published online Nov 13, 2020. PubMed CrossRef
- Kowalski JM, Kopec KT, Lavelle J, et al. A novel agent for management of agitated delirium: a case series of ketamine utilization in the pediatric emergency department. Pediatr Emerg Care. 2017;33(9):e58–e62. PubMed CrossRef
- Capino AC, Thomas AN, Baylor S, et al. Antipsychotic use in the prevention and treatment of intensive care unit delirium in pediatric patients. J Pediatr Pharmacol Ther. 2020;25(2):81–95. PubMed CrossRef
- Silver G, Doyle H, Hegel E, et al. Association between pediatric delirium and quality of life after discharge. Crit Care Med. 2020;48(12):1829–1834. PubMed CrossRef
- Colville G, Kerry S, Pierce C. Children’s factual and delusional memories of intensive care. Am J Respir Crit Care Med. 2008;177(9):976–982. Published online Jan 31, 2008. PubMed CrossRef
- Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: a systematic review. World J Crit Care Med. 2017;6(2):124–134. PubMed CrossRef
- Subedi D, DeBoer MD, Scharf RJ. Developmental trajectories in children with prolonged NICU stays. Arch Dis Child. 2017;102(1):29–34. PubMed CrossRef
- Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr Crit Care Med. 2014;15(7):640–648. PubMed CrossRef
- Dervan LA, Di Gennaro JL, Farris RWD, et al. Delirium in a tertiary PICU: risk factors and outcomes. Pediatr Crit Care Med. 2020;21(1):21–32. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top