Stress-induced changes in pharmacokinetics can significantly alter the plasma levels of some drugs such as clozapine. This report describes the case of a middle-aged man with schizoaffective disorder, bipolar type who showed sustained elevation in clozapine levels 3 days after discontinuation. Before the clozapine levels were drawn, he had developed acute bacterial pneumonia and signs of acute bacterial meningitis followed by neuroleptic malignant syndrome after he received multiple doses of intravenous haloperidol for worsening psychosis and aggressive behavior. Existing literature on this topic is also reviewed to investigate potential reasons for sustained clozapine levels during acute inflammatory stress and neuroleptic malignant syndrome.

Relationship Between Clozapine Levels and Acute Inflammatory Stress
ABSTRACT
Stress-induced changes in pharmacokinetics can significantly alter the plasma levels of some drugs such as clozapine. This report describes the case of a middle-aged man with schizoaffective disorder, bipolar type who showed sustained elevation in clozapine levels 3 days after discontinuation. Before the clozapine levels were drawn, he had developed acute bacterial pneumonia and signs of acute bacterial meningitis followed by neuroleptic malignant syndrome after he received multiple doses of intravenous haloperidol for worsening psychosis and aggressive behavior. Existing literature on this topic is also reviewed to investigate potential reasons for sustained clozapine levels during acute inflammatory stress and neuroleptic malignant syndrome.
Prim Care Companion CNS Disord 2020;22(4):19br02586
To cite: Wagner A, Shad MU. Relationship between clozapine levels and acute inflammatory stress. Prim Care Companion CNS Disord. 2020;22(4):19br02586.
To share: https://doi.org/10.4088/PCC.19br02586
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Good Samaritan Regional Medical Center, Corvallis, Oregon
bThe University of Toledo College of Medicine and Life Sciences, Toledo, Ohio
cDepartment of Psychiatry, Touro University, Vallejo, California
*Corresponding author: Mujeeb U. Shad, MD, MSCS, Napa State Hospital, 2100 Napa Vallejo Hwy, Napa, CA 94558 ([email protected]).
Clozapine is still considered a gold standard in the management of treatment-refractory schizophrenia. However, despite its proven efficacy, clozapine has not been accepted as a first-line treatment for schizophrenia due to multiple clinically serious adverse effects, including agranulocytosis and lowering of seizure threshold. Although these adverse effects are rare, they are well known to prescribers and closely monitored due to their clinical seriousness. But, there is less awareness of the risks associated with different types of drug interactions, including drug-disease interactions, in which an acute medical stress can alter the plasma level of drugs such as clozapine to further complicate psychiatric and physical health in medically vulnerable patients. Here, we review existing literature and describe the case of a middle-aged man with acute bacterial pneumonia/meningitis and haloperidol-induced neuroleptic malignant syndrome who developed sustained toxic levels of clozapine 3 days after discontinuation.
CASE REPORT
The patient was a 51-year-old white man with treatment-refractory schizoaffective disorder, bipolar type along with tobacco use disorder, obesity, hyperlipidemia, recurrent pneumonia, and chronic cough. He was stable enough on clozapine 450 mg/day augmented with lamotrigine 25 mg/day to live independently in an apartment. However, before admission to the hospital, he had presented to urgent care with flu-like symptoms along with unyielding auditory hallucinations. He was given acetaminophen 1,000 mg and oseltamivir 75 mg prior to being sent home. However, he continued to experience severe headache and auditory hallucinations and was taken to the emergency department.
At the hospital, the patient was found to have a fever of 102.6°F, tachycardia with a pulse of 117 bpm, and hypertension with a blood pressure of 146/77 mm Hg. He was only communicative with nonverbal cues and nodded in affirmation when asked about auditory hallucinations. He was observed repeatedly mimicking a smoking action with his pulse oximeter and on physical examination was found to have muscle rigidity of the neck and upper and lower extremities but with no change in muscle tone. Laboratory workup revealed mild leukocytosis (13.67 mm3) and a mildly toxic lactate level of 3.4 mmol/L; however, metabolic profile (except blood sugar level of 206 mg/dL), thyroid-stimulating hormone, T4, creatine kinase, ammonia, procalcitonin, urinalysis, and alcohol and urine drug screen findings were unremarkable. Blood cultures were positive for streptococcus pneumonia, and a chest x-ray revealed bilateral pulmonary infiltrates. The patient’s fever remained around 102.5°F during this time, probably due to repeated use of acetaminophen.
Medical staff attempted to perform a spinal tap, as the patient had neck rigidity, but could not do so successfully due to writhing movements. He was admitted to the medical unit for the treatment of severe sepsis, most likely secondary to bacterial pneumonia and potentially meningitis, and began treatment with broad-spectrum antibiotics. The patient’s fever, leukocytosis, and writhing movements decreased to allow for spinal tap, which revealed bacterial meningitis, most likely from the same bacteria that was found earlier in his blood cultures. However, his procalcitonin and lactate levels increased dramatically accompanied by encephalopathy, as reflected by agitation and verbal and physical aggression toward staff. These symptoms were treated with 5 consecutive doses of intravenous haloperidol 5 mg within 24 hours, which was followed by a relapse in fever with altered mental state, tachycardia, tachypnea, diaphoresis, increased blood pressure, tremors, confusion, and muscle rigidity. Neuroleptic malignant syndrome was diagnosed after laboratory values revealed a creatine kinase level of 3,200 U/L along with some elevation in liver enzymes (aspartate aminotransferase = 196 U/L, alanine aminotransferase = 126 U/L). However, instead of leukocytosis, a decline in leukocyte (9.92 to 6.90 mm3) and neutrophil (11.91 to 5.62 mm3) count was observed, possibly due to sepsis.1
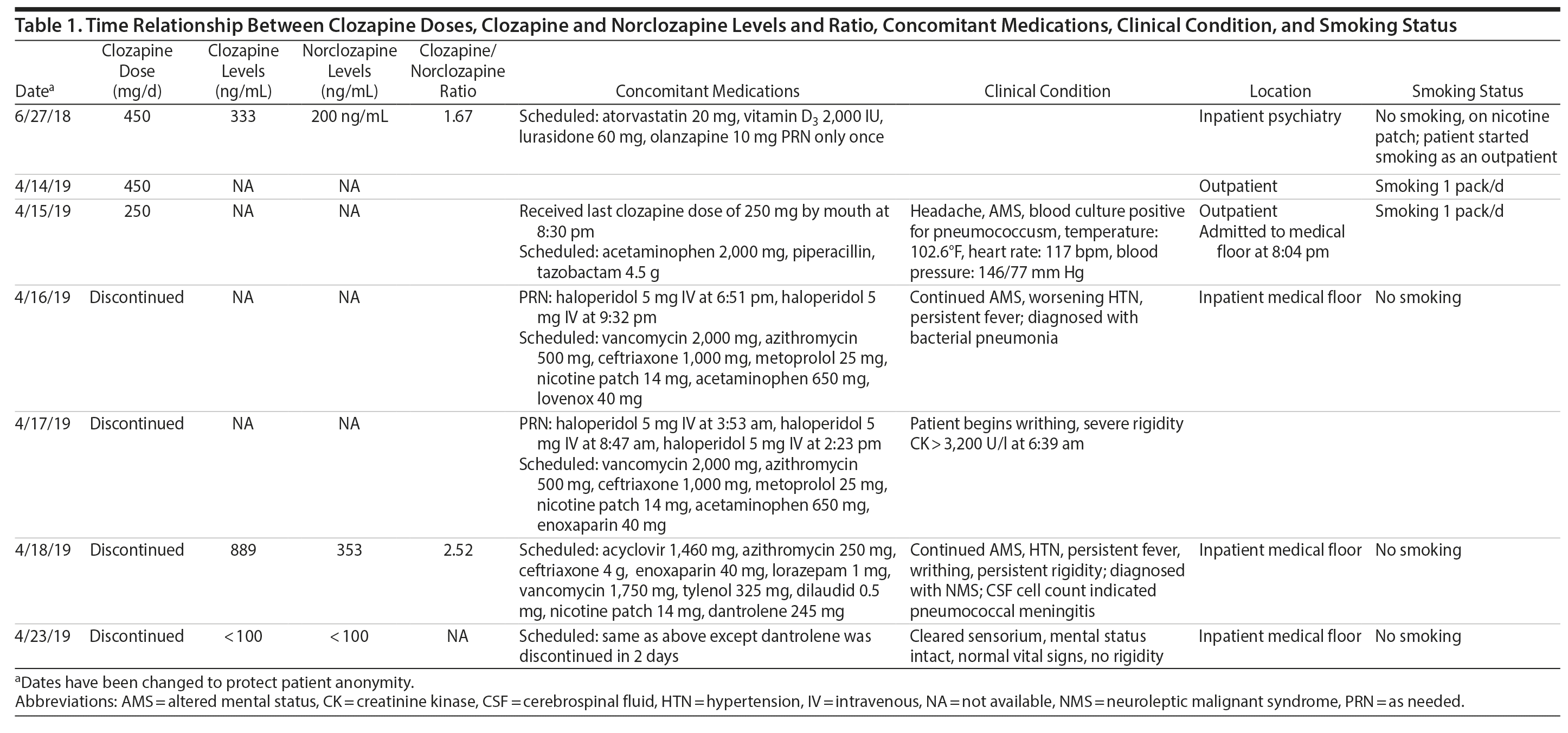
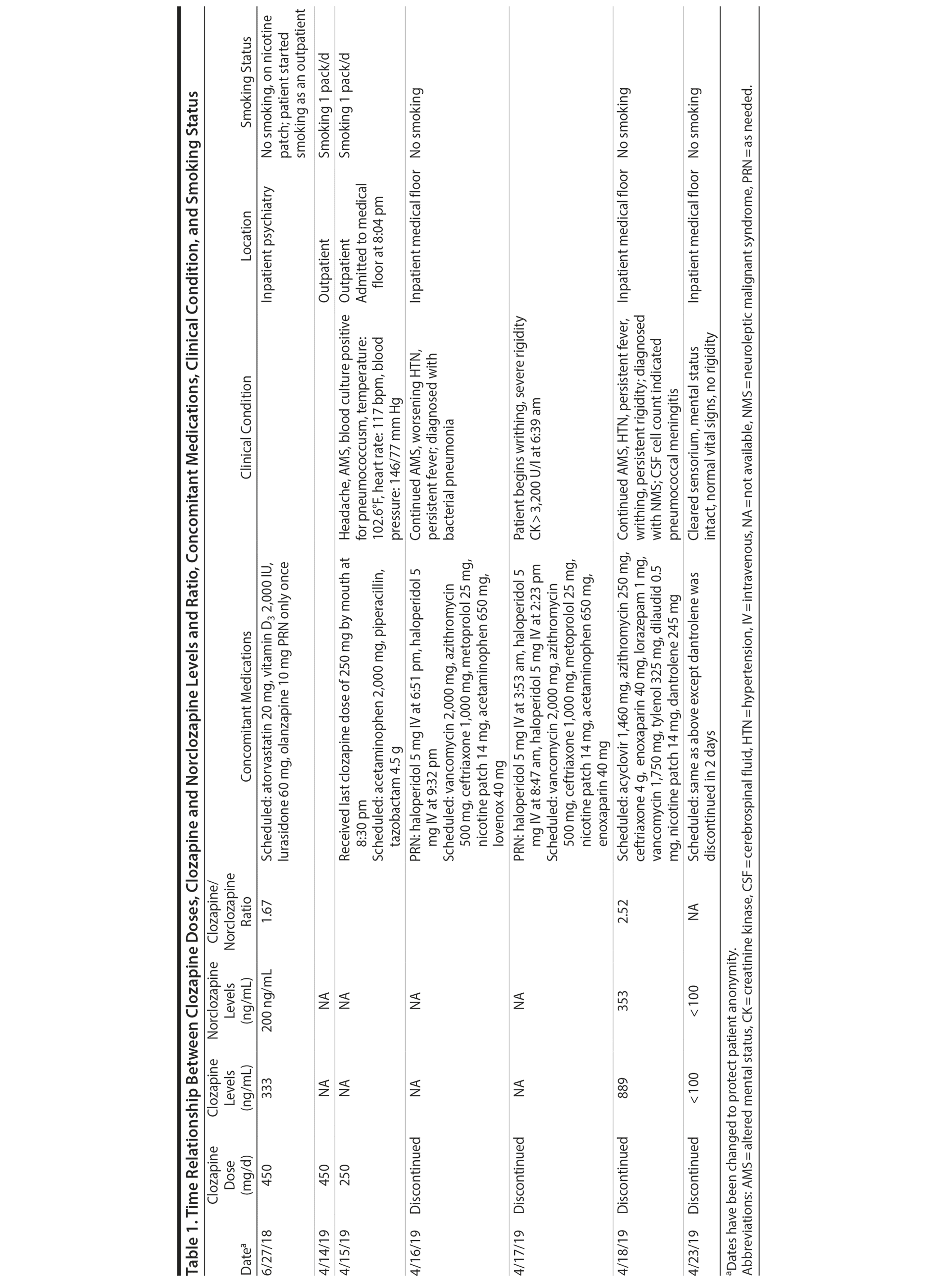
The patient was admitted to the intensive care unit to manage neuroleptic malignant syndrome with supportive care measures including lorazepam, cooling blankets, and close observation of vital signs. These measures failed to decrease his fever or leukocyte count, thus he was started on dantrolene. Treatment with dantrolene resulted in rapid improvement in fever, leukocytosis, and mental status as well as normalization of vital signs, and he was transferred out of the intensive care unit. Surprisingly, a clozapine level ordered 60 hours after the last bedtime dose of 250 mg revealed a clozapine and norclozapine level of 1,242 ng/mL. Of note, the patient had stopped smoking (ie, 1 pack/day) after his previous visit to the emergency department before this hospitalization. Interestingly, a similar dose of clozapine (ie, 450 mg/d) approximately 10 months ago produced a total clozapine and norclozapine level of 533 ng/mL (Table 1).
Clozapine Plasma Levels
The only baseline clozapine levels were 333 ng/mL for clozapine and 200 ng/mL for norclozapine on a daily dose of 450 mg/d. The second levels, drawn about 8 months later, were 889 ng/mL for clozapine and 353 ng/mL for norclozapine after 60 hours of a bedtime dose of clozapine 250 mg. The second level was assessed 1 to 2 days after the patient was diagnosed with bacterial pneumonia.
Clozapine/Norclozapine Ratio
The clozapine/norclozapine ratio is calculated by dividing clozapine with norclozapine plasma levels. This ratio was 1.67 at baseline. However, this ratio significantly increased to 2.52 at the second clozapine level assessment.
DISCUSSION
Several case reports and case series2-11 have documented changes in clozapine levels in response to acute inflammatory stress. Each of these cases, including the present case, has enhanced our understanding of various clinical aspects of this highly complex interaction between clozapine levels and inflammatory stress (drug-disease interaction). One such aspect that is unique to our case is that the unusual increase in clozapine levels could have been easily missed due to medical comorbidities and their treatment if clozapine levels were not requested 3 days after its discontinuation. It was the sustained clozapine level that initiated further investigation into this case (Table 1).

- There are clinically meaningful interactions between clozapine levels and acute inflammatory stress.
- Clozapine plasma levels may significantly increase during acute bacterial infections.
- Monitoring of clozapine levels may help in the detection of clozapine toxicity and prevention of complications in a medically vulnerable patient population.
To begin with, smoking is highly relevant to this case, as hydrocarbons in cigarette smoke are known to lower clozapine levels through induction of cytochrome P450 (CYP) enzyme 1A2, which provides the main metabolic pathway for clozapine.12,13 Hence, clozapine levels rebound after smoking is discontinued,2 as happened in our patient. However, this explanation is unlikely since our patient was within 60 hours of smoking discontinuation and increased clozapine levels are not observed earlier than 14 days after smoking discontinuation.14 Clozapine overdose could also explain unusually high clozapine levels before the patient presented to urgent care. However, the patient’s medical records revealed a long history of medication adherence. Nevertheless, an accidental clozapine overdose during an altered state of mind cannot be completely ruled out. Concomitant use of metoprolol and repeated doses of intravenous haloperidol are also unlikely to solely explain high clozapine levels, as CYP2D6, which metabolizes both metoprolol and haloperidol, does not provide the primary metabolic pathway for clozapine’s metabolism to norclozapine.12 Other concomitant medications (ie, analgesics and antibiotics) are also unlikely to alter clozapine levels significantly. In addition, an organ dysfunction secondary to bacterial sepsis and neuroleptic malignant syndrome could contribute toward an increase in clozapine levels, but a normal metabolic profile makes it unlikely.
A literature review15 revealed 2 plausible mechanisms underlying an unusual increase in clozapine levels in patients during inflammatory stress. The first mechanism is stress-induced increase in the clozapine-binding plasma protein α-1-acid glycoprotein.16 One case report8 found a 50% increase in α-1-acid glycoprotein during acute illness. This finding is significant because clozapine is about 95% protein bound and its metabolites N-desmethylclozapine and clozapine N-oxide are also 90% and 75% protein bound, respectively.4 Thus, an increase in α-1-acid glycoprotein can increase the binding capacity of both clozapine and norclozapine. The outcome is an increase in the protein-bound clozapine and norclozapine levels without affecting the plasma levels for unbound and biologically active clozapine.11 Since protein-bound clozapine is biologically inactive, any increase in protein-bound clozapine is unlikely to result in adverse effects.
The second underlying mechanism is inhibition of CYP1A2, which is responsible for 70% of clozapine’s metabolism7 by stress-induced increase in proinflammatory markers such as interleukin-6 (IL-6),4 IL-1β, tumor necrosis factor-α, and α or γ interferons.2 Since CYP1A2 mediates clozapine’s biotransformation to norclozapine, an increase in the clozapine/norclozapine ratio from 1.67 (based on levels drawn 10 months previously) to 2.52 in our patient suggests inhibited activity of CYP1A2, which is consistent with similar increases in the clozapine/norclozapine ratio observed in several other reports.5,8,9 In 1 review,7 clozapine metabolism seemed to decrease by a factor of 2, specifically during a respiratory infection. In another study,17 the total change in clozapine levels during inflammation was measured using the concentration per dose of clozapine levels in the setting of elevated levels of C-reactive protein (CRP), which is the most accurate marker available to monitor inflammation. The results revealed the highest increase (ie, 48%) in clozapine levels compared to risperidone (ie, 24.2%) and quetiapine (11.9%) levels.17 This finding17 suggests that CRP can be employed as a useful marker for inflammation and, in turn, toxic clozapine levels.
Unlike increase in protein-bound inactive clozapine, increase in biologically active clozapine due to inhibition of CYP1A2 by proinflammatory markers can be associated with adverse effects such as myoclonus, hypotension, sialorrhea, obtundation,8 aphasia, akinesia, incoherence of speech, and gait disturbance.9 Despite being masked by acute medical comorbidities, some of these adverse effects could have contributed to the bacterial infection in our patient. It is at least theoretically possible that the relatively mild leukocytosis observed in this patient, despite acute bacterial infection and neuroleptic malignant syndrome, could be due to neutropenic effects of clozapine. Another frequently observed factor in this patient population, which increases susceptibility to respiratory infections, is smoking and the negative effects it has on cilia.4 Clozapine toxicity can also be increased by its biotransformation to nitrenium ions, which can induce proinflammatory response, thus further contributing to the acute inflammatory stress and inhibitory effects of proinflammatory markers on clozapine metabolism.18 Furthermore, during acute inflammation, clozapine molecules can act as haptens, resulting in hypersensitivity reactions.4 For these reasons, it can be extremely difficult to differentiate adverse effects of clozapine toxicity from the signs and symptoms of acute medical comorbidities as observed in this report.
CONCLUSION
This case exemplifies the complex interplay between clozapine pharmacokinetics and acute inflammatory stress secondary to acute bacterial infections. Although the adverse effects from toxic clozapine levels in our patient are difficult to separate from those caused by acute medical comorbidities, monitoring of clozapine levels during acute bacterial infections may help detect clozapine toxicity and prevent further complications in medically vulnerable patients.
Submitted: December 21, 2019; accepted February 21, 2020.
Published online: July 2, 2020.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1.Bone RC, Balk RA, Cerra FB, et al; The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655. PubMed CrossRef
2.ten Bokum EM, van de Oever HL, Radstake DW, et al. Clozapine intoxication due to cessation of smoking and infection. Neth J Med. 2015;73(7):345-347. PubMed
3.Takahashi T, Masuya Y, Ueno K, et al. Clozapine-related negative myoclonus associated with urinary tract infection: a case report. J Clin Psychopharmacol. 2015;35(2):205-206. PubMed CrossRef
4.Haack MJ, Bak ML, Beurskens R, et al. Toxic rise of clozapine plasma concentrations in relation to inflammation. Eur Neuropsychopharmacol. 2003;13(5):381-385. PubMed CrossRef
5.Raaska K, Raitasuo V, Arstila M, et al. Bacterial pneumonia can increase serum concentration of clozapine. Eur J Clin Pharmacol. 2002;58(5):321-322. PubMed CrossRef
6.Pfuhlmann B, Hiemke C, Unterecker S, et al. Toxic clozapine serum levels during inflammatory reactions. J Clin Psychopharmacol. 2009;29(4):392-394. PubMed CrossRef
7.de Leon J, Diaz FJ. Serious respiratory infections can increase clozapine levels and contribute to side effects: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1059-1063. PubMed CrossRef
8.Leung JG, Nelson S, Takala CR, et al. Infection and inflammation leading to clozapine toxicity and intensive care: a case series. Ann Pharmacother. 2014;48(6):801-805. PubMed CrossRef
9.Jecel J, Michel TM, Gutknecht L, et al. Toxic clozapine serum levels during acute urinary tract infection: a case report. Eur J Clin Pharmacol. 2005;60(12):909-910. PubMed CrossRef
10.Matthews CJ, Hall TL. A clozapine conundrum: clozapine toxicity in an acute medical illness. Australas Psychiatry. 2014;22(6):543-545. PubMed CrossRef
11.Lee LH, White RF, Barr AM, et al. Elevated clozapine plasma concentration secondary to a urinary tract infection: proposed mechanisms. J Psychiatry Neurosci. 2016;41(4):E67-E68. PubMed CrossRef
12.Pirmohamed M, Williams D, Madden S, et al. Metabolism and bioactivation of clozapine by human liver in vitro. J Pharmacol Exp Ther. 1995;272(3):984-990. PubMed
13.Rostami-Hodjegan A, Amin AM, Spencer EP, et al. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol. 2004;24(1):70-78. PubMed CrossRef
14.Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574. PubMed CrossRef
15.Hochepied T, Berger FG, Baumann H, et al. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003;14(1):25-34. PubMed CrossRef
16.Man WH, Wilting I, Heerdink ER, et al. Unbound fraction of clozapine significantly decreases with elevated plasma concentrations of the inflammatory acute-phase protein alpha-1-acid glycoprotein. Clin Pharmacokinet. 2019;58(8):1069-1075. PubMed CrossRef
17.Hefner G, Shams ME, Unterecker S, et al. Inflammation and psychotropic drugs: the relationship between C-reactive protein and antipsychotic drug levels. Psychopharmacology (Berl). 2016;233(9):1695-1705. PubMed CrossRef
18.Darling P, Huthwaite MA. Infection-associated clozapine toxicity. Clin Schizophr Relat Psychoses. 2011;5(3):159-160. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top