Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

Case Conference
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Stead Family Memory Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows. The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2020;22(5):20alz02812
To cite: Gopalakrishna G, Hendrie KA. Spotting the zebra: keeping an open mind when evaluating cognitive impairment. Prim Care Companion CNS Disord. 2020;22(5):20alz02812.
To share: https://doi.org/10.4088/PCC.20alz02812
© Copyright 2020 Physicians Postgraduate Press, Inc.
Ganesh Gopalakrishna, MD, is a geriatric psychiatrist and dementia specialist at Banner Alzheimer’s Institute and a clinical associate professor of psychiatry at the University of Arizona College of Medicine, Phoenix, Arizona.
Kyle Angus Hendrie, DO, is a resident physician in the Department of Psychiatry, University of Arizona, Phoenix, Arizona.
Published online: October 22, 2020.
*Corresponding author: Ganesh Gopalakrishna, MD, Stead Memory Clinic, Banner Alzheimer’s Institute, 901 E Willetta St, Phoenix AZ 85006 ([email protected]).
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
- Use appropriate tools in the differential diagnosis of cognitive impairment
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in October 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through October 31, 2022. The latest review of this material was October 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Acadia, Allergan, Eisai, Merck, Supernus, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears on the next page.
HISTORY OF PRESENTING ILLNESS
Ms A is an 82-year-old white woman who presented to Banner Alzheimer’s Institute complaining of occasional short-term memory loss and associated feelings of sadness. For her initial visit, she was accompanied by her husband and son-in-law, both of whom were reliable informants. Ms A reported lapses in memory over the last few years, but these lapses were particularly worse in recent months. She reported difficulty recalling recent events and details of conversations and found herself repeating stories. She sometimes had difficulty understanding others, making judgments, and multitasking. She was occasionally forgetting to take her medications and had difficulty remembering names of acquaintances. She remained exceptional at organizing and had no trouble keeping track of dates or appointments. Her family had noticed no changes in her speech, handwriting, or ability to navigate. She was driving with no safety concerns. She continued to cook with no difficulty and had no change in her ability to manage electronics. She took care of all household finances and was able to file taxes that year. The husband noted, however, that managing finances had become frustrating for her lately, and he planned to take over soon. She was independent in managing her medications and organized her husband’s medications as well. She was able to shop independently. She was independent in personal care and grooming.
Ms A reported that she had become easily tearful over the last 2 years. She reported periods of tearfulness and crying that were sudden in onset with no specific trigger. These episodes happened about 3 to 4 times a week and lasted for about 15 minutes each. She felt frustrated when she had these episodes and could not control the crying without hugging her husband. She reported no laughing episodes or inappropriate smiling. She also denied history of shortness of breath, palpitation, chest pain, or tingling in her limbs during these periods of crying. Ms A reported that her mood was good other than feeling sad during these episodes. She reported poor sleep, particularly difficulty initiating and maintaining sleep, as a chronic issue for the past 5 years. She identified herself as a “worry wort.” Her energy and appetite were unchanged, and she denied weight changes. Her husband noted that she had not been inviting family over as much recently. He and the son-in-law identified anxiety as a significant factor affecting her perception of the future and stress levels. She denied any auditory or visual hallucinations and endorsed no delusions.
PAST MEDICAL HISTORY
Ms A has a history of hypertension and hyperlipidemia. She denied history of head injury, stroke, or seizures.
ALLERGIES
Ms A had no known allergies.
MEDICATIONS
Ms A’s medications included oral tablet atorvastatin 20 mg daily, hydrochlorothiazide-losartan 25 mg-100 mg daily, ibandronate daily, and oral tablet trazodone 50 mg daily.
SOCIAL HISTORY
Ms A completed some college. She worked part time as an accountant before retiring 18 years prior. She reported varied interests including cooking and writing. She has 2 daughters and a son. She currently lives with her husband.
SUBSTANCE USE HISTORY
Ms A reported a 50-pack/year smoking history but quit in 2002. She reported consuming about 8 alcoholic beverages per week and denied history of excessive alcohol use. She denied history of illicit drug use.
FAMILY HISTORY
Ms A’s mother had dementia with onset about 18 years before her death. Ms A frequently mentioned she did not want to be like her mother, in reference to her mother’s struggle with dementia. The patient and her family reported that her brothers also had suspected memory impairment. Her sister was diagnosed with dementia when she was in her 70s.
Lifetime risk for developing dementia for an individual with a family history of dementia is 20%, whereas lifetime risk for developing dementia in the general population is 10% (Loy et al, 2014). Alzheimer’s disease accounts for 60%-80% of all dementia diagnoses in the United States according to the report “2020 Alzheimer’s Disease Facts and Figures,” which details national statistics related to Alzheimer’s disease (Alzheimer’s Association, 2020). A person with a first-degree relative with Alzheimer’s disease is more likely to develop the disease than one without such a first-degree relative. Having more than 1 first-degree relative with Alzheimer’s disease places a person at even higher risk. Additionally, having a parent with dementia increases the chance that a person is carrying a known genetic risk factor for Alzheimer’s disease, such as the APOE-e4 allele (Green et al, 2002).
Ms A’s neurologic examination was unremarkable. Her mental status examination was also normal. Ms A’s physical examination showed mild hypertension of 164/75 mm Hg, a heart rate of 75 bpm, and weight of 72 kg (159 lb).
Ms A scored 22/30 on the MMSE (Folstein et al, 1975). Her score showed impairment in orientation (4 points lost), delayed recall (3 points lost), and drawing (1 point lost).
Ms A scored 19/30 on the MoCA. She showed impairment in visuospatial/executive function (3 points lost), attention (1 point lost), language (1 point lost), abstraction (1 point lost), delayed recall (5 points lost), and orientation (1 point lost).
The MoCA has been shown to have a better sensitivity and specificity in detecting subtle cognitive impairments, such as mild cognitive impairment (MCI), compared to the MMSE. Nasreddine et al (2005) found that the MMSE had a sensitivity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. In the mild Alzheimer’s disease group, the MMSE had a sensitivity of 78%, whereas the MoCA detected 100%. Specificity was excellent for both the MMSE and MoCA (100% and 87%, respectively).
Dementia can be classified as probable Alzheimer’s disease when core clinical criteria for all-cause dementia are met and there are characteristics of an insidious or gradual onset of cognitive symptoms over months to years, not sudden over hours or days, with clear-cut history of worsening reported or observed by others (McKhann et al, 2011). The initial and most prominent cognitive deficits most commonly reflect short-term memory dysfunction, with impairment in learning and recall of recently learned information. There should also be evidence of cognitive dysfunction in at least 1 other cognitive domain such as reasoning and handling of complex tasks, visuospatial abilities, language functions, or changes in behavior or comportment. Less commonly, nonamnestic presentations occur when the most prominent deficits are in language, visuospatial skills, or executive function.
Ms A was noted to have some difficulty with visuospatial tests during the screening at her initial visit. Considering the periods of confusion reported by the patient and family, Lewy body dementia was considered as a potential etiology. The performance on her initial screening tests could also be impacted due to executive dysfunction related to white matter disease. The episodes of crying and tearing could be related to anxiety or pseudobulbar affect.
THE TREATING PHYSICIAN’ S PLAN
The treating physician’s plan included ordering the following laboratory tests to rule out reversible causes of dementia: vitamin B12, folate, complete blood count, comprehensive metabolic panel, and thyroid-stimulating hormone. The physician recommended neuropsychological evaluation to quantify cognitive deficits and to establish a cognitive baseline. A magnetic resonance image (MRI) brain scan was ordered to rule out organic causes for cognitive changes. To help with the patient’s anxiety, the treating physician started Ms A on escitalopram 5 mg by mouth daily with the plan to increase to 10 mg by mouth daily after the first 2 weeks. If Ms A had a bad reaction to escitalopram at an appropriate treatment dose, the treating physician also considered replacing this medication with dextromethorphan hydrobromide and quinidine sulfate for possible pseudobulbar affect. The physician planned on discussing a referral to a social worker once the patient’s diagnosis was clarified.
LABORATORY AND RADIOGRAPHIC FINDINGS
Results of Ms A’s complete blood count, comprehensive metabolic panel, and thyroid-stimulating hormone, free T4, vitamin B12, folate, and methylmalonic acid tests performed after the initial visit were within normal limits.
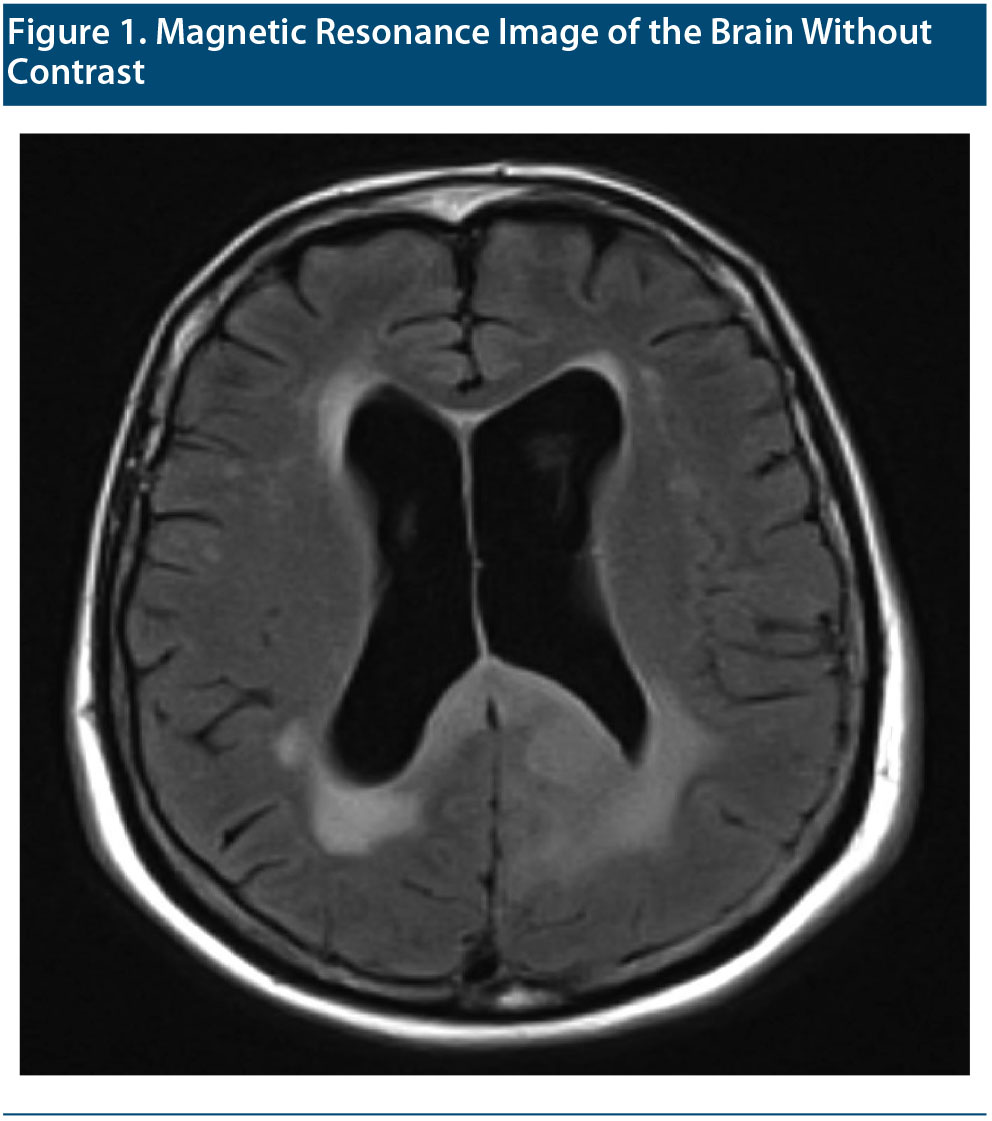
The MRI of the brain without contrast taken shortly after the visit is shown in Figure 1. After reviewing the MRI, the radiologist concluded that Ms A had chronic microvascular ischemic white matter disease. Cortical atrophy (with focal volume loss in the right anterior temporal lobe) and prominence of the lateral and third ventricles were also noted. Clinical correlation for normal pressure hydrocephalus was recommended. A remote lacunar infarct with volume loss in the right anterior temporal lobe was noted.
On the basis of the MRI report, no further radiologic testing was indicated. PET-FDG is indicated in the differentiation of Alzheimer’s disease from frontotemporal degeneration or other atypical presentations of Alzheimer’s disease. CTA of the head and fMRI of the brain are not indicated for routine evaluations of cognitive impairment.
NEUROPSYCHOLOGY EVALUATION
The patient’s neuropsychological evaluation was suggestive of amnestic mild cognitive impairment, multiple domains (memory, attention). Her neuropsychological profile demonstrated deficits in memory (verbal, visual) and attention as well as set switching and semantic fluency.
Ms A showed a relative weakness in processing speed. There was no substantial decline in intellectual functioning relative to the estimated premorbid level.
UPDATE ON Ms A’s CLINICAL PICTURE
A few months after Ms A’s initial interview, her husband called the office complaining she had worsening confusion, question repetition, and trouble completing self-hygiene tasks. He also reported worsening depression and crying spells. Ms A was leaving the stove on, unable to work the TV remote, and had an episode where she was unable to find the bathroom. He noted that she often could not find items that were right in front of her. She also had 4 episodes of bowel incontinence. Her confusion and crying spells appeared to be worse in the evening. He further reported that she was sleeping well and denied any dysuria or urinary frequency. There seemed to be a temporal correlation between the development of these symptoms and the increase in Ms A’s escitalopram dose from 5 mg daily to 10 mg daily.
The rapid progression of Ms A’s condition does not correlate with the typical progression related to dementia, especially Alzheimer’s disease. In the evaluation of acute or subacute confusion, it is important to rule out common infective metabolic and pharmacologic etiologies. Acute changes in mental status could be related to a cerebrovascular accident and are likely to be associated with localizing signs. Based on Ms A’s worsening symptoms, the treating physician decreased her escitalopram dose from 10 mg to 5 mg daily and ordered a urinalysis to rule out acute encephalopathy secondary to urinary tract infection. A few days after this change, her symptoms had not improved. There was no evidence of urinary tract infection. Ms A’s husband began showing notable caregiver fatigue during appointments secondary to the increased burden of caring for his wife in her worsened condition.
At this time, another clinician on the treatment team reviewed Ms A’s recently completed MRI brain scan with a neurologist. Both had concern for possible cerebral edema noted on the left side of the brain with attenuation of the sulci, raising suspicion for a brain tumor. The family was notified and brought Ms A to the emergency department for further evaluation.
Brain MRI with contrast is the preferred method for identification of brain tumors. CT images are lower resolution and more appropriate for emergency situations, for situations when evaluation of bony structures is necessary, or for patients who have a contraindication for MRI study (Wong and Wu, 2020). Contrast is used, as this makes lesions more conspicuous, and aspects of contrast enhancement increase specificity of a tumor diagnosis (Young and Knopp, 2006).
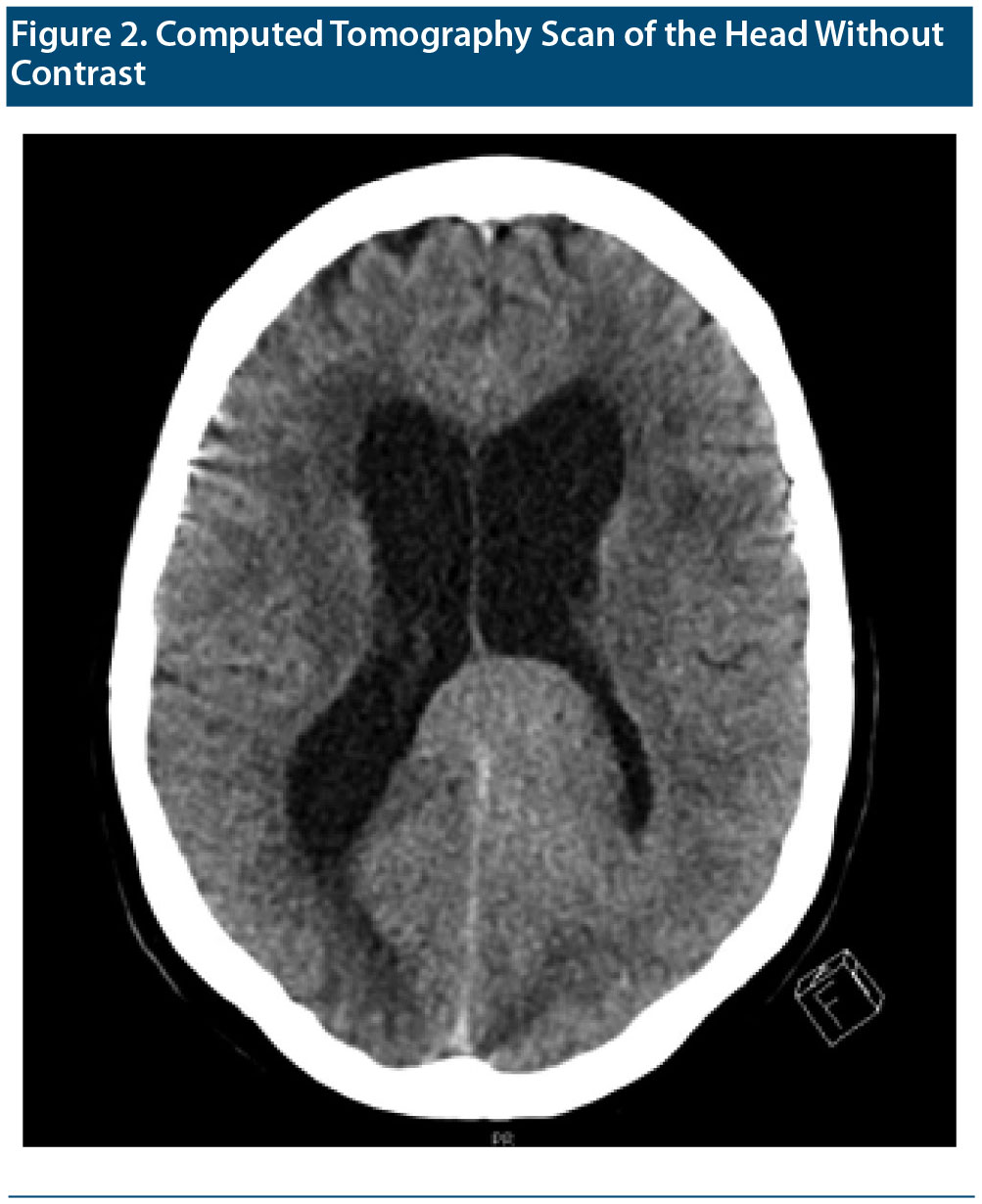
A CT scan (Figure 2) performed in the emergency department showed a probable 4.5 ×— 4.5 ×— 2.5-cm mass in the left parietal lobe with mass effect on the left lateral ventricle. Persistent dilatation of the lateral, third, and fourth ventricles was consistent with hydrocephalus. Ms A was admitted to the hospital for further evaluation. On the basis of the initial CT scan in the emergency department, neurosurgery staff determined that the tumor was nonresectable, and the medical oncology department was consulted.
IMAGING RESULTS
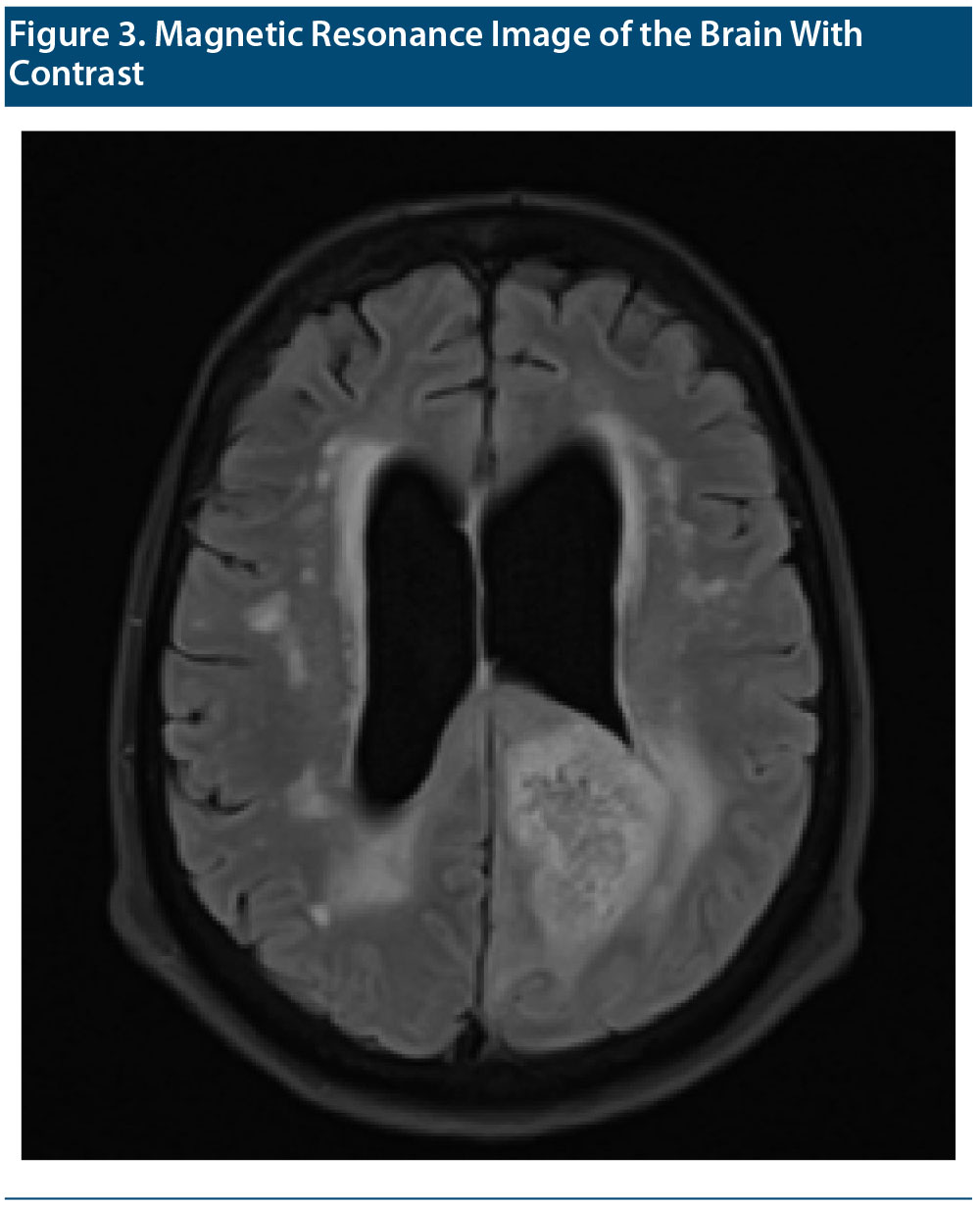
MRIs of the brain with and without contrast were performed and compared to the patient’s MRI without contrast performed a few months earlier (Figure 3 shows the MRI with contrast). The same radiologist who read the initial MRI read the second study. The report concluded that a left parietal lobe mass was causing mass effect on the atria of the left lateral ventricle with heterogeneous enhancement and surrounding peritumoral edema. The lesion crossed midline via the splenium of the corpus callosum and involved the posterior aspect right parietal lobe favoring a butterfly glioma (World Health Organization [WHO] grade 4 astrocytoma). Differential considerations included primary central nervous system lymphoma, cerebral toxoplasmosis if the patient is immunocompromised, and, less likely, metastatic disease. There was also a deep white matter hyperintense T2 and T2 fluid-attenuated inversion recovery signal suggestive of chronic microvascular ischemic white matter disease.
A left parietal lobe brain biopsy was performed, with the final result of glioblastoma WHO grade 4. Ms A was diagnosed with glioblastoma multiforme. During the hospital course, she was confused and impulsive, leading to the use of soft restraints. She was initiated on intravenous dexamethasone, and palliative care was consulted. The decision was made to place Ms A in inpatient hospice, with additional diagnoses of acute encephalopathy, acute urinary retention, and hydrocephalus.
DISCUSSION
This case provides several learning opportunities. We highlight some of the key takeaway points for the team at Banner Alzheimer’s Institute. First, this case underscores the need for an imaging study as part of the evaluation of any patient presenting with cognitive impairment. The initial presentation, absent the emotional incontinence, looks very similar to a patient presenting with dementia. American Academy of Neurology and American Psychiatric Association guidelines suggest MRI or CT scan of the brain for the workup of cognitive impairment (Knopman et al, 2001; Wippold et al, 2015).
Second, it is important to revisit the patient’s history and workup when the clinical course does not correlate with the working diagnosis. When a patient presents with confusion or other mental status changes, it is important to rule out causes that may precipitate or perpetuate such changes. If these investigations turn out to be negative, a deliberate effort must be made to revisit previous assumptions and workup.
Finally, this case presents a learning opportunity to address errors in medical care. Medical errors happen and are not without consequences. According to an excerpt from the consensus study report To Err is Human: Building a Safer Health System published by the Institute of Medicine in 2000, as many as 98,000 people die in any given year secondary to medical errors that occur in hospital settings. At the time that report was published, this was a greater number than those dying from motor vehicle accidents, breast cancer, or AIDS despite these issues appearing to receive more media attention. A main idea conveyed in that report is that the problem is not necessarily that health care workers are bad people but that good people are working in bad systems that need to be made safer.
Adrian P. Brady succinctly outlined the difference between errors and discrepancies in a 2017 publication (Brady AP, 2017). If a radiology interpretation is labeled as an error, it is implied that the radiologist’s read was incorrect and no room is left for difference in opinion among experts in the field regarding what the correct read should have been. Labeling the same impression as a discrepancy implies that if another radiologist were to have a different impression of the same MRI scan, these impressions would fall within the realm of reasonable differences of opinions between responsible, diligent practitioners. Errors do happen in radiologic interpretation just like in any other branch of medicine, but many situational aspects should be considered when analyzing diagnostic discrepancies. In the case presented here, the error of missing the tumor on the initial MRI, by both the radiologist and clinician, led to the lack of a follow-up MRI of the brain with contrast.
Just how often do these differences in opinion among radiologists occur? According to a review of radiologic errors and malpractice, the frequency in which radiologists make errors in reading imaging studies is reported as a wide range based on how you define the error or measure. If one is considering how often a radiologist makes an error in interpreting an abnormal study, it tends to be around 30%. If one considers normal and abnormal studies, which is more characteristic of the day-to-day job of a radiologist, discrepancies between readers average between 3.5% and 4% (Berlin L, 2007a; Berlin L, 2007b). In studies looking at correlations between clinical and pathological diagnoses, sensitivity ranged from 70.9% to 87.3% and specificity ranged from 44.3% to 70.8%. Neurologists of the National Institute of Aging-funded Alzheimer’s Disease Centers had higher predictive accuracy when they diagnosed Alzheimer’s disease in demented subjects than when they diagnosed dementing diseases other than Alzheimer’s disease (Beach et al, 2012). This finding highlights the risk of bias and error in all modalities of patient care. There is also the phenomenon of “hindsight bias,” which is commonplace in lawsuits related to negligence. Leonard Berlin (2000) defines hindsight bias as “a tendency for people with knowledge of the actual outcome of an event to believe falsely that they would have predicted the outcome.”p600
One way to combat medical errors is by using the systems approach. This approach was pioneered by the British psychologist James Reason (2000) and uses what is now referred to as the “Swiss cheese” model of medical errors. In contrast to tradition, wherein medical errors were treated as the failing of an individual, in the systems approach most errors reflect predictable human failings in the context of poorly designed systems. This approach sees human error as inevitable, especially in systems as complex as health care. This approach of course would not apply to instances of intentional neglect or reckless behavior. The systems approach differentiates between active errors and latent errors. Latent errors would include things such as faulty protocols, procedures, caseload, or an inexperienced workforce leading to errors. The goal of the systems approach is to identify situations or factors that are likely to give rise to human error. Once identified, the underlying system of care can be changed to reduce the occurrence of the errors or minimize their impact on patients. The systems approach includes many specific techniques that can be used to analyze errors. These techniques can be either retrospective or prospective. A specific example of a retrospective method that is often used is root cause analysis, also called systems analysis. The process of root cause analyses involves identifying the event to be investigated, chartering and selecting team facilitators and team members, describing the event that happened, identifying contributing factors to the event, identifying root causes of the event, designing and implementing changes to eliminate the root cause of the event, measuring the success of the implemented changes, and, lastly, communicating the results. In a published example of a root cause analysis analyzing errors in radiology, root causes identified included lack of manpower, issues with equipment, the radiologist’s standard setting, failures in team working, failures in error analysis, and poor performance of individuals (Fitzgerald R, 2001).
CONCLUSION
This report presents the case of an 82-year-old woman with a chief complaint of deficits in short-term memory and executive functioning along with mood and anxiety disorder symptoms. The patient’s clinical presentation was suggestive of a neurodegenerative disease like dementia. Although a complete workup for reversible organic causes of the symptoms was conducted, a major organic cause of these symptoms was missed and discovered later after the patient showed a significant change in clinical condition. Medical errors are commonplace in the current health system and use of a systems-based approach can help identify the potential pitfalls and improve outcomes.
Published online: October 22, 2020.
Disclosure of Off-Label Usage
The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial Disclosure
Drs Gopalakrishna and Hendrie have no personal affiliations or financial relationships with any commercial interests to disclose relative to the article.
FUNDING/SUPPORT
None.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
ADDITIONAL INFORMATION
Information about the patient case has been de-identified to protect anonymity.
Clinical Points
- Diagnosis of a patient with cognitive impairment should include consideration of a broad range of possible etiologies.
- It is important to revisit a patient’s initial history and workup when the clinical course does not correlate with the working diagnosis.
- A brain imaging study should be part of the workup for any patient presenting with cognitive impairment.
- Medical errors are common and should be addressed using a systems approach.
References
Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. PubMed CrossRef
Beach TG, Monsell SE, Phillips LE, et al. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. PubMed CrossRef
Berlin L. Accuracy of diagnostic procedures: has it improved over the past five decades? AJR Am J Roentgenol. 2007a;188(5):1173-1178. PubMed CrossRef
Berlin L. Hindsight bias. AJR Am J Roentgenol. 2000;175(3):597-601. PubMed CrossRef
Berlin L. Radiologic errors and malpractice: a blurry distinction. AJR Am J Roentgenol. 2007b;189(3):517-522. PubMed CrossRef
Brady AP. Error and discrepancy in radiology: inevitable or avoidable? Insights Imaging. 2017;8(1):171-182. PubMed CrossRef
Fitzgerald R. Error in radiology. Clin Radiol. 2001;56(12):938-946. PubMed CrossRef
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed CrossRef
Green RC, Cupples LA, Go R, et al; MIRAGE Study Group. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329-336. PubMed CrossRef
Institute of Medicine Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143-1153. PubMed CrossRef
Loy CT, Schofield PR, Turner AM, et al. Genetics of dementia. Lancet. 2014;383(9919):828-840. PubMed CrossRef
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed CrossRef
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed CrossRef
Reason J. Human error: models and management. BMJ. 2000;320(7237):768-770. PubMed CrossRef
Robinson PJ. Radiology’s Achilles’ heel: error and variation in the interpretation of the Röntgen image. Br J Radiol. 1997;70(839):1085-1098. PubMed CrossRef
Wippold FJ 2nd, Brown DC, Broderick DF, et al. ACR Appropriateness Criteria Dementia and Movement Disorders. J Am Coll Radiol. 2015;12(1):19-28. PubMed CrossRef
Wong ET, Wu JK. Overview of the clinical features and diagnosis of brain tumors in adults. UpToDate website. https://www.uptodate.com/contents/overview-of-the-clinical-features-and-diagnosis-of-brain-tumors-in-adults? Accessed June 10, 2020.
Young RJ, Knopp EA. Brain MRI: tumor evaluation. J Magn Reson Imaging. 2006;24(4):709-724. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top