ABSTRACT
Objective: Data are scarce regarding the incidence of neuropsychiatric events (NPEs) in people living with human immunodeficiency virus (HIV)–1 taking integrase inhibitor (INI)– or protease inhibitor (PI)–based regimens. This study evaluated the prevalence, incidence, and economic burden of NPEs among people living with HIV-1 who were newly treated with INI- or PI-based regimens in a Medicaid population.
Methods: A retrospective cohort study was conducted using administrative claims from the IBM MarketScan Multi-State Medicaid Database (January 1, 2014–December 31, 2018). Treatment-naive and treatment-experienced adults with HIV-1 newly treated with an INI- or PI-based regimen were included. Outcomes included NPE prevalence during the 12-month baseline period, prevalence of existing and incidence of new-onset NPEs during the 6-month post-index period, and total all-cause and NPE-related costs between treatment cohorts. Baseline characteristics between the 2 cohorts were balanced using inverse probability treatment weighting.
Results: In the INI (n = 3,929) and PI (n = 3,916) cohorts, mean (SD) ages were 44.87 (12.81) and 44.36 (11.85) years, and 41.7% and 41.3% were female, respectively. High proportions of patients in both cohorts had NPEs during the 12-month baseline period. Among patients with no baseline NPEs, adjusted NPE incident rate ratios (95% CIs) during the post-index period were as follows: any, 1.15 (1.00–1.33); chronic, 1.18 (0.98–1.42); and acute, 1.16 (0.96–1.39). Mean all-cause and NPE-related costs were similar between cohorts.
Conclusions: In this study of the Medicaid population, the prevalence and incidence of NPEs, as well as health care costs, were similar among people living with HIV-1 newly treated with an INI- or PI-based regimen.
Prim Care Companion CNS Disord 2023;25(2):22m03374
To cite: Song J, Donga P, Holt J, et al. The burden of neuropsychiatric disorders in Medicaid patients living with HIV-1 treated with integrase inhibitor or protease inhibitor antiretroviral therapies. Prim Care Companion CNS Disord. 2023;25(2):22m03374.
To share: https://doi.org/10.4088/PCC.22m03374
© 2023 Physicians Postgraduate Press, Inc.
aJanssen Scientific Affairs, LLC, Titusville, New Jersey
bNow with Gilead Sciences, Inc, Foster City, California
*Corresponding author: Ji Song, PhD, 1125 Trenton-Harbourton Rd, Titusville, NJ 08560 ([email protected]).
Neuropsychiatric disorders, such as depression or anxiety, occur more frequently among people living with human immunodeficiency virus (HIV)–1 than in the general population.1–4 The pathophysiology of such conditions is complex and may be due to several factors, including characteristics of HIV-1, social implications of living with HIV-1, and adverse reactions from antiretroviral therapy (ART).1 The high burden of neuropsychiatric disorders among people living with HIV-1 may contribute to challenges across the HIV continuum of care, including suboptimal ART adherence and reduced retention in care.5 Furthermore, these behaviors can lead to regimen discontinuation, development of ART resistance, disease progression, and increased mortality risk.5–7
The US Department of Health and Human Services recognizes that neuropsychiatric disorders are an important consideration in selecting an ART regimen, as some antiretroviral agents may exacerbate these conditions.5 Indeed, previous studies have shown that certain antiretroviral agents, most notably efavirenz and rilpivirine, can worsen psychiatric symptoms.1,5,8 Additionally, neuropsychiatric adverse events (AEs) leading to discontinuations have been seen in phase 3 studies of integrase inhibitor (INI)–based regimens.9,10
The high burden of neuropsychiatric disorders among people with HIV-1 is accompanied by a high economic burden, including both all-cause and neuropsychiatric event (NPE)–related costs.11 In particular, costs are substantially greater for people with HIV-1 who have a serious mental illness compared to patients with either HIV-1 or a serious mental illness alone.12–14 In the US general population, neuropsychiatric disorders are more common among Medicaid beneficiaries than in those with commercial health insurance.15 Moreover, Medicaid is one of the largest payers for HIV-1 therapy in the United States, covering approximately 45% of people with HIV-1.16 In a recent retrospective cohort study of Medicaid patients, those with HIV-1 who were newly treated with ART were found to have a higher 6-month period prevalence of NPEs than patients without HIV-1, although these differences were not stratified by ART class.17 There are limited data to demonstrate how modern protease inhibitor (PI)–based regimens compare to INI-based regimens with respect to the incidence of NPEs. In this study, the prevalence, incidence, and economic burden of NPEs in patients with HIV-1 who were newly treated with INI- or PI-based regimens were assessed in the Medicaid population.
METHODS
Study Design and Population
A retrospective cohort study was conducted using US administrative claims data from the IBM MarketScan Multi-State Medicaid Database collected between January 1, 2014, and December 31, 2018. The adjudicated claims data included outpatient diagnoses and procedures, hospital discharge diagnoses, and outpatient pharmacy claims. The intake period was from January 1, 2015, to June 30, 2018. The index date was defined as the date on which the earliest prescription for INI- or PI-based ART was filled during the intake period. Patients were followed for 6 months from the index date. The study design is summarized in Supplementary Figure S1.
Eligible patients were treatment-naive or treatment-experienced adults who were newly treated with an INI- or PI-based regimen during the intake period. Key inclusion criteria were adults (aged ≥ 18 years) with ≥ 1 diagnosis code for HIV-1 during the 12-month period preceding the index date (baseline period), ≥1 prescription fill for INI- or PI-based ART during the intake period, and ≥ 12 months of continuous health plan enrollment prior to the index date. Key exclusion criteria included having ≥ 1 prescription fill for the same antiretroviral class as the index regimen (ie, INI or PI) at any time prior to the index date or a non-nucleoside reverse transcriptase inhibitor (NNRTI) as part of the index regimen.
All data collected were deidentified in compliance with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. As this study consisted of secondary data analyses of deidentified patient records, informed consent and institutional review board approval were not required.
Outcomes
NPEs of interest included chronic and acute NPEs (Supplementary Table S1). The prevalence of NPEs of interest was evaluated during the 12-month baseline period and 6-month post-index period. The prevalence of NPEs of interest was calculated by dividing the number of patients having comorbid NPEs of interest by the total cohort population.
During the 6-month post-index period, the incidence of NPEs of interest was evaluated using incidence rate ratio (IRR) for 2 subgroups of patients. One subgroup (IRR 1) assessed the incidence of NPEs among patients who did not have chronic NPEs during the 12-month baseline period and/or did not have acute NPEs during the 3-month baseline period, while the other subgroup (IRR 2) assessed the incidence of NPEs among patients who did not have chronic NPEs during the 12-month baseline period and also did not have acute NPEs during the 3-month baseline period.
Per-patient per-month (PPPM) total all-cause and NPE-related health care resource utilization and costs between treatment cohorts were assessed during the 6-month post-index period. Total all-cause and NPE-related costs (total medical costs + total pharmacy costs) were reported in 2018 US dollars. Medical costs included inpatient hospitalizations, emergency department visits, physician office visits, outpatient service encounters, and skilled nursing facility/long-term care visits.
Statistical Analysis
Descriptive statistics, including frequencies and proportions, were used to report categorical variables, and means (SDs) were reported for continuous variables. Outcomes were compared between those initiating an INI-based regimen versus those initiating a PI-based regimen. Inverse probability treatment weighting (IPTW) was used to create more comparable treatment cohorts by accounting for confounding factors. IPTW-related weights were calculated using a logistic regression model adjusting for baseline covariates, including age, gender, region, race, index year, Quan-Charlson Comorbidity Index18 score, total number of baseline comorbidities, total number of concomitant medications during the baseline period, and baseline HIV-1–related procedures. Using standardized differences, the balance between cohorts was assessed, with a standardized difference < 10% considered well balanced.19
RESULTS
Baseline Period
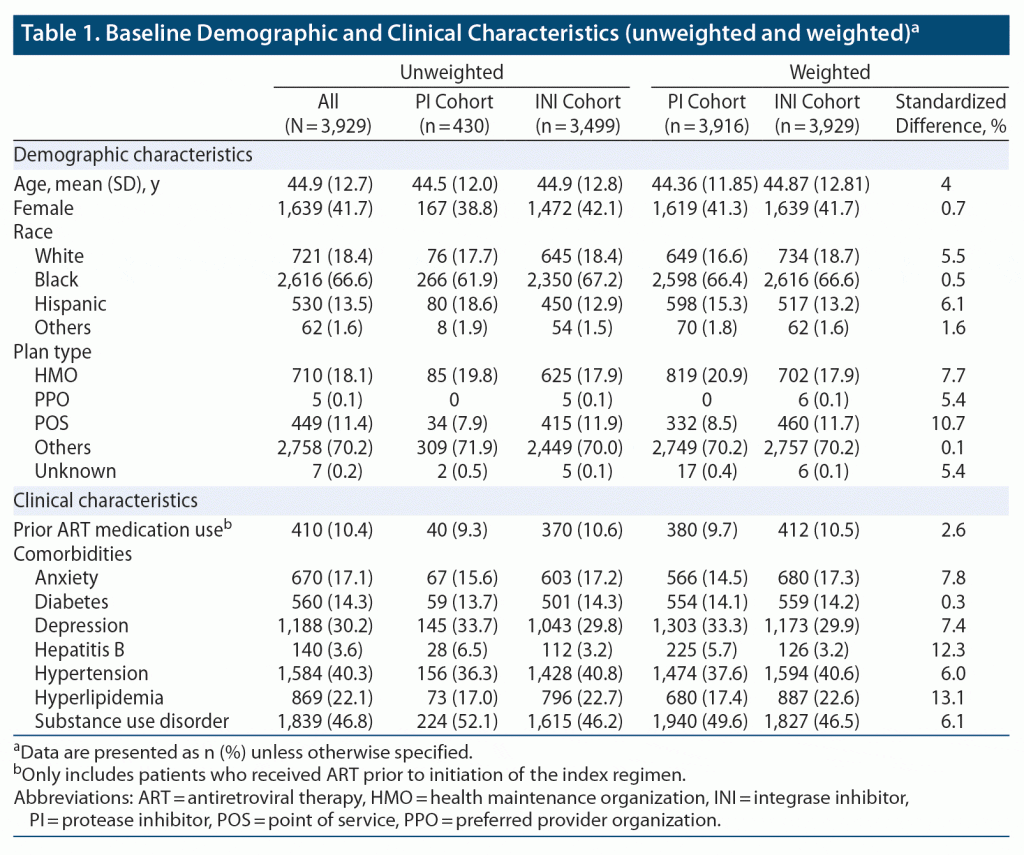
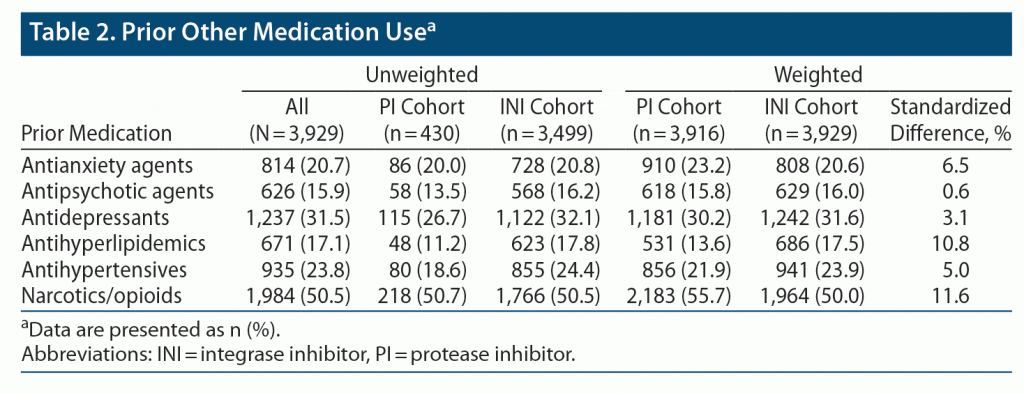
Of the total 3,929 patients included in the study, 3,499 were receiving an INI-based regimen and 430 were receiving a PI-based regimen (Supplementary Figure S2). The most frequently prescribed INI- and PI-based regimens were dolutegravir (48%) and darunavir (77%), respectively (Supplementary Table S2). After weighting the cohorts using IPTW, 3,929 patients were included in the INI cohort, and 3,916 patients were included in the PI cohort. In the INI and PI cohorts, the mean (SD) ages were 44.87 (12.81) and 44.36 (11.85) years, and 41.7% and 41.3% of patients were female, respectively. At baseline, the proportions of patients with hyperlipidemia, hepatitis B, and receiving narcotics/opioids were significantly different between treatment cohorts (Table 1, Table 2). Overall, 412 (10.5%) patients in the INI cohort and 380 (9.7%) patients in the PI cohort were treatment experienced (defined as ART medication use prior to baseline; Table 1). Among patients in the INI cohort, 187 (4.8%) previously received a PI-based regimen, 174 (4.4%) previously received an NNRTI-based regimen, and 69 (1.8%) previously received other ART agents. Among patients in the PI cohort, 104 (2.6%) previously received an INI-based regimen, 144 (3.7%) previously received an NNRTI-based regimen, and 143 (3.7%) previously received other ART agents.
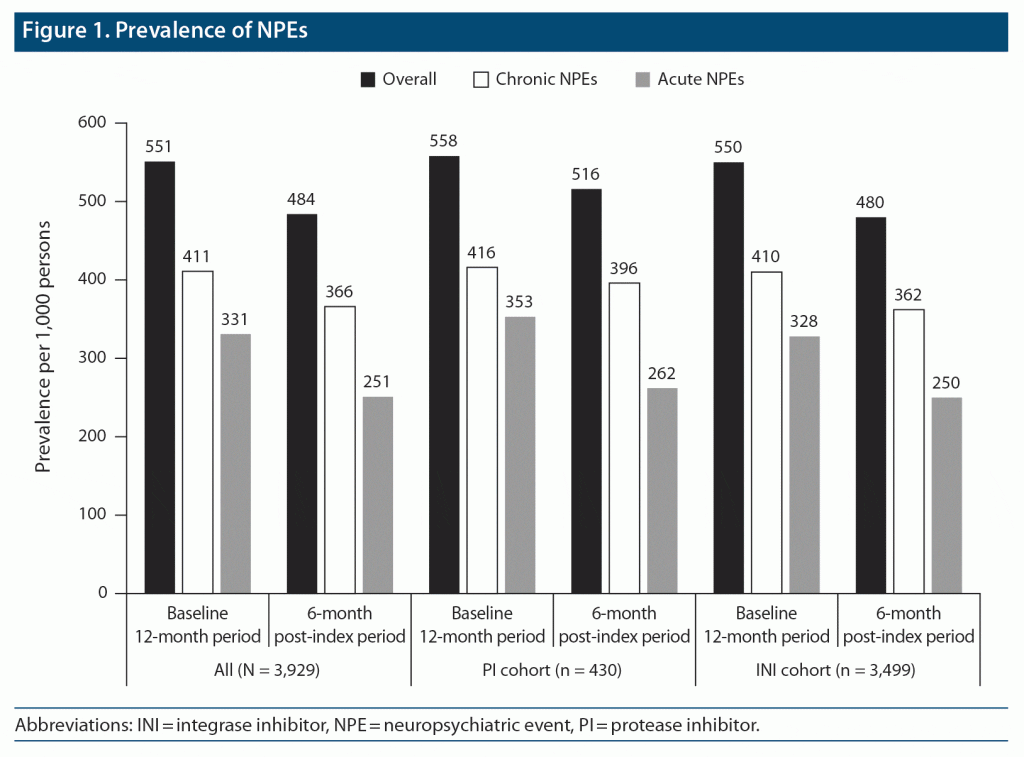
During the 12-month baseline period, high proportions of patients had NPEs, with chronic NPEs being numerically more common than acute NPEs across treatment cohorts. Among patients in the INI cohort, the prevalence of any, chronic, or acute NPEs was 55%, 41%, and 33%, respectively. For the PI cohort, the prevalence of any, chronic, or acute NPEs was 56%, 42%, and 35%, respectively.
Post-Index Period: NPEs
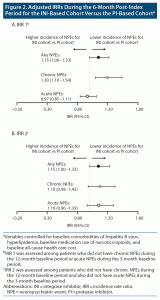
The prevalence of both chronic and acute NPEs decreased during the 6-month post-index period, regardless of treatment cohort (Figure 1). Overall, the incidence of new NPEs during the 6-month post-index period was similar for the INI- and PI-based cohorts. Among patients who did not experience chronic NPEs during the 12-month baseline period or acute NPEs during the 3-month baseline period (IRR 1), the adjusted IRRs (95% CI) were 1.15 (1.00–1.33) for any NPEs, 1.30 (1.10–1.54) for chronic NPEs, and 0.97 (0.85–1.11) for acute NPEs (Figure 2A). For patients who did not experience chronic NPEs during the 12-month baseline period and also did not experience acute NPEs during the 3-month baseline period (IRR 2), the adjusted IRRs (95% CI) for any NPEs, chronic NPEs, and acute NPEs were 1.15 (1.00–1.33), 1.18 (0.98–1.42), and 1.16 (0.96–1.39), respectively (Figure 2B).
Post-Index Period: Costs
During the 6-month post-index period, PPPM total all-cause and NPE-related costs were similar for the INI and PI cohorts. The mean (SD) adjusted PPPM total all-cause health care costs were $5,022 ($12,671) for patients in the INI cohort and $4,787 ($8,346) for patients in the PI cohort (Supplementary Figure S3A). Mean (SD) adjusted PPPM total NPE-related medical costs were $321 ($1,802) for patients in the INI cohort and $272 ($1,135) for patients in the PI cohort (Supplementary Figure S3B).
DISCUSSION
In this study of the Medicaid population, people who were newly treated with an INI- or PI-based regimen had a high prevalence of existing NPEs, with chronic NPEs being more common than acute NPEs at baseline. Additionally, regardless of treatment cohort, the prevalence of NPEs decreased between baseline and the 6-month post-index period. The incidence of any new-onset NPEs did not significantly differ between those receiving an INI-based regimen and those receiving a PI-based regimen. Total PPPM all-cause and NPE-related costs during the 6-month post-index period were similar across the INI- and PI-based cohorts. Notably, all-cause health care spending was higher for the INI- and PI-based cohorts compared to that observed for the general Medicaid population.20 This finding is consistent with previous studies21–23 that found considerable costs associated with HIV care as well as neuropsychiatric disorders.
In addition to increased health care spending, NPEs may lead to challenges across the HIV continuum of care.5 As certain antiretroviral agents have been associated with a propensity to cause or worsen NPEs,8,24–26 the incidence of NPEs is an important consideration when selecting or switching an ART regimen. In a pooled analysis of 2 phase 3 studies, efavirenz was found to be associated with greater neurologic and psychiatric AEs than rilpivirine.8 Additionally, studies24–26 comparing INI agents showed that dolutegravir was associated with neuropsychiatric AEs and discontinuations due to these AEs more frequently than other INIs. Conversely, an analysis of 2 phase 3 studies of a darunavir-based, single-tablet regimen demonstrated that patients with baseline NPEs did not have higher rates of new-onset study drug–related neurologic or psychiatric AEs than those without baseline NPEs.27
In the current study, no clinically meaningful differences were seen in the incidence of new-onset NPEs or all-cause and NPE-related costs between the INI- and PI-based cohorts. One potential explanation is the relatively short duration of follow-up, which may have precluded NPEs from presenting. Similar costs and health care resource utilization may have been observed across treatment cohorts due to the high underlying costs associated with the management and treatment of HIV-1.
This study has some limitations to consider. As with all claims data sources, Medicaid data may contain billing inaccuracies or omissions in diagnoses and other variables. Additionally, not all NPEs may have been captured in the claims data, given the complexity with coding. The majority of patients included in this study were treatment naive, so findings may not be generalizable to all patients with HIV-1. Furthermore, the short duration of follow-up may have precluded acute NPEs from presenting or becoming chronic. Notably, this study was conducted prior to the coronavirus disease 2019 pandemic, which has been associated with increases in mental health disorders.28
In summary, the prevalence and incidence of NPEs, as well as all-cause and NPE-related costs, were similar among people living with HIV-1 newly treated with an INI- or a PI-based regimen. Findings from this analysis underscore the high NPE burden as well as the considerable economic costs associated with NPEs among people living with HIV-1.
Submitted: July 22, 2022; accepted October 11, 2022.
Published online: April 4, 2023.
Author contributions: All authors contributed to the analysis and interpretation of the data, participated in the drafting of this manuscript, approved the final version, and take responsibility for the integrity of the work as a whole.
Relevant financial relationships: All authors were employees of Janssen Scientific Affairs, LLC, at the time of the study and may be stockholders in Johnson & Johnson.
Funding/support: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Janssen Scientific Affairs, LLC, and the authors were employees of Janssen Scientific Affairs, LLC, at the time of the study.
Role of the sponsor: The study sponsor was involved in the design, analysis, interpretation, and publication of this study.
Acknowledgments: Medical writing support was provided by Courtney St. Amour, PhD, of Lumanity Communications Inc, and was funded by Janssen Scientific Affairs, LLC. Dr St. Amour has no other conflicts of interest related to the subject of this article.
Additional information: The data that support the findings of this study are available from IBM MarketScan Research Databases. Restrictions apply to the availability of these data, which were used under license for this study. Interested individuals may visit https://www.ibm.com/products/marketscan-research-databases for more information on accessing data from the IBM MarketScan Multi-State Medicaid Database.
ORCID: Ji Song: https://orcid.org/0000-0002-4929-0745
Supplementary material: See accompanying pages.
Clinical Points:
- Neuropsychiatric disorders occur more frequently among people with human immunodeficiency virus (HIV)–1 than in the general population; this high clinical burden may contribute to challenges across the HIV continuum of care and is often accompanied by a high economic burden.
- Data are scarce regarding the incidence of neuropsychiatric events in people with HIV-1 taking integrase inhibitor– or protease inhibitor–based antiretroviral regimens.
- In this study of the Medicaid population, the prevalence and incidence of neuropsychiatric events, as well as health care costs, were similar among people with HIV-1 newly treated with an integrase inhibitor– or protease inhibitor–based regimen.
References (28)

- Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423–431. PubMed CrossRef
- Lowther K, Selman L, Harding R, et al. Experience of persistent psychological symptoms and perceived stigma among people with HIV on antiretroviral therapy (ART): a systematic review. Int J Nurs Stud. 2014;51(8):1171–1189. PubMed CrossRef
- Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171. PubMed CrossRef
- Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosom Med. 2005;67(2):260–269. PubMed CrossRef
- Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Panel on Antiretroviral Guidelines for Adults and Adolescents web site. Accessed April 24, 2022. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf
- Saag LA, Tamhane AR, Batey DS, et al. Mental health service utilization is associated with retention in care among persons living with HIV at a university-affiliated HIV clinic. AIDS Res Ther. 2018;15(1):1. PubMed CrossRef
- Bhatia MS, Munjal S. Prevalence of depression in people living with HIV/AIDS undergoing ART and factors associated with it. J Clin Diagn Res. 2014;8(10):WC01–WC04. PubMed CrossRef
- Mills AM, Antinori A, Clotet B, et al; ECHO and THRIVE study groups. Neurological and psychiatric tolerability of rilpivirine (TMC278) vs efavirenz in treatment-naïve, HIV-1-infected patients at 48 weeks. HIV Med. 2013;14(7):391–400. PubMed CrossRef
- Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–e372. PubMed CrossRef
- Walmsley SL, Antela A, Clumeck N, et al; SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. PubMed CrossRef
- Song J, Hardy H, Chow W, et al. The burden of neuropsychiatric events in patients living with HIV-1 treated with antiretroviral therapies–a perspective from US Medicaid data. Presented at the 10th International Workshop on HIV & Aging; October 10-11, 2019; New York, NY. Poster number 51.
- Rothbard AB, Metraux S, Blank MB. Cost of care for Medicaid recipients with serious mental illness and HIV infection or AIDS. Psychiatr Serv. 2003;54(9):1240–1246. PubMed CrossRef
- Rothbard AB, Miller K, Lee S, et al. Revised cost estimates of Medicaid recipients with serious mental illness and HIV-AIDS. Psychiatr Serv. 2009;60(7):974–977. PubMed CrossRef
- Rothbard AB, Lee S, Blank MB. Cost of treating seriously mentally ill persons with HIV following highly active retroviral therapy (HAART). J Ment Health Policy Econ. 2009;12(4):187–194. PubMed
- Gibson TB, Lee TA, Vogeli CS, et al. A four-system comparison of patients with chronic illness: the Military Health System, Veterans Health Administration, Medicaid, and commercial plans. Mil Med. 2009;174(9):936–943. PubMed CrossRef
- Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project, United States, 2017 Cycle (June 2017–May 2018). HIV Surveillance Special Report 23. Centers for Disease Control and Prevention. Accessed April 17, 2020. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- Chow W, Hardy H, Song J, et al. The burden of neuropsychiatric disorders in patients living with HIV-1 treated with antiretroviral therapies—a perspective from US Medicaid data. Int J STD AIDS. 2022;33(3):275–281. PubMed
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. PubMed CrossRef
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. PubMed CrossRef
- NHE fact sheet. Centers for Medicare and Medicaid Services. Accessed June 14, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Yeung H, Krentz HB, Gill MJ, et al. Neuropsychiatric disorders in HIV infection: impact of diagnosis on economic costs of care. AIDS. 2006;20(16):2005–2009. PubMed CrossRef
- Gebo KA, Fleishman JA, Conviser R, et al; HIV Research Network. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24(17):2705–2715. PubMed CrossRef
- Hajat C, Siegal Y, Adler-Waxman A. Clustering and healthcare costs with multiple chronic conditions in a US study. Front Public Health. 2021;8:607528. PubMed CrossRef
- Peñafiel J, de Lazzari E, Padilla M, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72(6):1752–1759. PubMed CrossRef
- Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63. PubMed CrossRef
- Hoffmann C, Llibre JM. Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Rev. 2019;21(1):4–10. PubMed CrossRef
- Bushen J, Luo D, Simonson RB, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment-naive (AMBER) and virologically suppressed (EMERALD) patients with neurologic and/or psychiatric comorbidities: week 96 subgroup analysis. Presented at the 23rd International AIDS Conference (AIDS 2020); July 6–10, 2020; Virtual.
- Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top